- *Corresponding Author:
- Dong Xia
Department of Gastrointestinal Surgery, Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan 646000, China
E-mail: 13378253517@163.com
| This article was originally published in a special issue, “Innovations in Biomedical Research and Drug Development” |
| Indian J Pharm Sci 2023:85(3) Spl Issue “199-210” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Gastric cancer is one of the most common gastrointestinal tumors, annually accounting for about 10 % of all diagnosed cancers and cancer mortalities worldwide. Scutellariae barbata Herba is one of the most commonly used Chinese medicines to treat gastric cancer. Although numerous experiments have been conducted to decipher the mechanism of Scutellariae barbata Herba, it has not been fully elucidated. Therefore, we constructed a pharmacological and molecular docking network to understand the mechanism of action of Scutellariae barbata Herba. The active components and targets of Scutellariae barbata Herba were screened with traditional Chinese medicine systems pharmacology. Gastric cancer related targets were screened using online mendelian inheritance in man and GeneCards Suite database platforms. The intersection target genes of Scutellariae barbata Herba and gastric cancer were retrieved by R software. The Cytoscape software was utilized to draw a drug-compound gene-disease visualization network diagram. The string online analysis platform was incorporated to construct the target-protein interaction network for screening the core targets. Gene ontology and Kyoto encyclopedia of genes and genomes enrichment analyses were performed on the core targets. PyMoL and other software were utilized to verify the molecular docking between the key active components of Scutellariae barbata Herba and the key targets. Our results elucidate the active components, associated targets, biological processes and signaling pathways of Scutellariae barbata Herba during gastric cancer treatment. This study deepens our understanding of the potential role of Scutellariae barbata Herba in gastric cancer and provides novel ideas for treating gastric cancer using Scutellariae barbata Herba
Keywords
Gastric cancer, Scutellariae barbata Herba, network pharmacology, molecular docking
Gastric cancer is essential worldwide, ranking 5th in incidence and 4th in mortality globally in 2020, causing more than 1 million new cases and an estimated 769 000 deaths[1]. The exact cause of gastric cancer is unclear. However, regional environment, dietary and lifestyle factors, Helicobacter pylori, chronic diseases and genetic factors are associated with the incidence of gastric cancer. Gastric cancer treatments include endoscopy, surgery, radiotherapy, chemotherapy and immunotherapy. Although significant progress has been made in diagnostic techniques and treatment strategies, patients having early gastric cancer often do not show any specific symptoms. They are usually diagnosed in the middle and advanced stages with distant metastasis. In addition, the cost of anticancer drugs, side effects and other factors increase the treatment burden of patients. Therefore, finding cost-effective drugs with low toxicity and side effects is necessary.
After thousands of years of development, Traditional Chinese Medicine (TCM) has formed its unique theory, diagnosis and treatment system. Can Chinese traditional medicine in cancer prevention and treatment play an important role, such as postoperative recovery time and having received radiotherapy or chemotherapy[2]. TCM can treat cancer from several aspects, such as inhibiting tumor angiogenesis, inducing cancer cell differentiation and apoptosis, using the poison to destroy tumor cells, immune and androgen way adjustment, etc.,[3]. Scutellariae barbata Herba (SBH) is a whole dried plant of the Lamiaceae family[4]. SBH has the functions of clearing heat and detoxifying, enhancing blood circulation and removing blood stasis, benefiting water and reducing swelling[5]. It is also used in treating primary liver, lung, cervical, colorectal, gastric adenocarcinoma and ovarian cancer, and in combination with other TCM to treat more cancer types[5,6]. Despite a large amount of published literature on treating cancer using SBH, the potential mechanism of action of SBH in the treatment of gastric cancer has not been systematically assessed.
TCM has the characteristics of multi-component, multi-target and multi-path synergism. Thus, traditional experimental methods have limitations in studying the molecular mechanism of the treatment of diseases[7-9]. Network pharmacology uses multi-disciplinary technologies and contents to develop a multi-level network of "disease, phenotype, gene and drug" similar to the overall philosophy of TCM. It provides feasible methods for exploring TCM[10,11].
Molecular docking is the most common method of drug design depending on computational structure[12], used to explore the ligand conformation adopted in the binding site of macromolecular targets and estimate the ligand-receptor binding free energy by assessing the critical phenomena involved in the intermolecular recognition process[13]. It could reduce research costs and enhance drug discovery.
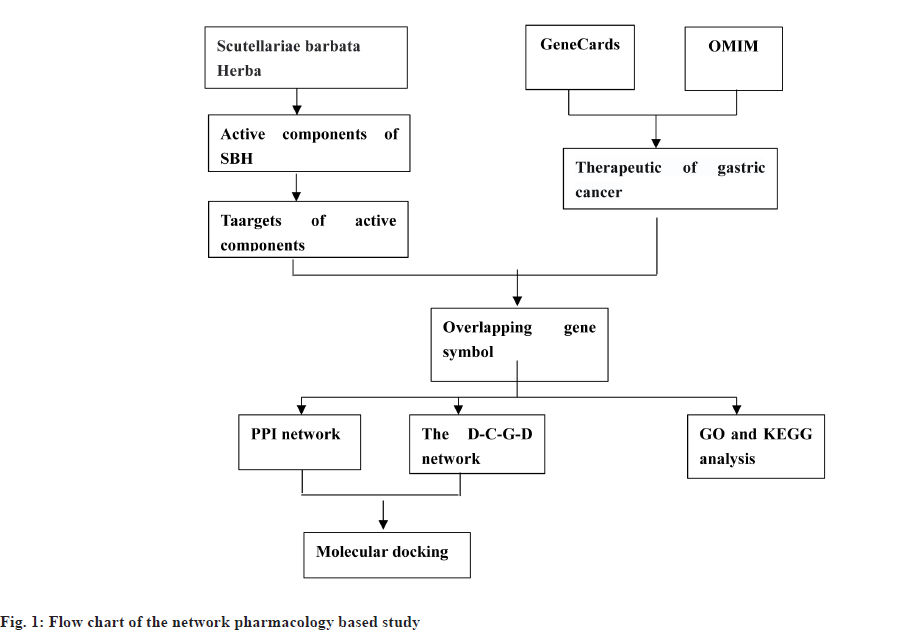
This study uses network pharmacology and molecular docking methods to investigate the mechanism of action of SBH in treating gastric cancer. We evaluated the Oral Bioavailability (OB) and Drug-Like (DL) to screen the active components in SBH and then obtained the targets of the active components in SBH. The potential gastric cancer target genes were screened from two databases (Online Mendelian Genetic Database (OMIM) and GeneCards) and then a network was constructed by analyzing the potential interactions between each target node. Additionally, Protein-Protein Interaction (PPI) data were retrieved from the String database and enrichment analysis (Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG)) was performed to identify the potential mechanism of anti-gastric cancer in SBH. Therefore, this study aimed to identify potential targets and pathways for treating gastric cancer with SBH using network pharmacology and molecular docking methods, and to systematically elucidate the mechanism of action of SBH in treating gastric cancer. Furthermore, molecular docking technology was utilized to verify the affinity relationship between active components and target proteins, providing a theoretical and scientific basis for future research as shown in fig. 1.
Materials and Methods
Active components and targets of SBH:
From the Pharmacology Database and Analysis platform of TCM Systems Pharmacology (TCMSP) (http://tcmspw.com), the active ingredients and their target proteins of SBH were obtained. TCMSP comprises many herbal items and can identify drug targets and drug-disease networks[14]. Based on the most commonly used standards in the TCM database, the active ingredients having OB ≥30 % and DL ≥0.18 % were selected for subsequent study. Then, the active components and their targets were screened out. Perl script was used to import all the screened targets within the UniProt database (http:// www.uniprot.org) and all the names were corrected to their official names.
Screening of potential target genes in gastric cancer:
Genes associated with gastric cancer were retrieved from two databases; first was GeneCards (https:// www.Org/, version 5.9.0), and the second was the OMIM (http://www.omim.org/), providing information on more than 15 000 genes and associated diseases based primarily on published literature[15]. After synthesizing the target genes from the two databases and removing the duplication, the target genes associated with gastric cancer were obtained.
Acquisition of potential targets for the interaction between SBH and gastric cancer:
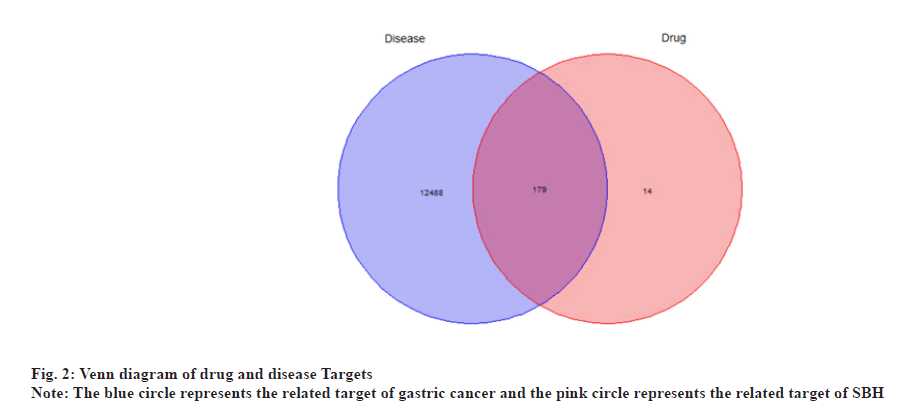
The Venn diagram data package of R software 4.1.3 was utilized to conduct a Venn analysis on the datasets of effective chemical components and targets associated with gastric cancer and plot the Venn diagram to obtain the intersection targets and preliminaries for exploring potential targets of SBH in treating gastric cancer.
Drug-Compounds-Genes-Disease (D-C-G-D) network construction:
The above intersection targets were imported into the Cytoscape software (V.3.7.2, https://cytoscape. org/) for visual analysis of data. D-C-G-D[16] was constructed, viz., the TCM regulatory network diagram of the SBH active component intersection target gastric cancer.
Protein interaction network construction:
The intersection target was uploaded to STRING[17] (https://stringdb.org/, version 11.5), the biological species were selected as "Homo sapiens," the "highest confidence" was set as 0.9 and the PPI network was obtained after searching. The obtained Tab Separated Value (TSV) file of the PPI relationship was imported into R software 4.1.3 to identify the core genes of the PPI network.
GO enrichment analysis and KEGG pathway analysis:
GO bio functional analysis is primarily used to describe the function of gene targets, such as Molecular Function (MF), Cellular Component (CC) and Biological Process (BP). KEGG enrichment analysis could identify the signal pathways enriched within the common targets of SBH and gastric cancer. The R package ("cluster Profiler, "based on the criteria of P value cutoff=0.05 and Q value cutoff=0.05) enriched the GO and KEGG pathways. The analyses were summarized, and the bar charts and bubble charts of GO and KEGG enrichment analyses were drawn.
Molecular docking:
The target sites with the highest PPI ranking and more research were selected for molecular docking to verify the relationship between active ingredients and target sites. From the PubChem database (http:// pubchem.ncbi.nim.nih.gov/) the Two-Dimensional (2D) structure was downloaded using Chem Three- Dimensional (3D) to convert 2D structure into a 3D structure and the molecular force field was performed on the small molecule structure optimization. Moreover, the 3D structure of the target protein was downloaded from Protein Data Bank (PDB) (https:// www.rcsb. Org/), and PyMoL was used to remove the water molecule and small molecule ligand of the target protein. Then the target protein was imported into AutoDockTools 1.5.7 for hydrogenation, charge distribution and atomic type addition. Auto Dock Vina 1.1.2 was utilized for molecular docking and PyMoL software was used to optimize the results to establish the molecular docking diagram.
Results and Discussion
Twenty-nine medicinal chemical components of SBH were screened from TCMSP under the conditions of OB ≥30 % and DL ≥0.18, as depicted in Table 1. The selected components were matched to TCMSP and their targets were mined. 193 potential targets were obtained after removing duplicates through the UniProt database transformation.
| MOL ID | Compound | MW | OB % | DL |
|---|---|---|---|---|
| MOL001040 | (2R)-5,7-dihydroxy-2-(4-hydroxyphenyl) chroman-4-one | 272.27 | 42.36 | 0.21 |
| MOL012245 | 5,7,4'-trihydroxy-6-methoxyflavanone | 302.3 | 36.63 | 0.27 |
| MOL012246 | 5,7,4'-trihydroxy-8-methoxyflavanone | 302.3 | 74.24 | 0.26 |
| MOL012248 | 5-hydroxy-7,8-dimethoxy-2-(4-methoxyphenyl) chromone | 328.34 | 65.82 | 0.33 |
| MOL012250 | 7-hydroxy-5,8-dimethoxy-2-phenyl-chromone | 298.31 | 43.72 | 0.25 |
| MOL012251 | Cluysin-5-methylether | 268.28 | 37.27 | 0.2 |
| MOL012252 | 9,l 9-cyclolanost-24-en-3-ol | 426.8 | 38.69 | 0.78 |
| MOL002776 | Baicalin | 446.39 | 40.12 | 0.75 |
| MOL012254 | Campesterol | 400.76 | 37.58 | 0.71 |
| MOL000953 | Campesterol/Lathosterol Ratio (CLR) | 386.73 | 37.87 | 0.68 |
| MOL000358 | Beta-sitosterol | 414.79 | 36.91 | 0.75 |
| MOL012266 | Rivularin | 344.34 | 37.94 | 0.37 |
| MOL001973 | Sitosterol acetate | 456.83 | 40.39 | 0.85 |
| MOL012269 | Stigmasta-5,22-dien-3-ol-acetate | 454.81 | 46.44 | 0.86 |
| MOL012270 | Stigrnastan-3,5,22-triene | 394.75 | 45.03 | 0.71 |
| MOL000449 | Stigrnasterol | 412.77 | 43.83 | 0.76 |
| MOL000173 | Wogomn | 284.28 | 30.68 | 0.23 |
| MOL001735 | Dinatin | 300.28 | 30.97 | 0.27 |
| MOL001755 | 24-Ethylcholest-4-en-3-one | 412.77 | 36.08 | 0.76 |
| MOL002714 | Baicalein | 270.25 | 33.52 | 0.21 |
| MOL002719 | 6-Hydroxynaringenin | 288.27 | 33.23 | 0.24 |
| MOL002915 | Salvigenin | 328.34 | 49.07 | 0.33 |
| MOL000351 | Rhamnazin | 330.31 | 47.14 | 0.34 |
| MOL000359 | Sitosterol | 414.79 | 36.91 | 0.75 |
| MOL005190 | Eriodictyol | 288.27 | 71.79 | 0.24 |
| MOL005869 | Daucostero_qt | 414.79 | 36.91 | 0.75 |
| MOL000006 | Luteolin | 286.25 | 36.16 | 0.25 |
| MOL008206 | Moslosooflavone | 298.31 | 44.09 | 0.25 |
| MOL000098 | Quercetin | 302.25 | 46.43 | 0.28 |
Table 1: Potential Effective Ingredients of SBH
Using "gastric cancer" as the keyword, we searched GeneCards and OMIM databases and 12 667 genes associated with gastric cancer-related diseases were mined after removing duplications.
By matching disease-related genes using potential drug target genes, a Venn map was designed, and 179 potential targets in the treatment of gastric cancer with SHB were obtained, particularly the intersection of drug and disease targets as shown in fig. 2.
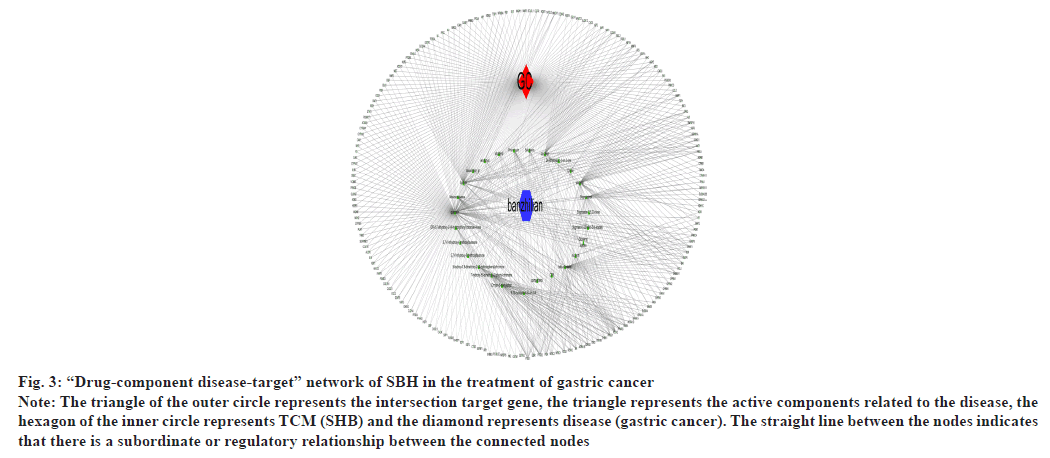
The above data were input into Cytoscape 3.7.2 software to construct a visual network diagram of chemical components of SBH and its target genes associated with treating gastric cancer. It had a total of 208 nodes with 621 edges as shown in fig. 3.
Fig. 3: “Drug-component disease-target” network of SBH in the treatment of gastric cancer
Note: The triangle of the outer circle represents the intersection target gene, the triangle represents the active components related to the disease, the
hexagon of the inner circle represents TCM (SHB) and the diamond represents disease (gastric cancer). The straight line between the nodes indicates
that there is a subordinate or regulatory relationship between the connected nodes
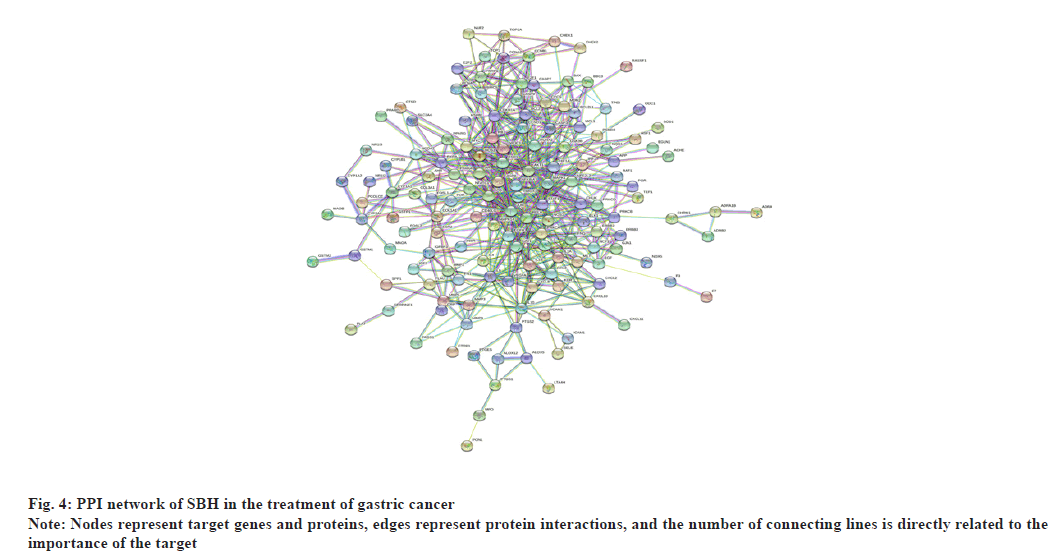
Values with scores greater than 0.9 were selected as the high confidence basis for protein interactions. The interaction network had 148 nodes and 1164 edges. Fig. 4 simultaneously, TSV data were exported and the R script was used to rank the genes in the PPI graph based on the number of connected nodes to identify the top 30 genes in the sum of the number of connected nodes and a bar graph was constructed (fig. 5). It could be observed that Jun Proto-Oncogene (JUN), Mitogen-Activated Protein Kinase (MAPK)- 1, AKT Serine/Threonine Kinase 1 (AKT1), RELA, Fos Proto-Oncogene (FOS), Estrogen Receptor 1 (ESR1), MAPK14, Interleukin-6 (IL-6), MYC, RB Transcriptional Corepressor 1 (RB1) and so on ranked at the top and were the core genes.
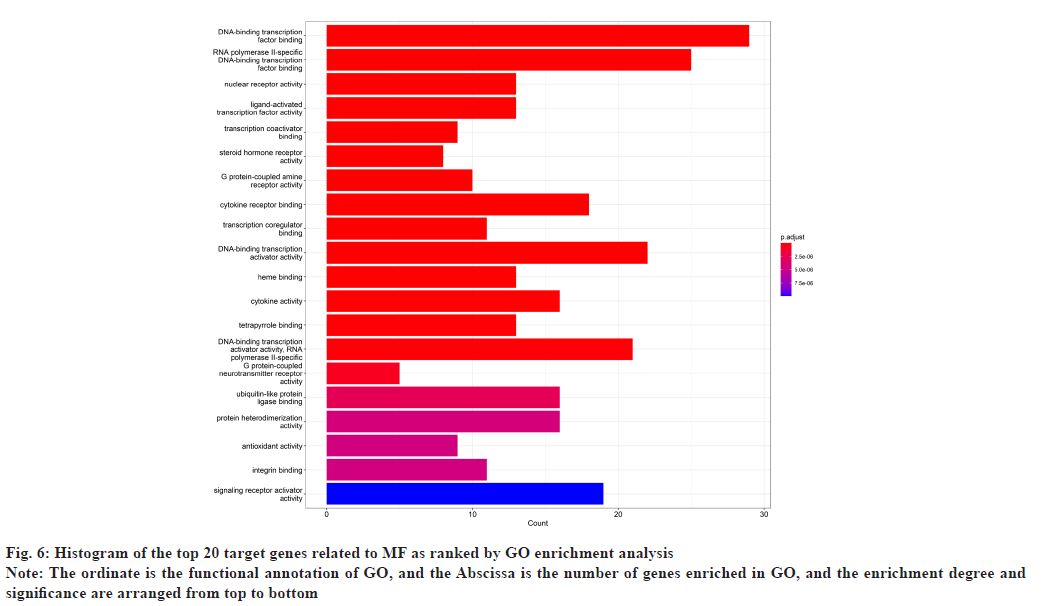
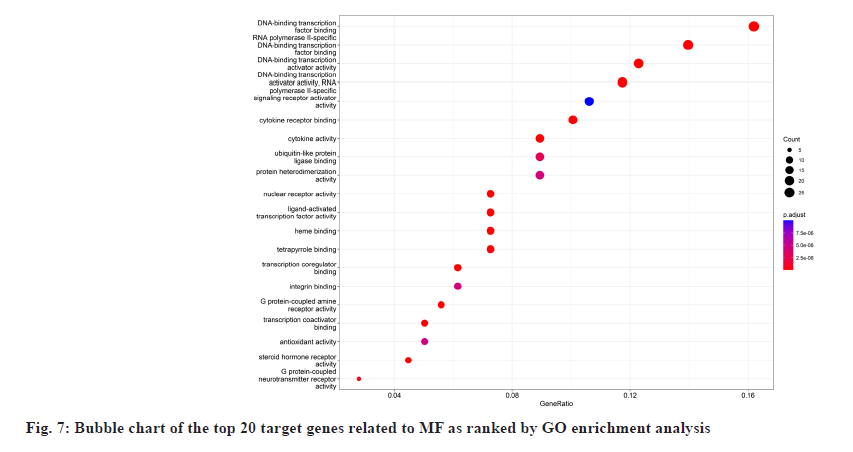
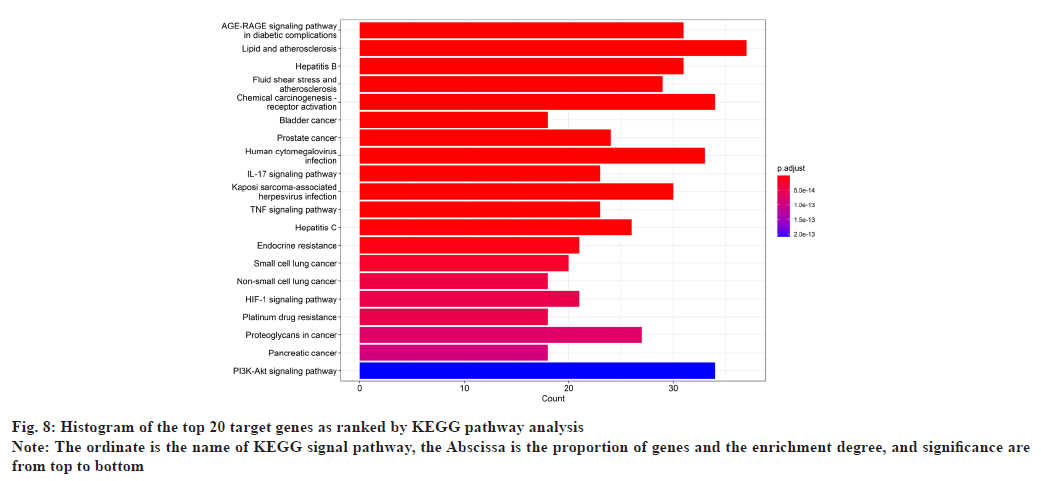
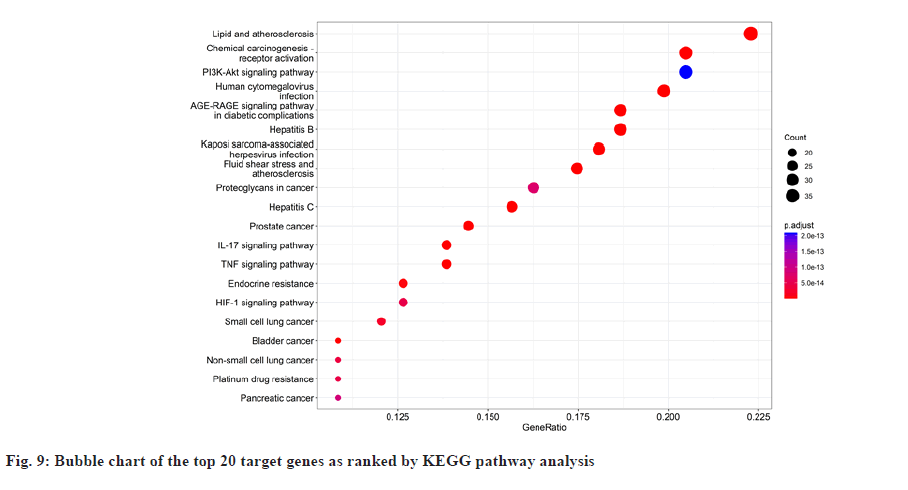
In GO enrichment analysis, 168 BP were screened. It primarily included Deoxyribonucleic Acid (DNA)- binding transcription factor binding, Ribonucleic Acid (RNA) polymerase II-specific DNA-binding transcription factor binding, etc. The top 20 enrichment results were selected depending on the enrichment ranking and R language 4.1.3 was used to design bubble charts and bar charts for visualization (fig. 6 and fig. 7). There were 169 signaling pathways in KEGG (fig. 8 and fig. 9), including the Advanced Glycation Endproducts-Receptor For Advanced Glycation Endproducts (AGE-RAGE) signaling pathway in diabetic complications, lipid atherosclerosis, Hepatitis B, fluid shear stress atherosclerosis, chemical carcinogens-receptor activation, human cytomegalovirus infection, etc.
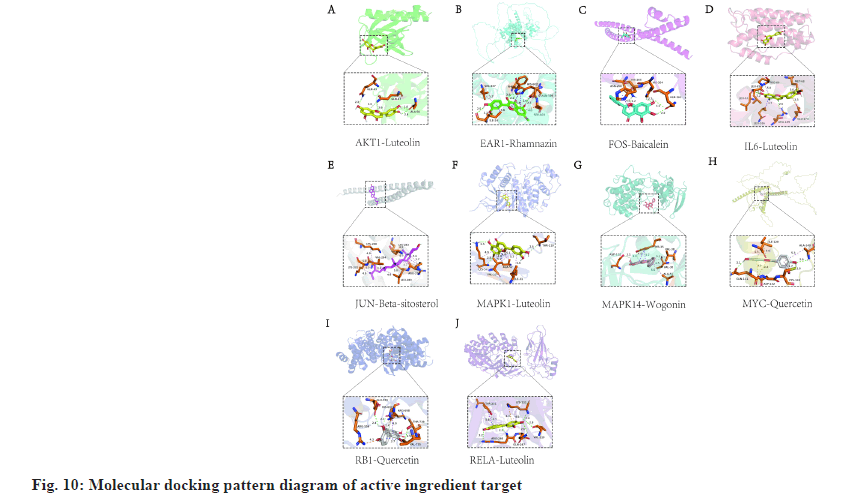
The binding energy can be combined with the parameters of the ligand and the receptor if a negative value indicates that the two can be combined. It is generally believed that ligand and receptor binding energy is lower in combination with a more stable system, showing a greater chance of working. In this study, we performed molecular docking verification on the top-ranked and well-studied core targets inside the PPI network with their matched active ingredients to determine the binding potential of proteins with ligands. The combination with the lowest binding energy was used to construct the 3D diagram of the molecular docking model, as shown in fig. 10 and Table 2. It showed that the predicted key active ingredients of SBH depicted a good binding activity with the key action targets for treating gastric cancer, confirming that the prediction of this study was reliable.
| Receptor | PDB ID | Ligand | PubChem ID | Energy (kcal/mol) |
|---|---|---|---|---|
| AKT1 | lunq | Luteolin | 5280445 | -6.1 |
| EAR l | Rhamnazin | 5320945 | -7.4 | |
| FOS | 2wt7 | Baicalein | 5281605 | -6.3 |
| IL-6 | lil6 | Luteolin | 5280445 | -7.2 |
| JUN | 5t01 | Beta-sitosterol | 222284 | -6.3 |
| MAPK l | ltvo | Luteolin | 5280445 | -8.7 |
| MAPK 14 | 7bdo | Wogonm | 5281703 | -8.5 |
| MYC | Quercetin | 5280343 | -6.0 | |
| RB I | 4elj | Quercetin | 5280343 | -7.9 |
| RELA | lnfi | Luteolin | 5280445 | -9.1 |
Table 2: Molecular Docking Results of Target and Active Compounds
Gastric cancer is a common malignant tumor of the digestive tract, severely threatening human life and health. SHB has been used in TCM for thousands of years[18] because it is believed to clear heat and detoxify toxins. With the rapid development of TCM, SHB has been established to play a positive role in treating various cancers. Especially the emergence of network pharmacology has introduced new research ideas and technical means for studying and elucidating its mechanism of action.
In this study, the network pharmacology approach was utilized to explore the relevant targets and pathways in treating gastric cancer with SHB and clarify its mechanism of action. The regulatory network of "TCM-ingredient-disease-target" showed that 27 effective active ingredients of SBH were obtained. Among them, quercetin, luteolin, Wogonin (WOG), Beta-Sitosterol (BS) and baicalein matched the most targets, which are active ingredients for treating gastric cancer. Quercetin can inhibit cell growth, induce cell death through different mechanisms, such as Endoplasmic Reticulum (ER) stress, Mitogen-Activated Protein Kinase (MAPK), Phosphatidylinositol 3-Kinase (PI3K), and Protein Kinase C (PKC) and also have anti-Helicobacter pylori activity[19]. Studies have shown that quercetin decreased the expression of anti-apoptotic proteins Myeloid Cell Leukemia-1 (MCL-1) and BC1- 2 and increased the expression of pro-apoptotic proteins Bas, Bax, Bak and Bid; thus, increasing the apoptosis of human Gastric Adenocarcinoma (AGS) cells[20]. Notably, quercetin can inhibit the growth of human gastric cancer stem cells by inhibiting the Phosphatidylinositol-3-Kinase (PI3K)/Akt signaling pathway to induce mitochondria-dependent apoptosis and has anti-proliferation and anti-angiogenesis effects[21]. Moreover, quercetin has also been proven to be a chemical sensitizing agent in daunorubicin resistance against mutant EPG85-257RDB cells by reduced P-glycoprotein expression, blocked drug transport and down-regulated ATP Binding Cassette Subfamily B Member 1 (ABCB1) gene expression. Therefore, quercetin could be considered a drug to overcome the resistance of gastric cancer cells[22]. Luteolin is a natural flavonoid having various pharmacological effects such as anticancer, antioxidant, anti-inflammatory, immunomodulatory and cardiac protection[23]. Wu et al.[24] demonstrated that luteolin partially decreased the expression of Bcl-2 by up regulating the expression of microRNA (mir-34a), thereby inducing apoptosis of human gastric cancer cells. Signal Transducer Activator of Transcription 3 (STAT3) and its target genes are over activated and/or overexpressed in drug-resistant gastric cancer cells. The inhibition of STAT3 function can significantly reduce cisplatin resistance and enhance apoptosis of drug-resistant cells[25]. Luteolin selectively kills gastric cancer cells showing high STAT3 activation by enhancing the binding of STAT3 and SHP-1[26]. Simultaneously, the combination of luteolin and oxaliplatin can inhibit the proliferation and induce apoptosis of gastric cancer cells by altering the cell cycle ratio and enhancing their sensitivity to oxaliplatin through the Cytochrome c (Cytc)/caspase pathway[27]. Luteolin can also control multiple signaling pathways and miRNAs in gastric cancer cells to inhibit cell viability, induce cell cycle arrest, colony formation, proliferation, migration and invasion, promote apoptosis in vitro and in vivo[28]. WOG mediates its anti-tumor activity by inducing apoptosis of cancer cells, blocking the tumor cell cycle, inhibiting tumor angiogenesis, releasing dangerous signal molecules, promoting dendritic cell phagocytosis and developing tumor-specific immune responses, other cellular and molecular mechanisms[29]. Studies have revealed that WOG elevates the exposure of Reactive Oxygen Species (ROS) and calreticulin/Annexin A1 in mouse gastric cancer Mouse Forestomach Carcinoma (MFC) cells in a Protein Kinase R-like Endoplasmic Reticulum Kinase (PERK)/Akt-dependent manner and decreases tumor growth in the MFC xenograft mouse model[30]. Qin et al.[31] showed that novel platinum (IV) complex coupled with WOG derivatives mediated its anticancer activity against SGC7901 cells by down-regulating Cyclooxygenase (COX)-1, COX-2 and Bcl-2 and up regulating ROS, P53 and cleaved caspase-3. Studies have demonstrated that BS induces apoptosis of AGS cells via AMP-Activated Protein Kinase (AMPK) activation mediated expression of Phosphatase and Tensin Homolog (PTEN). BS also inhibits Hsp90, a chaperone protein that is associated with apoptosis. Due to its role in stabilizing cell proliferation, cell cycle progression and other proteins required for survival[32]. BS could significantly inhibit the growth and induce apoptosis of human gastric cancer cells in vitro by reducing BCL2/Bax ratio and DNA damage[33]. Baicalein inhibits the proliferation and migration of gastric cancer cells through the down-regulation of the AKT/mammalian Target of Rapamycin (mTOR) signaling pathway and Focal Adhesion Kinase (FAK) interaction[34]. Li et al.[35] described that baicalein induced apoptosis and autophagy of gastric cancer cells through the AKT/mTOR and Nuclear factor erythroid 2–related factor 2 (Nrf2)/Keap 1 pathways and enhanced the sensitivity of gastric cancer cells to cisplatin. Baicalein could also inhibit FAK through PI3K/ AKT signaling pathway to affect the proliferation, metastasis and angiogenesis of gastric cancer cells, and inhibit gastric cancer progression[36]. Several studies have confirmed that quercetin, luteolin, WOG, BS, baicalein and other components are increasingly important in treating gastric cancer. It shows anticancer effects by inducing apoptosis, blocking the tumor cell cycle, inhibiting tumor angiogenesis, producing specific antigens, etc. Simultaneously, it also has good potential in combating the resistance of chemotherapy drugs, including platinum, which can enhance chemotherapy sensitivity.
From the D-C-G-D network, it can be observed that the active components of Barista have many common targets, indicating that they could have some synergistic effects on the treatment process of gastric cancer. It can be seen from the PPI network that JUN, MAPK1, AKT1, RELA, FOS, ESR1 and so on rank high, which could be the core targets playing a crucial role in treatment. JUN is the main subfamily of Activator Protein 1 (AP-1). FOS and JUN are both AP-1 subfamily proteins. JUN protein can bind FOS protein and ATF subfamily members. JUN and FOS can facilitate cells to synthesize DNA and promote the cell cycle through the Growth (G) 0-G1 transition[37]. Laminin subunit beta1 (Lamβ1) is significantly overexpressed in gastric cancer tissues, promoting tumor growth and invasion, and migration of gastric cancer cells. Studies have revealed that c-Jun can bind to the Lamb1 promoter region and act as a transcription factor to control the expression of the Lamb1 gene in gastric cancer. However, its transcriptional activation remains to be studied[38]. MAPK1 regulates cancer-related cell activities such as migration and proliferation. Studies have depicted that MAPK1 is highly expressed in gastric cancer tissues and cells, and can inhibit the expression of miR-585–3p through LINC01436 and Enhancer of Zeste Homolog 2 (EZH2) and up regulate MAPK1 expression to promote gastric cancer progression[39]. The expression of Ubiquitin- Specific Protease (USP21) in gastric cancer tissues positively correlates with the grade of gastric cancer. It can stimulate the proliferation, migration, invasion and stem cell differentiation of gastric cancer cells through the USP21/GATA3/MAPK1 axis[40]. AKT1 is an active kinase that contributes to the progression of gastric cancer and promotes the proliferation of gastric cancer cells. AKT1 is significantly up regulated in gastric cancer tissues. RELA gene is an essential target of the Nuclear Factor (NF)- Kappa B pathway, and its encoded RELA (p65) is a crucial member of the Rel family. RELA directly promotes the transcription of multiple oncogenes and enhances tumourogenesis and cancer progression. However, Zhang et al.[41] confirmed that RELA is a transcription factor of the tumor suppressor gene LXRa and a negative regulator of the Wnt pathway, which is a proto-cancer pathway involved in the proliferation, invasion, metastasis, drug resistance and angiogenesis of tumor cells. The ESR1 gene is closely associated with breast cancer, primarily involved in estrogen metabolism[42]. Currently, there is no definite study on the relationship between ESR1 and gastric cancer.
GO functional enrichment analysis and KEGG pathway enrichment analysis revealed that the active components of SBH may treat gastric cancer by controlling different BP and pathways, primarily mainly involved in nuclear receptor activity, cytokine activity, transcription factor activity and other aspects. The pathways involved include the Age- RACG signaling pathway, chemical carcinogenesis receptor activation, NF-Kappa B pathway and so on. Previous studies have reported that Miller Fisher Syndrome (MFS) and signaling pathways are closely related to the above essential target genes. However, the specific regulatory mechanisms are unclear, which would be the main direction for future research. Quercetin, luteolin, WOG, BS and baicalein, the active components of SBH, also induce apoptosis of gastric cancer cells. JUN, MAPK1, AKT1, RELA, FOS and ESR1 are essential in tumor cell apoptosis among the above targets. The KEGG pathway may be the main pathway of active components in treating gastric cancer.
Therefore, this study mainly explored the main active components of SBH. It analyzed the main targets of gastric cancer through network pharmacological methods based on the related ideas of multi-component, multi-target and multi-pathway. Quercetin, luteolin, WOG, BS, baicalein and so on is the essential effective components of SBH in treating gastric cancer. JUN, MAPK1, AKT1, RELA, FOS and ESR1 are necessary to target genes in treating gastric cancer. The synergistic effects of multiple active components and target genes on multiple MFs and signaling pathways could achieve the effect of gastric cancer treatment, providing a new idea for the treatment of gastric cancer by SBH.
Availability of data and materials:
All data and materials used in this study are available in the TCM Systems Pharmacology Database and Analysis Platform (TCMSP, http://tcmspw.com) and Gene Cards (https://www.Org/, version 5.9.0), and the Online Mendelian Genetic Database (OMIM) (http://www.omim.org/) and STRING (https:// stringdb.org/, version 11.5).
Authors’ contributions:
Dong Xia and Ping Tang have conceived and designed the studies.
Conflict of interests:
The authors declared no conflict of interests.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-49.
[Crossref] [Google Scholar] [PubMed]
- Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang Z, et al. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Biosci Trends 2015;9(1):16-34.
[Crossref] [Google Scholar] [PubMed]
- Lin H, Liu J, Zhang Y. Developments in cancer prevention and treatment using traditional Chinese medicine. Front Med 2011;5(2):127-33.
[Crossref] [Google Scholar] [PubMed]
- Qi X, Xu H, Zhang P, Chen G, Chen Z, Fang C, et al. Investigating the mechanism of Scutellariae barbata herba in the treatment of colorectal cancer by network pharmacology and molecular docking. Evid Based Complement Altern Med 2021;2021:3905367.
[Crossref] [Google Scholar] [PubMed]
- Chen Q, Rahman K, Wang SJ, Zhou S, Zhang H. Scutellaria barbata: A review on chemical constituents, pharmacological activities and clinical applications. Curr Pharm Des 2020;26(1):160-75.
[Crossref] [Google Scholar] [PubMed]
- Gong B, Kao Y, Zhang C, Sun F, Zhao H. Systematic investigation of Scutellariae barbatae herba for treating hepatocellular carcinoma based on network pharmacology. Evid Based Complement Altern Med 2018;2018:4365739.
- Song Y, Wang H, Pan Y, Liu T. Investigating the multi-target pharmacological mechanism of Hedyotis diffusa Willd acting on prostate cancer: A network pharmacology approach. Biomolecules 2019;9(10):591.
[Crossref] [Google Scholar] [PubMed]
- Huang J, Guo W, Cheung F, Tan HY, Wang N, Feng Y. Integrating network pharmacology and experimental models to investigate the efficacy of coptidis and Scutellaria containing Huanglian Jiedu decoction on hepatocellular carcinoma. Am J Chin Med 2020;48(1):161-82.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Wu J, Zhang D, Wang K, Duan X, Zhang X. A network pharmacology approach to uncover the multiple mechanisms of Hedyotis diffusa Willd. on colorectal cancer. Evid Based Complement Altern Med 2018;2018.
[Crossref] [Google Scholar] [PubMed]
- Shao LI, Zhang B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin J Nat Med 2013;11(2):110-20.
[Crossref] [Google Scholar] [PubMed]
- Liu ZH, Sun XB. Network pharmacology: New opportunity for the modernization of traditional Chinese medicine. Yao Xue Xue Bao 2012;47(6):696-703.
[Google Scholar] [PubMed]
- Stanzione F, Giangreco I, Cole JC. Use of molecular docking computational tools in drug discovery. Prog Med Chem 2021;60:273-343.
[Crossref] [Google Scholar] [PubMed]
- Ferreira LG, Dos Santos RN, Oliva G, Andricopulo AD. Molecular docking and structure-based drug design strategies. Molecules 2015;20(7):13384-421.
[Crossref] [Google Scholar] [PubMed]
- Ru J, Li P, Wang J, Zhou W, Li B, Huang C, et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J Cheminform 2014;6:13.
[Crossref] [Google Scholar] [PubMed]
- Amberger JS, Hamosh A. Searching online mendelian inheritance in man (OMIM): A knowledgebase of human genes and genetic phenotypes. Curr Protoc Bioinformatics 2017;58(1):1-2.
[Crossref] [Google Scholar] [PubMed]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res 2003;13(11):2498-504.
[Crossref] [Google Scholar] [PubMed]
- Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, et al. The STRING database in 2021: Customizable protein–protein networks and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res 2021;49(D1):D605-12.
[Crossref] [Google Scholar] [PubMed]
- Wang L, Chen W, Li M, Zhang F, Chen K, Chen W. A review of the ethnopharmacology, phytochemistry, pharmacology and quality control of Scutellaria barbata D. Don. J Ethnopharmacol 2020;254:112260.
[Crossref] [Google Scholar] [PubMed]
- Haghi A, Azimi H, Rahimi R. A comprehensive review on pharmacotherapeutics of three phytochemicals, curcumin, quercetin and allicin, in the treatment of gastric cancer. J Gastrointestinal Cancer 2017;48(4):314-20.
[Crossref] [Google Scholar] [PubMed]
- Shang HS, Lu HF, Lee CH, Chiang HS, Chu YL, Chen A, et al. Quercetin induced cell apoptosis and altered gene expression in AGS human gastric cancer cells. Environ Toxicol 2018;33(11):1168-81.
[Crossref] [Google Scholar] [PubMed]
- Shen X, Si Y, Wang Z, Wang J, Guo Y, Zhang X. Quercetin inhibits the growth of human gastric cancer stem cells by inducing mitochondrial-dependent apoptosis through the inhibition of PI3K/Akt signaling. Int J Mol Med 2016;38(2):619-26.
[Crossref] [Google Scholar] [PubMed]
- Borska S, Chmielewska M, Wysocka T, Drag-Zalesinska M, Zabel M, Dziegiel P. In vitro effect of quercetin on human gastric carcinoma: Targeting cancer cells death and MDR. Food Chem Toxicol 2012;50(9):3375-83.
[Crossref] [Google Scholar] [PubMed]
- Ma J, Chen X, Zhu X, Pan Z, Hao W, Li D, et al. Luteolin potentiates low-dose oxaliplatin-induced inhibitory effects on cell proliferation in gastric cancer by inducing G2/M cell cycle arrest and apoptosis. Oncol Lett 2022;23(1):16.
[Crossref] [Google Scholar] [PubMed]
- Wu H, Huang M, Liu Y, Shu Y, Liu P. Luteolin induces apoptosis by up-regulating miR-34a in human gastric cancer cells. Technol Cancer Res Treat 2015;14(6):747-55.
[Crossref] [Google Scholar] [PubMed]
- Huang S, Chen M, Shen Y, Shen W, Guo H, Gao Q, et al. Inhibition of activated Stat3 reverses drug resistance to chemotherapeutic agents in gastric cancer cells. Cancer Lett 2012;315(2):198-205.
[Crossref] [Google Scholar] [PubMed]
- Song S, Su Z, Xu H, Niu M, Chen X, Min H, et al. Luteolin selectively kills STAT3 highly activated gastric cancer cells through enhancing the binding of STAT3 to SHP-1. Cell Death Dis 2017;8(2):e2612.
[Crossref] [Google Scholar] [PubMed]
- Ren LQ, Li Q, Zhang Y. Luteolin suppresses the proliferation of gastric cancer cells and acts in synergy with oxaliplatin. Biomed Res Int 2020;2020:9396512.
[Crossref] [Google Scholar] [PubMed]
- Pu Y, Zhang T, Wang J, Mao Z, Duan B, Long Y, et al. Luteolin exerts an anticancer effect on gastric cancer cells through multiple signaling pathways and regulating miRNAs. J Cancer 2018;9(20):3669.
[Crossref] [Google Scholar] [PubMed]
- Xiao W, Wu K, Yin M, Han S, Ding Y, Qiao A, et al. Wogonin inhibits tumor-derived regulatory molecules by suppressing STAT3 signaling to promote tumor immunity. J Immunother 2015;38(5):167-84.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Li XJ, Chen Z, Zhu XX, Wang J, Zhang LB, et al. Wogonin induced calreticulin/Annexin A1 exposure dictates the immunogenicity of cancer cells in a PERK/AKT dependent manner. PLoS One 2012;7(12):e50811.
[Crossref] [Google Scholar] [PubMed]
- Qin X, Xu G, Chen F, Fang L, Gou S. Novel platinum (IV) complexes conjugated with a wogonin derivative as multi-targeted anticancer agents. Bioorg Med Chem 2017;25(8):2507-17.
[Crossref] [Google Scholar] [PubMed]
- Shin EJ, Choi HK, Sung MJ, Park JH, Chung MY, Chung S, et al. Anti-tumour effects of beta-sitosterol are mediated by AMPK/PTEN/HSP90 axis in AGS human gastric adenocarcinoma cells and xenograft mouse models. Biochem Pharmacol 2018;152:60-70.
[Crossref] [Google Scholar] [PubMed]
- Zhao Y, Chang SK, Qu G, Li T, Cui H. β-sitosterol inhibits cell growth and induces apoptosis in SGC-7901 human stomach cancer cells. J Agric Food Chem 2009;57(12):5211-8.
[Crossref] [Google Scholar] [PubMed]
- Qiao D, Jin J, Xing J, Zhang Y, Jia N, Ren X, et al. Baicalein inhibits gastric cancer cell proliferation and migration through a FAK interaction via AKT/mTOR signaling. Am J Chin Med 2021;49(02):525-41.
[Crossref] [Google Scholar] [PubMed]
- Li P, Hu J, Shi B, Tie J. Baicalein enhanced cisplatin sensitivity of gastric cancer cells by inducing cell apoptosis and autophagy via Akt/mTOR and Nrf2/Keap 1 pathway. Biochem Biophys Res Commun 2020;531(3):320-7.
[Crossref] [Google Scholar] [PubMed]
- Qiao D, Xing J, Duan Y, Wang S, Yao G, Zhang S, et al. The molecular mechanism of baicalein repressing progression of gastric cancer mediating miR-7/FAK/AKT signaling pathway. Phytomedicine 2022;100:154046.
[Crossref] [Google Scholar] [PubMed]
- Trop-Steinberg S, Azar Y. AP-1 expression and its clinical relevance in immune disorders and cancer. Am J Med Sci 2017;353(5):474-83.
[Crossref] [Google Scholar] [PubMed]
- Lee H, Kim WJ, Kang HG, Jang JH, Choi IJ, Chun KH, et al. Up regulation of LAMB1 via ERK/c-Jun axis promotes gastric cancer growth and motility. Int J Mol Sci 2021;22(2):626.
[Crossref] [Google Scholar] [PubMed]
- Xu Y, Dong M, Wang J, Zhao W, Jiao M. LINC01436 inhibited miR-585-3p expression and up regulated MAPK1 expression to promote gastric cancer progression. Dig Dis Sci 2021;66:1885-94.
[Crossref] [Google Scholar] [PubMed]
- Guo Q, Shi D, Lin L, Li H, Wei Y, Li B, et al. De-ubiquitinating enzymes USP21 regulate MAPK1 expression by binding to transcription factor GATA3 to regulate tumor growth and cell stemness of gastric cancer. Front Cell Dev Biol 2021;9:641981.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Zhang D, Sun X, Li S, Sun Y, Zhai H. Tumor suppressor gene XEDAR promotes differentiation and suppresses proliferation and migration of gastric cancer cells through up regulating the RELA/LXRα axis and deactivating the Wnt/β-catenin pathway. Cell Transplant 2021;30:0963689721996346.
[Crossref] [Google Scholar] [PubMed]
- Oesterreich S, Davidson NE. The search for ESR1 mutations in breast cancer. Nat Genet 2013;45(12):1415-6.
[Crossref] [Google Scholar] [PubMed]