- *Corresponding Author:
- Jing Tong
Department of Otorhinolaryngology, The Fifth Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang 830011, China
E-mail: tj81218@126.com
| Date of Received | 19 January 2023 |
| Date of Revision | 07 September 2023 |
| Date of Acceptance | 22 April 2024 |
| Indian J Pharm Sci 2024;86(2):531-536 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The expression and correlation of exogenous interleukin-10 and GATA binding protein-3 in the nasal mucosa of allergic rhinitis rats were analyzed. Twenty male Sprague-Dawley rats were randomly divided into two groups; normal control group (group A) and allergic rhinitis group (group B). The interleukin-10 and GATA binding protein-3 in the nasal mucosa of the two groups were determined by immunohistochemistry. The expression levels of GATA binding protein-3 and interleukin-10 were measured by Western blot. Pearson correlation test was used to analyze the correlation between the expression of exogenous interleukin-10 and GATA binding protein-3 in the rat model of allergic rhinitis. The scores of sneezing, runny nose and itching in the group B were reduced than group A. In group A, the epithelial cells of the nasal mucosa were arranged in order, the cilia were complete, and there were a few inflammatory cells and glands under the basement membrane. In group B, the epithelial cells of the nasal mucosa were disordered, the cilia were inverted and absent, the goblet cells in the epithelium were increased, a large number of inflammatory cells such as type selection granulocytes could be seen under the basement membrane, and the gland hyperplasia and vasodilation were obvious. The expression level of interleukin-10 and GATA binding protein-3 in the group B was decreased than control group. The interleukin-10 was positively correlated with GATA binding protein-3. The expression level of interleukin-10 and GATA binding protein-3 in the nasal mucosa of rats with allergic rhinitis was significantly lower than that of rats with allergic rhinitis.
Keywords
Interleukin-10, GATA binding protein-3, allergic rhinitis, nasal mucosa, immunity
Allergic Rhinitis (AR), also known as anaphylactic rhinitis, is a common allergic disease in clinical practice, which is mainly a type I allergic reaction mediated by Immunoglobulin E (IgE). In recent years, the incidence of AR is increasing year by year, although it is not life-threatening, but it seriously affects people’s quality of life and increases the burden on society. The pathogenesis of AR is complex and not yet clear. It is believed that it may be caused by an imbalance between T helper (Th) 1 cells and Th2 immune responses, which may involve a large number of cytokines and inflammatory mediators in this process[1]. Recognition and regulation of mammalian innate immunity is primarily achieved by recognizing pathogen-associated molecular patterns or damage-associated molecular patterns expressed on pathogenic microorganisms[2]. These receptors recognize invading pathogens and their types, and then activate effector molecules to form inflammatory complexes through a number of signaling pathways, thereby initiating effector mechanisms of innate immunity, releasing regulatory factors, and inducing B-cell or T-cell differentiation to regulate adaptive immune responses[3]. At present, there are many studies on pattern recognition receptors in inflammatory diseases, allergic diseases, tumors and autoimmune diseases, especially Toll-like receptors distributed on the cell surface and specific organelles, which can recognize allergens on the cell surface and thus play an important role in allergic reactions[4]. Some studies have found that Interleukin (IL)- 10 and GATA Binding Protein-3 (GATA-3) play critical role in allergic diseases, and their roles in the occurrence and development of AR cannot be ignored[5]. Sprague-Dawley (SD) rats were used to establish a rat model of AR, and the expression and correlation of exogenous IL-10 and GATA-3 in the Nasal Mucosa (NM) of the rat model of AR were investigated and analyzed.
Materials and Methods
Experimental animals:
Twenty healthy male SD rats of clean grade were randomly selected (purchased from Guangzhou Frebo Biotechnology Co., Ltd., production license No: SCXK (Yue) 2018-0022). All rats weighed (205±15) g, aged about 7 w old, and were adaptively fed for 1 w in an environment with temperature of (22°±3°), humidity of (50±15) %, and diurnal alternation time ratio of 1:1, with free diet and water intake.
Experimental instruments and reagents:
Electrothermal constant-temperature water bath (Shanghai Shibei Instrument and Equipment Factory, model: Western blot-1-15), electronic balance (Shanghai Shunyu Hengping Scientific Instrument Co., Ltd., model: AE124), lowtemperature high-speed centrifuge (Hebei Huicai Technology Co., Ltd., model: A1301022), air bath constant-temperature oscillator (Changzhou Guowang Instrument Manufacturing Co., Ltd., model: THZ-82A), paraffin microtome (Beijing Shengke Xinde Technology Co., Ltd., model: RM2245), 80° ultra-low temperature refrigerator (Beijing Elix Biotechnology Co., Ltd., model: DW-86L626), inverted biomicroscope (Shanghai Wumu optical instrument Co., Ltd., model: WMS- 1081), real-time Polymerase Chain Reaction (PCR) detector (Xi'an Tianlong Technology Co., Ltd., model: Gentier 96E), absolute ethanol (Shanghai Mubai Chemical Co., Ltd.), ovalbumin (Shanghai Jizhi Biochemical Technology Co., Ltd.), 0.9 % sodium chloride injection (Qingshan Likang Pharmaceutical Co., Ltd., batch number: 20170019, and 100 ml hematoxylin & eosin stain (Chengdu).
Establishment of AR rat model:
The rats were randomly divided into normal control group (group A) and AR group (group B) according to random number table, with 10 rats in each group. In group B, 20 mg ovalbumin and 30 mg aluminum hydroxide were dissolved in 1 ml normal saline for intraperitoneal injection every other day for 8 consecutive injections, and 5 % concentration ovalbumin normal saline solution was instilled into the nasal cavity of rats for stimulation from the 16th d, 10 μl per measurement, for 10 d. Rats in the control group were intranasally instilled with normal saline.
Observation indicators:
By stimulation, animal models developed symptoms such as nasal itching, scratching the nose, scratching the face, sneezing, and runny nose. They were observed for 30 min after the last stimulation. The scores of nasal itching, sneezing and runny nose are 1~3 points, among which, nasal itching; 1 point for light rubbing nose, 2 points for scratching nose and face, 3 points for rubbing everywhere; in sneezing 1~3 points for 1 sneeze, 2 points for 4~10 sneezes, 3 points for more than 11 sneezes and in runny nose 1 point for running to anterior nostril, 2 points for exceeding anterior nostril, 3 points for full face of runny nose. The total score was superimposed and recorded, and a total score of more than 5 was considered a modeling success.
Rats in both groups were sampled after the last stimulation, and the rats were anesthetized, and the NM on both sides of the rats in both groups was taken and placed in 4 % formalin solution for fixation, and paraffin sections were routinely made.
Hematoxylin and Eosin (H&E) staining was used to determine the changes of NM in the two groups. The tissue sections were baked in a constant temperature of 60° for about 30 min to dissolve the wax. The sections were put into xylene and ethanol for transparency and dehydration, washed with phosphate buffer solution, and dried with filter paper to remove excess water. And then they were stained with H&E staining solution for 2~3 min and then rinsed with running water appropriately and kept the sections dehydrated and transparent, finally mounted with neutral gum.
The IL-10 and GATA-3 in the NM of the two groups of rats were determined by immunohistochemistry. Paraffin sections were deparaffinized and hydrated in xylene and absolute ethanol, respectively, rinsed with phosphate buffer for 3 times, 3 min each time, boiled with sodium citrate buffer for 10 min in a microwave oven and heated for 10 min, cooled for 5 min and then continued to heat for 5 min for antigen retrieval. They were cooled at room temperature, rinsed with phosphate buffer, dropped with serum blocking solution, added with chromogenic agent, then dropped with 3,3-Diaminobenzidine (DAB) for color development, counterstained with hematoxylin, rinsed with distilled water, kept the sections dehydrated and transparent, and then mounted. GATA-3 and IL-10 expression levels were measured by Western blotting in both groups of rats. Pearson correlation test was used to analyze the correlation of exogenous IL-10 and GATA-3 expression in a rat model of AR.
Statistical methods:
Statistical data analysis was performed using the Statistical Package for the Social Sciences (SPSS) 22.0 software. Independent sample t-test was used to compare measurement data between the two groups. H&E staining was used to determine the changes of NM in the two groups. The expression levels of IL-10 and GATA-3 in the NM of the two groups of rats were determined by immunohistochemistry. GATA-3 and IL-10 expression levels were measured by Western blotting in both groups of rats. Pearson correlation test was used to analyze the correlation of exogenous IL-10 and GATA-3 expression in a rat model of AR. Statistical results were considered statistically significant at p<0.05.
Results and Discussion
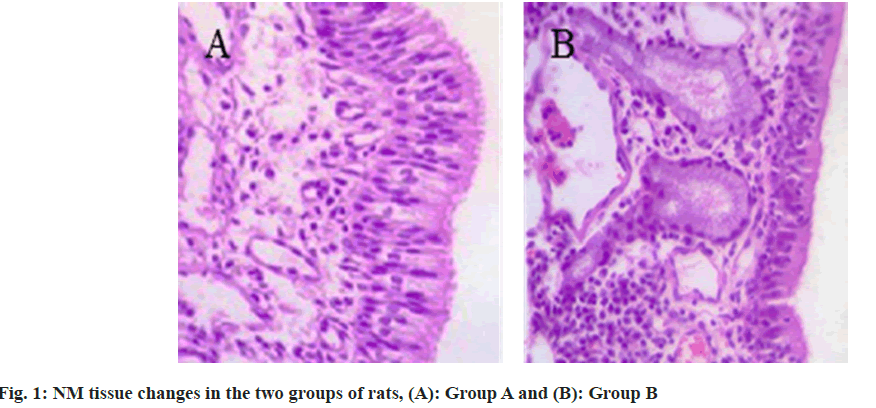
Compared with the group A, the scores of sneezing, runny nose, and nasal itching were reduced in the group B (Table 1).
| Group | Cases (n) | Sneeze | Runny nose | Nasal itching |
|---|---|---|---|---|
| A | 10 | 0.37±0.15 | 0.49±0.20 | 2.44±0.18 |
| B | 10 | 2.09±0.47 | 2.26±0.40 | 2.13±0.33 |
| t | 11.025 | 12.516 | 2.608 | |
| p | <0.001 | <0.001 | 0.018 |
Table 1: Symptom Scores (X̄±S)
In the group A, the epithelial cells of NM tissue in rats were in alignment. The cilia were intact. And there were a small number of inflammatory cells and glands under the basement membrane. In the AR rats, the epithelial cells of NM tissue were disorganized. The cilia were inverted and absent. The intraepithelial goblet cells were increased. A large number of inflammatory cell infiltrates such as selected granulocytes were observed under the basement membrane, and the glandular proliferation and vasodilatation were obvious as shown in fig. 1.
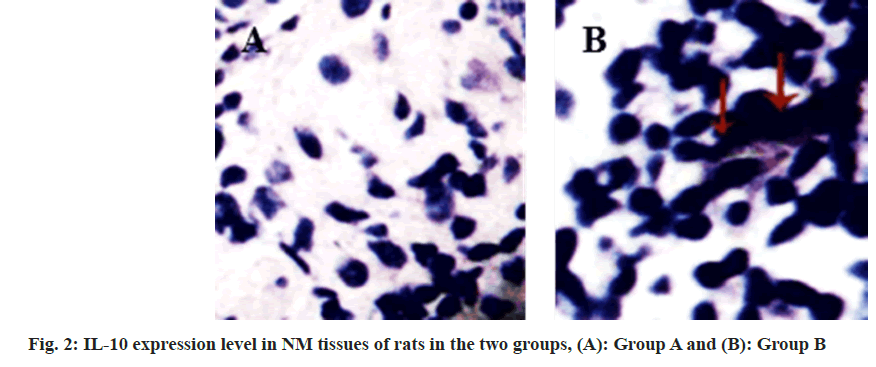
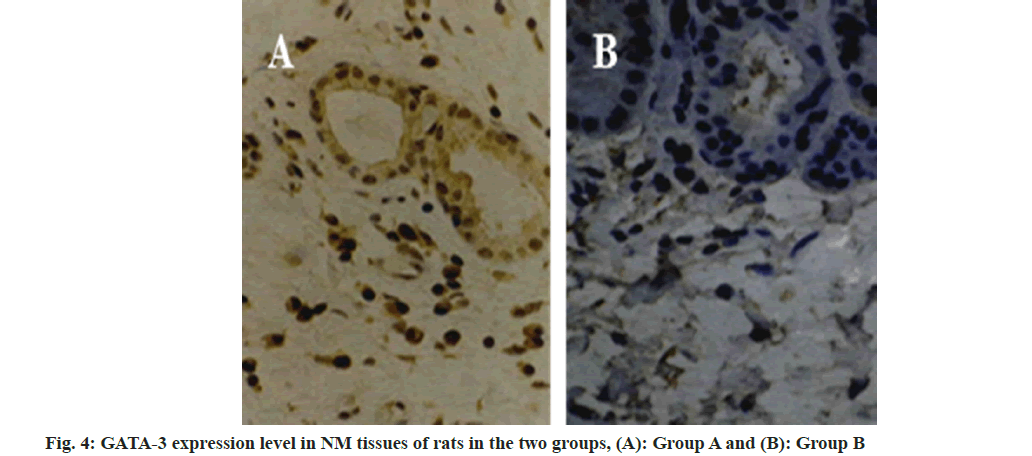
Compared with the group A, the IL-10 expression level was reduced in the group B (fig. 2 and fig. 3, and Table 2). Compared with the group A, GATA- 3 expression levels were increased in the group B (fig. 4 and fig. 5, and Table 3). After Pearson correlation test analysis, IL-10 expression levels were significantly positively correlated with GATA-3 (r=0.215, p<0.05)
| Group | Cases (n) | IL-10 |
|---|---|---|
| A | 10 | 686.10±176.27 |
| B | 10 | 147.03±61.05 |
| t | 9.138 | |
| p | <0.001 |
Table 2: Il-10 Expression Levels in Nm Tissues of Rats iIn the Two Groups (X±S)
| Group | Cases (n) | GATA-3 |
|---|---|---|
| A | 10 | 1.53±0.36 |
| B | 10 | 2.35±0.81 |
| t | 2.925 | |
| p | 0.009 |
Table 3: Gata-3 Expression Level in Nm Tissues of Rats in the Two Groups (X±S)
Clinically, AR is one of the most common chronic respiratory diseases, with nasal itching, episodic sneezing, a large number of watery nasal discharge, and nasal obstruction as the main clinical symptoms, and eosinophil, lymphocyte infiltration, NM edema, vasodilatation, and glandular cell proliferation as the main pathological changes[6]. In recent years, AR has become a global health problem, and with the development of global industrialization, its incidence has been increasing year by year. However, its pathogenesis is not clear, researchers have proposed the Th1/ Th2 factor imbalance theory and Treg/ Th17 cytokine imbalance theory[7]. The clinical treatment of AR is not based on surgery, and clinical specimens are not easy to obtain. Therefore, the establishment of AR model with corresponding clinical and pathological characteristics has an important role for related research. Guinea pigs are the first animals to be used to establish AR models due to their allergenic biological characteristics, abundant complement in serum and stable production of specific IgE. However, we still do not know the gene and immune system of guinea pigs, while we have a good understanding of the immune system of rats. They can be used not only for the technical study of modern genetic engineering, but also in combination with traditional experimental techniques for the study of histomorphology and immune mechanism. The reproductive capacity and ability of defensing respiratory disease of rats are strong. Besides, they are easy to obtain[8]. Therefore, SD rats were mainly selected as the study subjects in this study. To study the expression and correlation of exogenous IL-10 and GATA-3 in the NM tissue of a rat model of AR, ovalbumin was used as an allergen.
With the deepening of research, it has been found that a variety of chemokines and cytokines play critical role in AR and participate in the regulation of the local microenvironment, thus affecting the occurrence and outcome of AR[9]. Chemokines can promote the infiltration of inflammatory cells between tissues and mediate the participation of various inflammatory cells such as eosinophils, neutrophils and mast cells in local inflammation and allergic reactions. GATA-3, a member of the GATA protein family, was first discovered in T lymphocytes and embryonic brain, and can specifically bind to enhancers of the four subunits of the T-Cell Receptor (TCR) and regulate the expression of TCR genes, which is considered to be a T cell-specific transcription factor and is closely related to the early development of T cells[10]. It has been found that GATA-3 has some inhibitory effect on the development of Th1 cells, which may be inhibited by the signal transduction of IL-12 and Interferon Gamma (INF-γ)[11]. Shirkani et al.[12] found that the expression of GATA-3 was significantly up-regulated in the NM of patients with AR after antigenic stimulation. The results were the same as those in our studies. These results suggest that GATA-3 may serve as an important therapeutic target. Regulation of GATA- 3 function is beneficial to control the formation or development of AR. It plays an important role in the body’s immune function as a Th2 cytokine that inhibits the transformation of Th0 cells into Th1 cells, the migration of inflammatory cells, and the production of proinflammatory cytokines. Since Major Histocompatibility Complex (MHC) II and B7 mediators are required for the activation and proliferation of T cells, while IL-10 has a certain inhibitory effect on the expression of MHC II and B7. Thus IL-10 can thereby inhibit the activation, proliferation and further differentiation of T cells, and then inhibiting the occurrence of effector T cell-mediated IgE-secreting allergic reactions[13]. According to relevant reports, the inhibitory effect of IL-10 mainly includes inhibiting the secretion of cytokines of plasma cells, natural killer cells and some other cells, reducing the production of IgE and playing a protective role in allergeninduced nasal inflammation[14]. That IL-10 expression levels were reduced in AR rats than in normal rats. This may be due to the fact that IL- 10 is a negative immunoregulatory factor, and the reduction of IL-10 makes it unable to effectively inhibit Alkylphosphocholines (APCs), resulting in a reduced ability to deal with abnormal immune responses. Thus, the production of IgE in the body is not controlled, and a large number of mediators such as eosinophils in the local NM accumulate, which in turn aggravates the local lesions in patients with AR[15].
In summary, the IL-10 was reduced and the GATA- 3 was raised in the NM tissue of a rat model of AR. The two have a significant correlation and may be used as a new target for the treatment of AR.
Conflict of interests:
The authors declared no conflict of interests.
References
- Adegbiji WA, Olajide GT, Olajuyin AO, Aremu SK, Olusola AG. Pattern of allergic rhinitis among children in Ekiti, Nigeria. Int J Pediatr Otorhinolaryngol 2018;106:75-9.
[Crossref] [Google Scholar] [PubMed]
- Chen S, Guo S, Wang J, Ha E, Marmori F, Wang Y, et al. Effectiveness of moxibustion for allergic rhinitis: Protocol for a systematic review. BMJ Open 2015;5(5):e006570.
[Crossref] [Google Scholar] [PubMed]
- Zhang S, Liu Y, Shen C, Li G, Yang K, Shi X, et al. The role of Nods like receptors in the patients with allergic rhinitis. J Clin Otolaryngol 2015;29(15):1323-8.
[Google Scholar] [PubMed]
- Wong CK, Chu IM, Hon KL, Tsang MS, Lam CW. Aberrant expression of bacterial pattern recognition receptor NOD2 of basophils and microbicidal peptides in atopic dermatitis. Molecules 2016;21(4):471.
[Crossref] [Google Scholar] [PubMed]
- Kim BY, Park HR, Jeong HG, Kim SW. Berberine reduce allergic inflammation in a house dust mite allergic rhinitis mouse model. Rhinology 2015;53(4):353-8.
[Crossref] [Google Scholar] [PubMed]
- Ciprandi G, Marseglia GL, Castagnoli R, Valsecchi C, Tagliacarne C, Caimmi S, et al. From IgE to clinical trials of allergic rhinitis. Expert Rev Clin Immunol 2015;11(12):1321-33.
[Crossref] [Google Scholar] [PubMed]
- Xu F, Yu S, Qin M, Mao Y, Jin L, Che N, et al. Hydrogen-rich saline ameliorates allergic rhinitis by reversing the imbalance of Th1/Th2 and up-regulation of CD4+ CD25+ Foxp3+ regulatory T cells, interleukin-10, and membrane-bound transforming growth factor-β in guinea pigs. Inflammation 2018;41(1):81-92.
[Google Scholar] [PubMed]
- Liu T, Liang A, Yi Y, Li C, Zhao Y, Hao R, et al. Omparative study on allergen assessment animal models in Brown Norway rat and guinea pig. Zhongguo Zhong Yao Za Zhi 2009;34(4):472-5.
[Google Scholar] [PubMed]
- Bachert C, Maspero J. Efficacy of second-generation antihistamines in patients with allergic rhinitis and comorbid asthma. J Asthma 2011;48(9):965-73.
[Crossref] [Google Scholar] [PubMed]
- Kim SW, Kim SW, Kang JM. Interleukin-37 relieves allergic inflammation in a house dust mite allergic rhinitis murine model. Iranian J Allergy Asthma Immunol 2017;16(5):404-17.
[Google Scholar] [PubMed]
- Ren M, Tang Q, Chen F, Xing X, Huang Y, Tan X. Mahuang Fuzi Xixin decoction attenuates Th1 and Th2 responses in the treatment of ovalbumin-induced allergic inflammation in a rat model of allergic rhinitis. J Immunol Res 2017;2017:8254324.
[Crossref] [Google Scholar] [PubMed]
- Shirkani A, Mansouri A, Abbaszadegan MR, Faridhosseini R, Azad FJ, Gholamin M. GATA3 gene polymorphisms associated with allergic rhinitis in an Iranian population. Rep Biochem Mol Boil 2017;5(2):97.
- Nasiri R, Hirbod-Mobarakeh A, Movahedi M, Farhadi E, Ansaripour B, Amirzargar AA, et al. Gene polymorphisms of interleukin-10 and transforming growth factor beta in allergic rhinitis. Allergol Immunopathol 2016;44(2):125-30.
[Crossref] [Google Scholar] [PubMed]
- Zhao N, Liu Y, Liang H, Jiang X. Bone marrow-derived mesenchymal stem cells reduce immune reaction in a mouse model of allergic rhinitis. Am J Transl Res 2016;8(12):5628.
[Google Scholar] [PubMed]
- Li Z. Expression and clinical significance of CD19 and IL-10 in allergic rhinitis. Shanxi Med Univ 2013.