- *Corresponding Author:
- Shiwen Guo
Department of Neurosurgery, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi 710061, China
E-mail: rsc45678@126.com
| This article was originally published in a special issue, “Recent Developments in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(4) Spl Issue “269-278” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed u nder the identical terms
Abstract
This research aims to study the effect of interference with tripartite motif-containing protein 26 expression on the proliferation of intracranial glioma cells. This research randomly selected 60 cases of intracranial glioma specimens and the brain tissue of 10 cases of brain trauma patients as a normal control group. First, cell culture is performed to observe the growth status of the cells. Subsequently, the small interfering ribonucleic acid interference technique was used to interfere with the expression of tripartite motif-containing protein 26 on the glioma cell lines A172 and U251 cells. The cells were collected for tripartite motif-containing protein 26, Western blot and fluorescence quantitative polymerase chain reaction was used to verify the knockout effect, and the cell proliferation, clone formation, transfer, and invasion experiments were performed. Experimental data showed that among 60 glioma patients, tripartite motif-containing protein 26 samples were positive in 46 cases and 10 normal brain tissue samples were all negative. The performance of tripartite motif-containing protein 26 in glioma was compared with the performance in normal brain tissue. The difference was statistically significant (p<0.01). The results show that tripartite motif-containing protein 26 can reduce the protein level of pre-B-cell leukemia homeobox through the correlation of time gradient and concentration gradient. Tripartite motif-containing protein 26 can reduce the messenger ribonucleic acid level of ring finger protein 6 and the downstream target gene of pre-B-cell leukemia homeobox without affecting the messenger ribonucleic acid level of pre-B-cell leukemia homeobox.
Keywords
Tripartite motif-containing protein 26, glioma cells, cell proliferation, cell migration, pre-B-cell leukemia homeobox
Currently, intracranial glioma, as the most common malignant tumor in the brain, seriously endangers human life and health. In the clinical treatment of glioma, surgery, radiotherapy, chemotherapy and other methods are used, but the treatment effect is relatively poor. Most patients have a high brain tumor recurrence rate in a short period after operation. Most of the patients with malignant tumor survive less than 1 y. Therefore, improving the therapeutic effect of glioma is the focus of clinical and scientific research in neurosurgery world. This research aims to study the treatment effect of Tripartite Motif-Containing Protein 26 (TRIM26) expression on intracranial glioma.
The surgical resection of colloidal tumors and postoperative chemotherapy has not completely suppressed the occurrence of glioma. The recurrence rate of glioma is still very high and the prognosis is not optimistic. TRIM26 shares the physical location with Pre-B-Cell Leukemia Homeobox (PBX1) and is located in the nucleus. TRIM26 combines Spla and the Ryanodine Receptor (SPRY) structure region and PBX1, decomposes PBX1, inhibits its transcriptional activity, and promotes the proliferation of glioma cells. These have important significance and clinical value for glioma’s future theoretical research and clinical individual treatment guidance[1-4].
TRIM26 is a member of the triple coupled protein (Tripartite Motif-Containing Protein (TRIM)) in pan-coupling enzyme family. It has been found that its biological functions are mainly concentrated in two aspects. TRIM26 mediates the degradation of Interferon Regulatory Factor 3 (IRF3) through the polymerization of polyubiquitin chains linked via lysine 48 (K48) and further inhibits the production of Interferon beta (IFN-β), thereby inhibiting the antiviral response. Under the interference of the Ribonucleic Acid (RNA) virus, TRIM26 binds to the Nuclear Factor kappa B Essential Modulator (NEMO) protein after generalization and further binds the NEMO protein to the Trichome Birefringence-Like (TBL) to activate it, thereby having an effect on the RNA virus[5-8]. The multipotent transcription factor, Sex Determining Region Y-Box 2 (SOX2) is critical for the maintenance of glioblastoma stem cells and underlies tumor growth, treatment resistance, and recurrence. TRIM26 protects SOX and competes with WW domain containing E3 ubiquitin Protein ligase 2 (WWP2) for SOX2 binding, leading to inhibition of WWP2-mediated SOX2 ubiquitination and proteasome degradation[9].
These three domain family proteins have evolutionary and conserved structural regions and are considered to be important coordinators in cell biology. There are three N-terminal structural regions in TRIM members, including three zinc-binding structural regions, a ring structural region, a B-box 1 and B-box 2 and a coiled structural region. Therefore, it has many characteristics of opsin E3 universal ligase. TRIM protein affects cell proliferation, differentiation, non-specific immunity, cell death, cell migration, etc.,[9-12]. For example, TRIM27 and TRIM24 are oncogenes, while TRIM16 can block the cell cycle and inhibit the growth of aneurysms. TRIM28 has anti-proliferative activity by inhibiting the transcription factors of the E2F family in lung cancer. In gastrointestinal tumors, TRIM40 prevents cancer progression by promoting synaptic nuclear factor kappa B kinase regulatory subunit gamma inhibitors. In colon cancer, TRIM25 is highly expressed in colon cancer tissues, while TRIM25 is exogenously expressed in colon cancer cells. Transforming Growth Factor beta (TGF-β) signaling pathway regulates the growth and invasion of colon cancer cells, and its growth and migration rate is more than twice that of control cells[13-16].
TRIM26 belongs to the C-IV category of the TRIM series. In the protein structure, the TRIM family has a characteristic RING, B-box, Coiled-Coil (RBCC) structure at the N-terminus and the PRY-SPRY structure in which the substrate protein and substrate are combined at the C-terminus. TRIM26 cleaves the target into proteasomes, which are formed by the multiplication of substrate protein specifically mediated by pancreas ligase E3. TRIM26 mediates the formation of the K48-linked polyprimary chain of Nei Like Deoxyribonucleic Acid (DNA) Glycosylase 1 (NEIL1), which is related to the repair process of DNA damage, because mass spectrometry indicates that TRIM26 is a universal ligase for NEIL1 and NEIL1 may be degraded at this time [17-19].
Glioma is a malignant tumor of the central nervous system. It is produced by nerve interstitial cells and is divided into astrocytoma, medulloblastoma, ependymoma and pineoblastoma. The main symptoms of the patient are headache, neurological deficit, infiltration and brain tissue destruction caused by high cranial pressure[20-22].
Golgi Phosphoprotein 3 (GOLPH3) is also closely related to the glycosylation modification of proteins. The secreted protein formed by the small cell body enters from the front of the Golgi body and is transported between the membrane sacs. A series of orderly processing and modification takes place and most of the original sugar chain mannose is excised. However, various types of sugars were added in the order of various carbohydrate aminotransferases, resulting in specific carbohydrate aminotransferase modifications. GOLPH3 is attached to the N-terminus of ribose isoflavone kinase exposed on the cytoplasm. These lipases are fixed to the Golgi body along the mesenteric sac to regulate the processing and modification of carbohydrate proteins. The reduction of GOLPH3 may affect the fixation of pectin glycosyl aminotransferase, forming low-sterol secreted protein[23-27]. Changes in glycosylation of proteins are usually associated with tumor formation because changes in glycosylation of proteins may cause changes in cell proliferation, adhesion, invasiveness and immune cognition, as well as changes in intracellular signal transmission. Sterols play an important role in the transmission of membrane penetration signals caused by growth factors. Moreover, sterols modification may also change the activity of membrane receptors and affect the reaction with corresponding ligands. The glyceride modification fixes Epidermal Growth Factor Receptor (EGFR) on the intact membrane and protects the terminal nervous system of the cell, thereby increasing the role of growth factors.
As an important molecule in the cell, β-catechin has many functions closely related to the intracellular distribution. β-catechin is distributed in cell membrane, nucleus and cytoplasm. On the cell membrane, β-catechin and E-cadmium form a complex and participate in cell adhesion; β-catechin in the nucleus participates in intracellular signal transmission. Under normal circumstances, β-catechin in the cytoplasm is phosphorylated by Glycogen Synthase Kinase 3 (GSK3β) and decomposed by pantothenic acid, so the concentration of β-catechin in the cytoplasm is kept low. In some cases such as Wnt signal activation, the function of GSK3β is blocked, β-catechin in the cytoplasm cannot be phosphorylated and the concentration of β-catechin in the cytoplasm increases[28].
The main reason for the failure of colloid tumor treatment is relapse. Postoperative radiotherapy and chemotherapy are common treatments. Chemotherapy plays an important role in killing residual tumor cells. It is an important adjuvant therapy for the treatment of brain glioma tumors, which can easily cause severe liver damage and bone marrow suppression. However, radiation tolerance of tumor cells can cause recurrence of residual lesions in radiation therapy. Radiation therapy after surgery allows the survival time of patients to be extended, but the proportion of local recurrence is still high. As the dose increases, the patient’s cognitive dysfunction will continue to increase.
Effect of TRIM26 expression on glioma cell proliferation was explained here. Viral infection induces the emergence of TRIM26, which acts as a mechanism to avoid the natural immune system by regulating IRF3 and interferon[29]. In addition, the transfer group TRIM26 can directly decompose NEIL1 into viscose in vitro and then adjust the protein level of NEIL1 in vitro. The lack of TRIM26 greatly increases the resistance to Ionizing Radiation (IR) cells[30]. Some TRIM families target antiviral immunity may also be involved in the regulation of the main Base Excision Repair (BER) enzyme in the repair of cellular DNA damage[31]. As a new tumor suppressor of liver cancer, TRIM26 is significantly reduced in liver cancer compared with normal liver tissue and is related to the malignancy of liver cancer[30]. Moreover, TRIM26 can promote the proliferation and metastasis of Hepatocellular Carcinoma (HCC) cells. In Human Embryonic Kidney 293 (HEK293) cells, TRIM26 interacts with Tumor Necrosis Factor (TNF) Receptor Associated Factor-2 (TRAF2) through the optical coil domain, which plays an important role in regulating cell activation and tumor death[9].
Materials and Methods
This study is conducted in the neurosurgery department of 3201 Hospital Affiliated to Xi’an Jiaotong University. Before the study, the researchers have applied the ethical approval from the hospital. This study is approved by the ethical committee of the hospital and this experiment is obtained with the informed consent of all participants.
Specimen collection:
60 cases of glioma were randomly collected, including 15 cases of grade Ⅱ, 20 cases of grade Ⅲ neuropathy and 25 cases of grade Ⅳ neuropathy. After surgical resection, each patient’s specimen is divided into two parts. Some samples were fixed in 10 % formaldehyde solution. The remaining specimens are placed in sterile centrifuge tubes. Liquid nitrogen is kept frozen. Brain tissue was randomly selected from 10 brain trauma patients as a normal control group.
Experimental instruments and main reagents:
The main instruments and reagents used in the experiment are shown in Table 1 and Table 2.
| Serial number | Equipment name | Manufacturer |
|---|---|---|
| 1 | Ultra-clean workbench heal force safe 1200 | Germany Heraeus and Lishen |
| 2 | CO2 constant humidity incubator Heraeus BB 16 UV | Germany Heraeus and Lishen |
| 3 | Inverted phase contrast microscope Olympus CX43 | Olympus Corporation of Japan |
| 4 | Small desktop centrifuge 1-14 | American Sigma |
| 5 | Electronic balance CPA2245 | Germany Sartorius |
| 6 | Liquid nitrogen container YDS-35-125 | Leshan Dongya Electromechanical Industry and Trade Co. Ltd. |
| 7 | pH meter PHS-3C | Shanghai Jingke Company |
| 8 | Small desktop multifunctional oscillator IKA-MS 3 | Germany IKA |
| 9 | Micro-adjustable pipette (10, 100, 1000 μl) | American Gilson Corporation |
| 10 | Microplate reader Infinite M200 | American Tecan Company |
Table 1: Main Instruments Used in the Study
| Serial number | Reagent name | Manufacturer |
|---|---|---|
| 1 | Bicinchoninic Acid (BCATM) protein kit | American Pierce company |
| 2 | Rabbit anti-human Heat shock protein 90 (Hsp90) antibody | British Abeam |
| 3 | Horseradish Peroxidase (HRP) anti-rabbit secondary antibody | British Abeam |
| 4 | RNA reverse transcription kit | Toyobo |
| 5 | BCA protein concentration detection kit | Pierce |
| 6 | Phenylmethylsulfonyl Fluoride (PMSF) | Roche |
| 7 | Cocktail | Roche |
Table 2: Main Reagents Used in the Study
Preparation of reagents:
Mixing of Glial-cell line Derived Neurotrophic Factor (GDNF) is as follows. 1 mg Bovine Serum Albumin (BSA) was added into 1 ml Phosphate Buffered Saline (PBS) with 0.1 % concentration and then 10 μg GDNF was added to mix into 10 μg/μl GDNF mother liquor. After filtration and sterilization, it was frozen and stored in a refrigerator at -20°. When used, it can be diluted with 0.1 % BSA according to the required concentration.
The molecular weight of the 0.1 mg/ml polylysine acid solution (mother liquor) is 30 000 to 70 000. Dissolve 10 mg of polylysine in 100 ml of ultrapure water and use a 0.22 μm caliber disposable filter bacteria, store the prepared solution in the refrigerator at -20°.
Cell culture:
Preparation of culture medium: Add 10 % volume of fetal bovine serum and 1 % penicillin streptomycin antibiotic solution (100×) to the high glucose Dulbecco’s Modified Eagle Medium (DMEM), mix and store.
Cell recovery: Remove the frozen cells from the liquid nitrogen tank and pinch the cells with tweezers, taking care to avoid frostbite. Immediately place the frozen storage tube in a 37° water bath and shake it constantly. Thaw the cryopreservation solution into the cryopreservation tube in about 1 min. Under sterile conditions, absorb the ice and store the liquid. After discarding the liquid above, you can see the sedimented cells in the center. Blow dry and mix the cells evenly, add a small amount of medium to allow them to settle, then transfer to a cell culture flask and gently blow into the culture flask to mix. Transfer to 37° and incubate in 5 % Carbon dioxide (CO2) environment. The next day, change the medium and continue to grow and observe the growth.
Immunohistochemistry:
Insert the tissue slice into the slice frame, add distilled water to the washing box, place it on the shaker 3 times at a rate of 3 times/min, discard the distilled water, add PBS buffer and then place it on the shaker. Leave and wash 3 times for 3 min each time.
Insert the tissue slice into the slice box, put it into a washing box containing PBS buffer memory, put it into a blender and wash for 3 min.
Tilt the remaining PBS buffer in the tissue section to suspend the undiluted sheep serum solution on the whole tissue, seal at room temperature for 20 min and do not wash if tilted.
Using the 3,3'-Diaminobenzidine (DAB) developer kit, collect 1 ml of distilled water, add A, B and C reagents to the kit, one drop at a time, add 100 μl to the slice, and then add the slice to the microscope. Control reaction for 1-3 min. When the color is light, add 0.01 M PBS to stop the reaction and immediately wash the distilled water. Rinse with tap water and re-stain hematoxylin for 1 min, wash with ethanol for 3 to 5 s, and then rinse with tap water. After rinsing with ammonia, water turned blue and after rinsing for 10 s, the gradient alcohol was dehydrated and dried.
Statistical analysis and data statistics:
The results of Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Western blot were collected, processed and analyzed with Image J software, and the relevant data was statistically analyzed with Statistical Package for the Social Sciences (SPSS) 19.0. p<0.05 is statistically significant. The 3-(4,5-Dimethylthiazol-2- yl)-2,5-Diphenyltetrazolium Bromide (MTT) method was used to compare the cell absorption photometric values of the target group and the control group, and the floating control method was used to detect the mortality of the target group and the control group by unidirectional dispersion analysis.
Results and Discussion
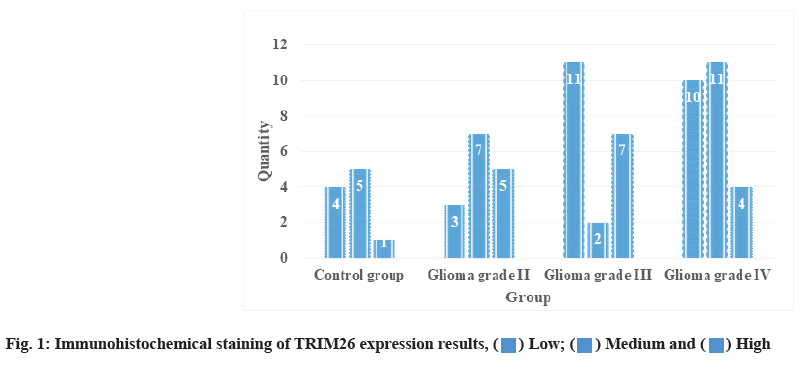
Expression analysis of TRIM26 in immunohistochemical staining is as follows. After immunohistochemical staining, the expression of TRIM26 in the control group and glioma group is shown in fig. 1 and Table 3. It can be seen that there was only one person with higher expression of TRIM26 in the control group, while 7 people with higher expression of gliomas III grade were significantly higher than the control group. The expression of TRIM26 in glioma tissue is mainly concentrated in the cytoplasm of tumor cells, but no expression in the nucleus. Cationic liposome method is the latest method in liposomemediated transfection method and is one of the nonviral transfection methods with the highest transfection efficiency and the least toxicity. This method obtains a high transfection efficiency that was previously unavailable in a variety of eukaryotic cell types and it is easy to use and guarantees reproducible and consistent results. It is used for cell lines for protein expression (such as fibroblast-like cell that was isolated from the kidney of an African green monkey (COS-7), Chinese Hamster Ovary (CHO) cells and HEK293), LipofectamineTM 2000 transfection reagent is particularly effective, and the efficiency can exceed 90 %. When the cationic liposome reagent is added to water under optimized conditions, it can form tiny (average size about 100- 400 nm) monolayer liposomes. Compared with other methods, negatively charged DNA automatically binds to positively charged liposomes to form DNA cationic liposome complexes. These complexes are positively charged and can be bound to the surface of negatively charged cell membranes by electrostatic action, and the captured DNA will be introduced into the cultured cells. Liposome-mediated transfection can be used to establish cell lines containing DNA that stably integrates the target DNA. Tumor cells have a strong proliferative ability, which is reflected in the increase of cells in the division phase in the cell cycle. After knocking out oncogenes, tumor cells can reduce cell division and reduce cell proliferation capacity.
| Group | Low | Medium | High |
|---|---|---|---|
| Control group | 4 | 5 | 1 |
| Glioma grade II | 3 | 7 | 5 |
| Glioma grade III | 11 | 2 | 7 |
| Glioma grade IV | 10 | 11 | 4 |
Table 3: TRIM26 Immunohistochemical Staining Results
The expression of TRIM26 in glioma and normal brain tissue was detected. The statistical results are shown in fig. 2. Of the 60 glioma samples, 46 cases were positive for TRIM26 protein; all 10 cases of normal brain tissue samples show negative expression; comparing the expression of TRIM26 in glioma with the expression in normal brain tissue, the difference was significant and statistically significant (p<0.01). In human cells, there are many tumor suppressor genes, such as p53, which is a protein with a half-life of about 30 min. If normal cells are damaged, p53 will become more stable after such damage and then the entire cell will die. There is a great correlation between DNA damage and the corresponding increase in p53. The final response level of p53 mainly depends on the actual nature of the entire damage. Studies have also shown that this is related to the corresponding p53 caused by the entire ubiquitin-proteasome system. The degradation ability is related to down regulation. Another series of studies suggest that if there is a heat-resistant E1 genotype in the cell line, p53 accumulation will occur and once the E1 gene is transferred, the accumulation of p53 will be blocked. Taking human herpes virus as an example, the E6 oncoprotein of high-risk human herpes virus cells binds to p53 through E6-Associated Protein (E6AP) and promotes the degradation of p53 through the ubiquitinated protease pathway. On the contrary, many viruses, such as low-risk viruses, cannot be combined with p53 and cannot be degraded. Under the action of E2 and E3 enzymes, the combination of ubiquitin and E6AP is mainly able to produce the corresponding thiol ester complex, and this substance has the function and effect of activating enzyme, so that p53 is finally compounded by the protease 26S body degradation.
Relationship between TRIM26 expression level and clinical parameters of glioma was shown here. A total of 60 cases of glioma patients were collected with complete clinical data. The statistical results are shown in fig. 3. Correlation analysis of TRIM26 expression level and clinicopathological characteristics (tumor size, tumor site, tumor grade, gender, age) found that TRIM26 expression level was correlated with tumor size (p<0.01, r=0.391) and tumor site, tumor grade, gender and age were not statistically different (p>0.05). Due to the infiltrative growth of gliomas, the particularity and functionality of brain tissue, it is difficult to cure it with surgery, radiotherapy and chemotherapy, and the recurrence rate is extremely high. The growth rate and anti-apoptosis ability of glioma determine its progress. Therefore, the research on the growth and apoptosis of glioma is of great significance for its treatment. Through the detoxification of the carbonyl group by the cell, it can regulate the balance and steady state of retinoic acid and regulate the synthesis of fatty acid and lipid metabolism, affect the proliferation, invasion and metastasis of tumors, and are closely related to the occurrence and development of tumors. TRIM26 is mainly expressed in Non-Small Cell Lung Cancer (NSCLC) (pulmonary squamous cell carcinoma and adenocarcinoma), and TRIM26 overexpression in lung squamous cell carcinoma is closely related to smoking. TRIM26 activates carcinogens such as Polycyclic Aromatic Hydrocarbons (PAH) in tobacco biological precursor, leading to lung tumors. In addition, TRIM26 promotes tumorigenesis in breast cancer and colon cancer by regulating lipid anabolism, protects tumor cells and regulates tumorigenesis by detoxifying carbonyl groups.
In cell growth, many cell growth-related proteins are the target substrates of ubiquitinated proteasome complexes, such as rapid and periodic B-cell degradation. In the study of cancer cells, it can be found that, when the entire cell cycle exits the rapid division period, cell cycle B, E and D1 basically show a certain overexpression in the process of cell carcinogenesis. Moreover, cell cycle B, E and D1 are also substrates of the proteasome complex after ubiquitination throughout the cell growth cycle. Their overexpression is caused by the decrease of ubiquitin-proteasome activity.
The expression of TRIM26 in glioma cell proliferation is shown in fig. 4.
The free amino and sulfhydryl groups in proteins and amino acids can form covalently modified compounds with carbonyl groups, that is, the cross-linking reaction of carbon and ammonia, which results in the inability of the protein to hydrolyze and loose its original function, leading to the disorder of cell structure and function; the protein modified by carbonyl group can be aggregated where it can form proteasome resistance, further prevent abnormal protein degradation and may cause tumors and other diseases through a series of biochemical reactions. Carbonyl groups can also mutate and damage DNA, leading to disease. Hematoxylin and Eosin (HE) staining is mainly used to evaluate the heterogeneity of Human Glioma cell line (CHG-5) and Uppsala 87 Malignant Glioma (U87) cells. The expression level of TRIM26 is an important indicator of the degree of differentiation of glioma cells and the degree of differentiation is inversely related to the degree of malignancy of tumor cells. The expression level of TRIM26 is an important indicator that reflects the malignant progress of gliomas. The expression level of TRIM26 is positively correlated with the malignant degree of gliomas. The glioma cells with chromosome 1 (1p)/chromosome 19 (19q) combined deletion are highly sensitive to Procarbazine, Lomustine, Vincristine (PCV) chemotherapy and the prognosis of these patients is better. In a further study of clinical glioma samples, it was found that Temozolomide (TMZ) simultaneous chemoradiotherapy and subsequent adjuvant therapy for patients with O6-Methylguanine-DNA Methyltransferase (MGMT) promoter methylation can significantly improve the patient’s prognosis and prolong the survival of patients, while the MGMT promoter is not. After methylated patients received the same condition of treatment, their prognostic survival level was significantly lower than that of patients with MGMT promoter methylation. Krüppel-like Zinc Finger Transcription Factor (KLF6) is an important regulatory pathway of Myocyte Enhancer Factor 2D (MEF2D) in hippocampal neurons. Down-regulation of KLF6 expression can promote the death of neurons and promote MEF2D to promote the apoptosis of hippocampal neurons through negative regulation. In addition, Myocyte Enhancer Factor 2 (MEF2) can regulate the expression of various genes in the human brain. MEF2 plays an important role in the regulation and development of the human nervous system. Among them, epilepsy, autism, mental retardation and other diseases are related to mutations of MEF2 factors and MEF2 target genes. In the case of insufficient MEF2C single dose, severe mental retardation can be formed and when certain genes regulated by MEF2 are mutated, it can lead to imbalance of synaptic excitation and induce epilepsy. In the human lung Adenocarcinoma cell line (A549) with low expression of TRIM26, overexpression of TRIM26 will degrade the protein level of PBX1 and promote the proliferation of A549 cells, while knocking down TRIM26 in Human Lung Cancer Cell Line (H226) with high expression of TRIM26 will upregulate the protein level of PBX1 and inhibit the proliferation of H226 cells. Furthermore, after knocking down TRIM26 in lung cancer cell line H226 with high expression of TRIM26 and overexpressing TRIM26 in lung cancer cell line A549 with low expression of TRIM26, clone formation experiments were conducted. Experimental results show that knocking down TRIM26 inhibits the formation of H226 cell clones and overexpressing TRIM26 can promote the formation of A549 cell clones. In summary, the high expression of TRIM26 in lung cancer tissue is related to the low expression of PBX1. TRIM26 promotes the proliferation of NSCLC by inducing the degradation of PBX1.
TRIM26 can inhibit proliferation and promote apoptosis of glioma cells. However, glioma U251 and U87 cells are Phosphatase and Tensin Homolog (PTEN)-deficient cell lines. Therefore, the mechanism of TRIM26 exerting tumor suppressive effect in glioma cells remains to be explored. Inositol Polyphosphate 4-Phosphatase type II B (INPP4B) is a member of the inositol polyphosphate phosphatase family, about 105 KD, has two subtypes, appearing at 514 base pair (bp) and 395 bp, it has a recognized phosphatase catalytic site and a highly conserved C2 structural site. Its structure is highly similar in rodents and humans, and is expressed in brain tissue and many other tissues. TRIM26 phosphorylates the 336 tyrosine site of PTEN to maintain the stability of PTEN and play a role in cancer suppression. INPP4B has a specific phosphorylation site. Therefore, in gliomas, TRIM26 may play a role in suppressing cancer through INPP4B. Wingless-Related Integration Site (Wnt) plays an important role in various systems of the body and is an important way to regulate the growth and development of cancer cells. Therefore, it is reasonable to speculate that TRIM26 may affect the function of Wnt signaling and cause cancer-related activities. Wnt gene is a proto-oncogene. The Wnt egg self-sufficient secreted glycoprotein encodes a hydrophobic signal sequence, followed by several glycosylation sites and is rich in cysteine. Knocking out TRIM26 can directly increase cell proliferation and promote cell transfer. Bioinformatics analysis indicates that TRIM26 may be an important regulator of cancer cell metabolism.
This article mainly studies the effect of the interference of TRIM26 expression on the growth of glioma cells. Through immunoprecipitation experiments, it was found that the C-terminal SPRY structure region of TRIM26 bound to PBX1. After analyzing the patient’s samples, we found that the expression of TRIM26 messenger RNA (mRNA) in glioma tissue was significantly higher than that in normal brain tissue, which proved that TRIM26 can actually inhibit the transcription activity of PBX1. The expression of TRIM26 IRF3 protein in NSCLC was negatively correlated. Co-immunoprecipitation studies were conducted by rotating TRIM26 and IRF3 particles in H299 cells, and it was found that TRIM26 and IRF3 interacted.
The essence of cell carcinogenesis is the infinite proliferation of cells due to protein dysfunction related to the redirection of cell growth signals. Molecular targeted therapy has become one of the current hot spots in neuroma research. Molecular targeted therapy is a treatment method that regulates interference at the target level, such as tumor cell receptors, cell transduction pathways, regulatory factors and essential genes at the molecular level. Many members of the TRIM family target various substances such as cell cycle regulators, tumors or tumor suppressors and play an important role as regulators in cancer. The discovery of TRIM26 hindered the proliferation of human non-small cell lung carcinoma cell line (H1299) cells and promoted cell death. In addition, TRIM26 hindered the migration of H1299 cells and A549 cells, so it was confirmed that TRIM26 plays a role in promoting carcinogenesis in nerve cells. TRIM26 affects the stability of IRF3 through ubiquitination and may reduce the amount of PBX1 bound to other factors.
The results of this research show that TRIM26 can reduce the protein level of PBX1 through the correlation of time gradient and concentration gradient. TRIM26 can reduce the mRNA level of Ring Finger Protein 6 (RNF6), the downstream target gene of PBX1, without affecting the mRNA level of PBX1.
Ethical approval:
The Ethical Committee of 3201 Hospital Affiliated to Xi’an Jiaotong University approved the research design. The approval number was 201800168.
Author’s contributions:
Lin Zhu and Shiwen Guo conceived and designed the experiments; Lin Zhu and Shiwen Guo performed the experiments; Lin Zhu and Juan Lu have collected data; Lin Zhu and Zhijun Bao analyzed the data; Lin Zhu wrote the paper and Juan Lu finalized the paper.
Acknowledgements:
The research team hereby expresses the sincere gratitude to the leaders of the 3201 hospital for their support to the work.
Conflict of interests:
The authors declared no conflict of interest.
References
- Huang WL, Hsiung MH, Dai W, Hu SS. Rottlerin, BDNF and the impairment of inhibitory avoidance memory. Psychopharmacology 2021;238(2):421-39.
[Crossref] [Google Scholar] [PubMed]
- Martini S, Figini M, Croce A, Frigerio B, Pennati M, Gianni AM, et al. Selinexor sensitizes TRAIL-R2-positive TNBC cells to the activity of TRAIL-R2xCD3 bispecific antibody. Cells 2020;9(10):2231.
[Crossref] [Google Scholar] [PubMed]
- Ding L, Wang S, Wang W, Lv P, Zhao D, Chen F, et al. Tanshinone IIA affects autophagy and apoptosis of glioma cells by inhibiting phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling pathway. Pharmacology 2017;99(3):188-95.
[Crossref] [Google Scholar] [PubMed]
- Zhu D, Tu M, Zeng B, Cai L, Zheng W, Su Z, et al. Up‐regulation of miR‐497 confers resistance to temozolomide in human glioma cells by targeting mTOR/Bcl‐2. Cancer Med 2017;6(2):452-62.
[Crossref] [Google Scholar] [PubMed]
- Song H, Zhang Y, Liu N, Wan C, Zhang D, Zhao S, et al. miR-92b regulates glioma cells proliferation, migration, invasion and apoptosis via PTEN/Akt signaling pathway. J Physiol Biochem 2016;72(2):201-11.
[Crossref] [Google Scholar] [PubMed]
- Xiao ZQ, Yin TK, Li YX, Zhang JH, Gu JJ. MiR-130b regulates the proliferation, invasion and apoptosis of glioma cells via targeting of CYLD. Oncol Rep 2017;38(1):167-74.
[Crossref] [Google Scholar] [PubMed]
- Niu H, Liu Y, Wang Y, Tian Y, Jiang H, Cao S, et al. MiR-130b can suppress proliferation of glioma cells through targeting PTEN to regulate AKT pathway. J BUON 2020;25(4):2059-65.
[Google Scholar] [PubMed]
- Peng G, Liu Y, Yang C, Shen C. MicroRNA‑25 promotes cell proliferation, migration and invasion in glioma by directly targeting cell adhesion molecule 2. Exp Ther Med 2022;23(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Mahlokozera T, Patel B, Chen H, Desouza P, Qu X, Mao DD, et al. Competitive binding of E3 ligases TRIM26 and WWP2 controls SOX2 in glioblastoma. Nat Commun 2021;12(1):1-6.
- Tang JH, Ma ZX, Huang GH, Xu QF, Xiang Y, Li N, et al. Downregulation of HIF-1a sensitizes U251 glioma cells to the temozolomide (TMZ) treatment. Exp Cell Res 2016;343(2):148-58.
[Crossref] [Google Scholar] [PubMed]
- Liang HX, Sun LB, Liu NJ. Neferine inhibits proliferation, migration and invasion of U251 glioma cells by down-regulation of miR-10b. Biomed Pharmacother 2019;109:1032-40.
[Crossref] [Google Scholar] [PubMed]
- Sun L, Jin X, Xie L, Xu G, Cui Y, Chen Z. Swainsonine represses glioma cell proliferation, migration and invasion by reduction of miR-92a expression. BMC Cancer 2019;19(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Qiao D, Meyer K, Friedl A. Glypican 1 stimulates S phase entry and DNA replication in human glioma cells and normal astrocytes. Mol Cell Biol 2013;33(22):4408-21.
[Crossref] [Google Scholar] [PubMed]
- Antonietti P, Linder B, Hehlgans S, Mildenberger IC, Burger MC, Fulda S, et al. Interference with the HSF1/HSP70/BAG3 pathway primes glioma cells to matrix detachment and BH3 mimetic-induced apoptosis. Mol Cancer Ther 2017;16(1):156-68.
[Crossref] [Google Scholar] [PubMed]
- Gao L, Chen B, Li J, Yang F, Cen X, Liao Z, et al. Wnt/β-catenin signaling pathway inhibits the proliferation and apoptosis of U87 glioma cells via different mechanisms. PLoS One 2017;12(8):e0181346.
[Crossref] [Google Scholar] [PubMed]
- Sun J, Li B, Jia Z, Zhang A, Wang G, Chen Z, et al. RUNX3 inhibits glioma survival and invasion via suppression of the β-catenin/TCF-4 signaling pathway. J Neurooncol 2018;140(1):15-26.
[Crossref] [Google Scholar] [PubMed]
- Jiang Y, Sheng H, Meng L, Yue H, Li B, Zhang A, et al. RBM5 inhibits tumorigenesis of gliomas through inhibition of Wnt/β-catenin signaling and induction of apoptosis. World J Surg Oncol 2017;15(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Xie C, Liu S, Wu B, Zhao Y, Chen B, Guo J, et al. MiR-19 promotes cell proliferation, invasion, migration and EMT by inhibiting SPRED2-mediated autophagy in osteosarcoma cells. Cell Transplant 2020;29:1-10.
[Crossref] [Google Scholar] [PubMed]
- Zhou H, Tang H, Li N, Chen H, Chen X, Gu L, et al. MicroRNA-361-3p inhibit the progression of lymphoma by the Wnt/β-catenin signaling pathway. Cancer Manag Res 2020;12:12375-84.
- Wang G, Liu M, Wang H, Yu S, Jiang Z, Sun J, et al. Centrosomal protein of 55 regulates glucose metabolism, proliferation and apoptosis of glioma cells via the Akt/mTOR signaling pathway. J Cancer 2016;7(11):1431-40.
[Crossref] [Google Scholar] [PubMed]
- Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics 2017;14(2):284-97.
[Crossref] [Google Scholar] [PubMed]
- Gusyatiner O, Hegi ME. Glioma epigenetics: From subclassification to novel treatment options. Semin Cancer Biol 2018;51:50-58.
[Crossref] [Google Scholar] [PubMed]
- Nitta Y, Shimizu S, Shishido‐Hara Y, Suzuki K, Shiokawa Y, Nagane M. Nimotuzumab enhances temozolomide‐induced growth suppression of glioma cells expressing mutant EGFR in vivo. Cancer Med 2016;5(3):486-99.
[Crossref] [Google Scholar] [PubMed]
- Sechi S, Frappaolo A, Karimpour-Ghahnavieh A, Piergentili R, Giansanti MG. Oncogenic roles of GOLPH3 in the physiopathology of cancer. Int J Mol Sci 2020;21(3):933.
[Crossref] [Google Scholar] [PubMed]
- Hu P, Wang K, Zhou D, Wang L, Zhao M, Wang W, et al. GOLPH3 regulates exosome miRNA secretion in glioma cells. J Mol Neurosci 2020;70(8):1257-66.
[Crossref] [Google Scholar] [PubMed]
- Wang DY, Wang J, Deng D. Golgi phosphoprotein-3 (GOLPH3) promote metastasis of nasopharyngeal carcinoma through regulating E-cadherin. Eur Rev Med Pharmacol Sci 2020;24(17):8871-9.
[Crossref] [Google Scholar] [PubMed]
- Zhao C, Zhang J, Ma L, Wu H, Zhang H, Su J, et al. GOLPH3 promotes angiogenesis of lung adenocarcinoma by regulating the Wnt/β-catenin signaling pathway. Onco Targets Ther 2020;13:6265-77.
[Crossref] [Google Scholar] [PubMed]
- Lin J, Song T, Li C, Mao W. GSK-3β in DNA repair, apoptosis and resistance of chemotherapy, radiotherapy of cancer. Biochim Biophys Acta 2020;1867(5):118659.
[Crossref] [Google Scholar] [PubMed]
- Fang G, Zhang P, Liu J, Zhang X, Zhu X, Li R, et al. Inhibition of GSK-3β activity suppresses HCC malignant phenotype by inhibiting glycolysis via activating AMPK/mTOR signaling. Cancer Lett 2019;463:11-26.
[Crossref] [Google Scholar] [PubMed]
- Dhawan T, Zahoor MA, Heryani N, Workenhe ST, Nazli A, Kaushic C. TRIM26 facilitates HSV-2 infection by downregulating antiviral responses through the IRF3 pathway. Viruses 2021;13(1):70.
[Crossref] [Google Scholar] [PubMed]
- Lu T, Bao Z, Wang Y, Yang L, Lu B, Yan K, et al. Karyopherin β1 regulates proliferation of human glioma cells via Wnt/β-catenin pathway. Biochem Biophys Res Commun 2016;478(3):1189-97.
[Crossref] [Google Scholar] [PubMed]
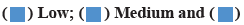 High
High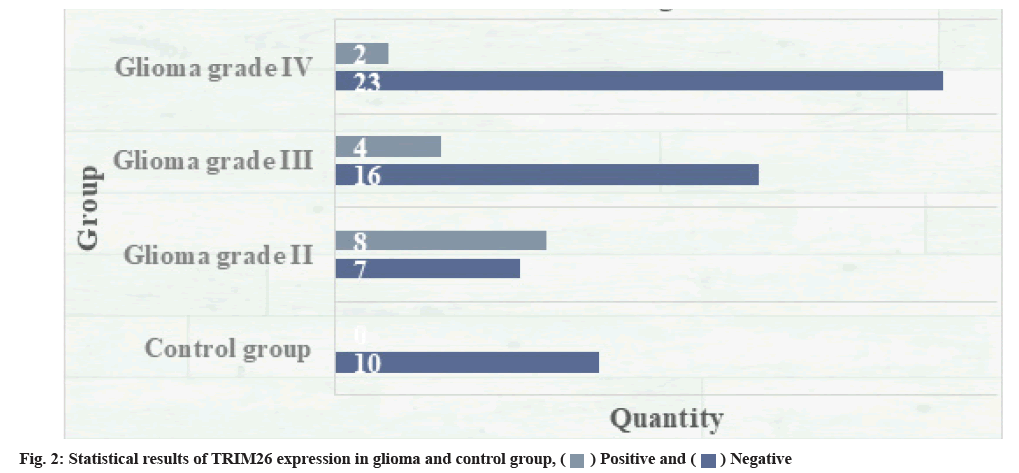
 Negative
Negative
 Tumor grade
Tumor grade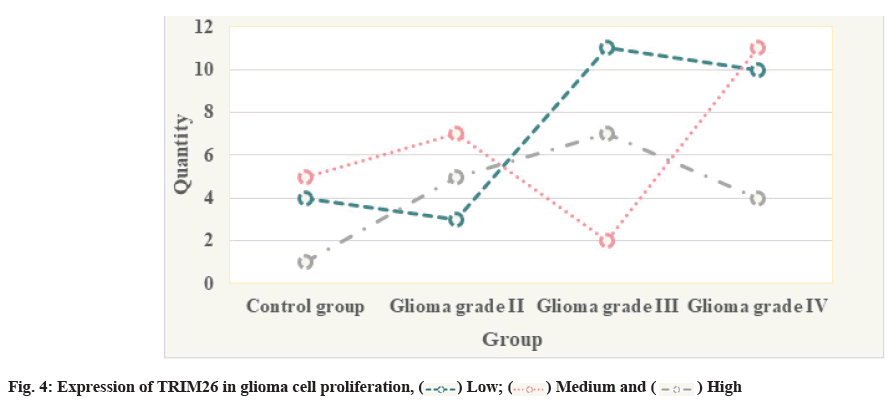
 High
High



