- *Corresponding Author:
- Zerrin Cantürk
Department of Pharmaceutical Microbiology, Pharmacy Faculty, Anadolu University, Eskisehir 26470, Turkey
E-mail: zkcanturk@anadolu.edu.tr
| Date of Received | 29 July 2025 |
| Date of Revision | 02 August 2025 |
| Date of Accepted | 10 October 2025 |
| Indian J Pharm Sci 2025;87(5):169-178 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Alpha humulene, the major active ingredient of the hops plant (Humulus lupulus L.), is a monocyclic sesquiterpene composed of three isoprene units containing three unpaired C=C double bonds. Beside hops plant, it is also commonly found in herbs such as cannabis, sage, basil, and ginseng. Alpha humulene is also known as alpha Caryophyllene and it has many effects such as anti-inflammatory, antimicrobial, antioxidant, anticancer and local anaesthetic. In this study, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide test was used to determine the concentration range of the cytotoxic effects of Alpha humulene in the SH-SY5Y cell line, a bone marrow derived human neuroblastoma cell line. In addition, the neuroprotective effects of Alpha humulene in the model of neurotoxicity induced by hydrogen peroxide, the half-maximal inhibitory concentration value of Alpha humulene on undifferentiated and retinoic acid induced differentiated SH-SH5Y was determined 221 μg/ml and >400 μg/ml cells using the Real-time cell analysis system. Apoptotic effect levels were determined by Acridine orange/Parkinson's disease, staining method. The SH-SY5Y cell line and the lipopolysaccharide stimulated Tohoku hospital pediatrics-1 cell line were incubated together to establish an in vitro co-culture model. In the in vitro co-culture model established, fluorescence measurements were taken in Cytation 3 multi-mode reader to determine the immunological effects of alpha humulene using lipopolysaccharide stimulation and multiple independent images were taken. The apoptotic effect caused by cellular damage caused by hydrogen peroxide alpha humulene was reduced.
Keywords
Alpha humulene, neuroprotective effect, co-culture model, real-time cell analysis system dual purpose
Cannabinoids are metabolites comprising a group of chemical compounds that act through cannabinoid receptors found in essential oils of species belonging to the Cannabaceae family, such as Eugenia caryophyllus, Humulus lupulus, Teucrium marum and Lantana achyranthifola. Cannabinoids work together with terpenes, which are secondary metabolites. Caryophyllenes are sesquiterpenes containing beta caryophyllene, alpha caryophyllene (Alpha humulene), and isocaryophyllene. Alpha humulen Humulus lupulus L. is a monocyclic sesquiterpene composed of three isoprene units found in the essential oils of the hop plant[1]. Alpha humulene has anti-inflammatory, antimicrobial, anticancer, antioxidant and local anesthetic effects[2].
Along with the evolutionary process, there is an increase in the incidence of neurodegenerative diseases with the prolongation of human life. Alzheimer's Disease (AD), Parkinson's Disease (PD), Huntington's Disease (HD), Amyotrophic Lateral Sclerosis (ALS), and Spinocerebellar Ataxia Disease (SCA) are common neurodegenerative diseases[3]. Unfortunately, there is no effective treatment for these diseases, even as a result of scientific studies carried out with the developing technology.
Oxygen (O2), which is important for the vital activities of organisms is used in mitochondrial redox reactions and as a result of these reactions, reactive oxygen derivatives are formed. In case of excessive Reactive Oxygen Species (ROS) production, oxidative stress occurs. Oxidative stress can cause macromolecular damage, leading to atherosclerosis, diabetes, cancer, neurodegenerative diseases and aging.
It is accepted in the literature that cannabinoids are a useful therapeutic agent due to their low toxicity and are suitable for use in the treatment of neurological diseases[4]. In the Central Nervous System (CNS) of rodents and humans; the location of the CB1 receptor in the hippocampus, amygdala, hypothalamus, cerebellum, brain stem and spinal cord was determined[5]. Cannabinoids can reduce oxidative damage by acting as scavengers of ROS[6]. Cannabinoids exert an effect on macrophage and microglial cells by modulating Interleukin (IL)-1, Tumor Necrosis Factor-Alpha (TNF-α) and IL-6 in neurodegeneration and neuroinflammatory states[7]. Cannabinoids have neuroprotective, anti-oxidative and anti-apoptotic effects against Aβ.
In order to investigate the antiproliferative and antiapoptotic effects of Alpha humulene within the scope of the study, first of all, toxic/nontoxic concentrations were determined on SHSY5Y cells by 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) method. After that Hydrogen peroxide (H2O2) induced neurotoxicity model was established on SH-SY5Y cells differentiated into neuronal phenotype and cell viability values of the neuroprotective effect of Alpha humulene were determined by both the MTT method and the xCELLigence Real-Time Cell Analysis System Dual Purpose (RTCA-DP). In addition to the neurotoxicity model, an in vitro co-culture model was established by co-incubating the SH-SY5Y cell line and the Lipopolysaccharide (LPS)-stimulated Tohoku Hospital Pediatrics-1 (THP-1) cell line.
In conclusion, the antiproliferative, neuroprotective and antiapoptotic effects of Alpha humulene on the SH-SY5Y cell line were determined within the scope of the study.
Materials and Methods
Cell lines development:
Roswell Park Memorial Institute Medium 1640 (RPMI-1640) medium containing 1 % Pen-Strep, 0.1 % Pyromycin, 10 % Fatal Bovine Serum (FBS) and 0.0004 %-mercaptoethanol was used to grow the THP-1 cell line.
The SH-SY5Y cell line was developed in 10 % FBS, 1 % penicillin-streptomycin, 1 % amphotericin-B, 0.1 % puromycin, and Dulbecco's Modified Eagle's Medium (DMEM) medium. For neuronal differentiation of SH-SY5Y cells, all-trans- Retinoic Acid (RA) was added a day after plating at final concentration 10 μM in DMEM with 1 % FBS and maintained for 5 d.
Differentiation of the SH-SY5Y human neuroblastoma cell line into neuronal phenotype:
SH-SY5Y cells were grown in DMEM medium containing 10 % FBS, 1 % penicillin/streptomycin until they reached a density of 70 %-80 %, then incubated for 5 d in differentiation medium containing 1 % FBS, 1 % penicillin/streptomycin, and 10 μM RA to differentiate. When SH-SY5Y human neuroblastoma cells are undifferentiated, they grow in clusters to express immature neuron markers and undergo rapid proliferation. When cells differentiate with RA, proliferation decreases, polarization and neurite formation begin.
Counting cells:
After visualizing with an inverted microscope that SH-SY5Y neuroblastoma cells reached a density of 70 %-80 %, the medium in the 75 cm2 flask was removed and washed with 5 ml of Phosphate Buffer Solution (PBS). After washing, PBS was removed and 1000 μl trypsin- Ethylenediamine Tetraacetic Acid (EDTA) was added into the flask and left to incubate in a Carbon dioxide (CO2) oven for 5 min. After incubation, the cells were removed from the surface by applying mechanical force to the flask. 5 ml of fresh medium was added to the flask and the suspended cell group was transferred to a 50 ml flask. Centrifuged at 1250 rpm for 5 min, the cells settle to the bottom and thus the supernatant was removed. Pipetting was performed by adding 1 ml of suitable fresh medium (DMEM) to the cell pellet remaining in the falcon. Homogeneously distributed SH-SY5Y cells after pipetting were counted on Cedex (Roche - Germany) using Trypan Blue dye.
THP-1 cells developed and contained in flasks of 75 cm2 were collected and transferred to 50 ml falcons and centrifuged at 1100 rpm for 5 min. After centrifugation, the supernatant was removed and 1 ml of fresh medium was added to the settled THP-1 cells and pipetting was performed. THP-1 cells showing a homogeneous distribution after pipetting were counted on Cedex (Roche-Germany) using Trypan Blue.
Cytotoxicity test (MTT test):
MTT test was performed both RA-differentiated and undifferentiated SH-SY5Y cells. After the cells were counted, 5×103 for RA-differentiated SH-SY5Y and 1×104 for undifferentiated SH-SY5Y cells were calculated in each well and 100 μl was distributed to 96-well plates. The plates were incubated for 24 h in a 5 % CO2 oven.
After 24 h of incubation, the media on the plates were withdrawn and discarded. For Alpha humulene dilution was prepared in the range of 400 μM-50 μM and given to each well in 100 μl medium. They were placed in the oven again with 5 % CO2 and left 24 h for undifferentiated and 5 d RA-differentiated SH-SY5Y incubation.
At the end of the incubation, the media were withdrawn and discarded. MTT prepared at a ratio of 1:9 in the dark was distributed to each well as 10 μl (5 mg/ml) and incubated in an oven with 5 % CO2 for 4 h.
At the end of the incubation, the media on the plates were withdrawn and discarded. For H2O2 dilution was prepared in the range of 400 μM-1 μM and given to each well in 100 μl medium. They were placed in the oven again with 5 % CO2 and left for 2 h incubation.
After 2 h of incubation, the media were withdrawn and discarded. MTT prepared at a ratio of 1:9 in the dark was distributed to each well as 10 μl (5 mg/ml) and incubated in an oven with 5 % CO2 for 4 h.
After 24 h of incubation, the media on the plates were withdrawn and discarded. For Alpha humulene dilution was prepared in the range of 400 μM-50 μM and given to each well in 100 μl medium. They were placed in the oven again with 5 % CO2 and left 24 h for undifferentiating and 5 d for RA-differentiation SH-SY5Y incubation. At the end of the incubation, the existing medium was changed with the medium containing the non-toxic concentration of H2O2, which was also determined by the MTT method, and it was left to incubate for 2 h.
After 2 h of incubation, the media were withdrawn and discarded. MTT prepared at a ratio of 1:9 in the dark was distributed to each well as 10 μl (5 mg/ml) and incubated in an oven with 5 % CO2 for 4 h.
After the cells were counted, 5×103 for RA-differentiated SH-SY5Y cells were calculated in each well and 100 μl was distributed to 96-well plates. The plates were incubated for 5 d in a 5 % CO2 oven. On the 3rd d, the medium was replaced with fresh medium. After the THP-1 cells reaching the appropriate density were counted, they were inoculated into 6-well plates at 2×105 cells per well.
While Escherichia coli (E. coli) LPS was added to one well of suspended THP-1 cells at a rate of 1 μg/ml and the other well at a rate of 100 ng/ml, 1 well was separated as a control group and left to incubate for 4 h. At the end of the incubation, the cell-medium in each well was collected in the same falcon and centrifuged at 1100 rpm for 5 min. At the end of centrifugation, concentrations of 400-50 μg/ml prepared from the stock solution of alpha humulene were applied on SH-SY5Y cells in the obtained supernatant. At the end of the incubation period, the non-toxic concentration of H2O2 was added to the medium and left for 2 h incubation. At the end of the incubation, MTT method was applied.
MTT dye was drawn off from SH-SY5Y cell line whose incubation period had expired. Later, 100 μl of Dimethyl Sulfoxide (DMSO) was given to each well and measured at 540 nm absorbance in an Enzyme-Linked Immunoassay (ELISA) device within half an hour.
Cell viability was calculated from the obtained data and plotted on GraphPad, and significant results were selected for SH-SY5Y cell line and tested on xCELLigence device.
Real-time cell analysis system (xCELLigence Technology):
For the SH-SY5Y cell line; 100 μl of clean DMEM medium was added to the special E-plates used for the xCELLigence instrument. The xCELLigence device was stopped to plant cells on the clean media that were taught, and after counting the cells in the Cedex device, 100 μl was distributed to each well except one well by calculating 1×105 for undifferentiated cells, 5×103 for RA-differentiated per well. Medium control was performed by adding 100 μl of clean medium to the empty well. The cells, which were kept in the sterile cabinet for 15 min, were placed in the xCELLigence device and the program continued to be run from where it left off.
When the number of cells measured with a clean medium for 24 h reached the highest point, the device was stopped and E-plaques were removed. 100 μl was withdrawn from the medium and the concentrations prepared in the range of 400 μl, 200 μM - 100 μM and 50 μM for Alpha humulene were given. The device was restarted and measurements were taken for 24 and 48 h.
Apoptosis Test (Acridine Orange (AO)-Propidium Iodide (PI) double staining):
AO/PI double staining was performed for RA-differentiated and undifferentiated SH-SY5Y cells. After the cells were counted, 2×105 for RA-differentiated SH-SY5Y and 5×105 for undifferentiated SH-SY5Y cells were calculated in each well and 1 ml was distributed to 6-well plates. 24 h for undifferentiated cells, 5 d for RA-differentiation left for incubation.
At the end of the incubation, the cells were counted, 2×105 THP-1 cells were calculated in each well and 1 ml was distributed to 6-well plates. 100 ng/ml of E.coli LPS was added to each well of the suspended THP-1 cells and incubated for 4 h. At the end of the incubation, the cell-medium in each well was collected in the same falcon and centrifuged at 1100 rpm for 5 min. A co-culture model was established with the SH-SY5Y cell line RA-differentiated into neuronal phenotype and THP-1 cells stimulated with E.coli LPS.
Alpha humulene dilution was prepared in the range of 400 μM-50 μM and given to each well in 1 ml medium. The plates were incubated for 24 h in a 5 % CO2 oven. In order to determine apoptosis with AO/PI staining after 24 h, it was incubated for 2 h with H2O2 IC50 value and staining procedure was applied at the end of the incubation period.
At the end of the applied procedure, fluorescence measurements at a wavelength of 525 excitation and 617 nm emission were taken, and multiple independent images were taken in the Cytation 3 multi-mode reader.
Results and Discussion
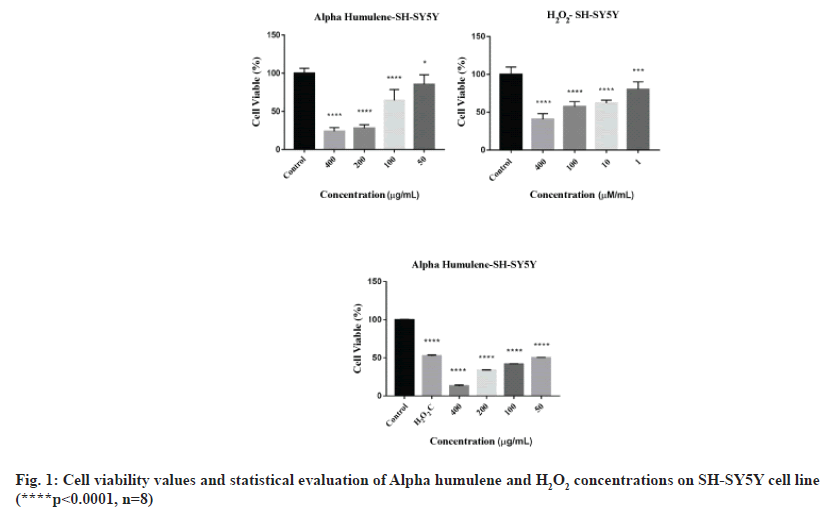
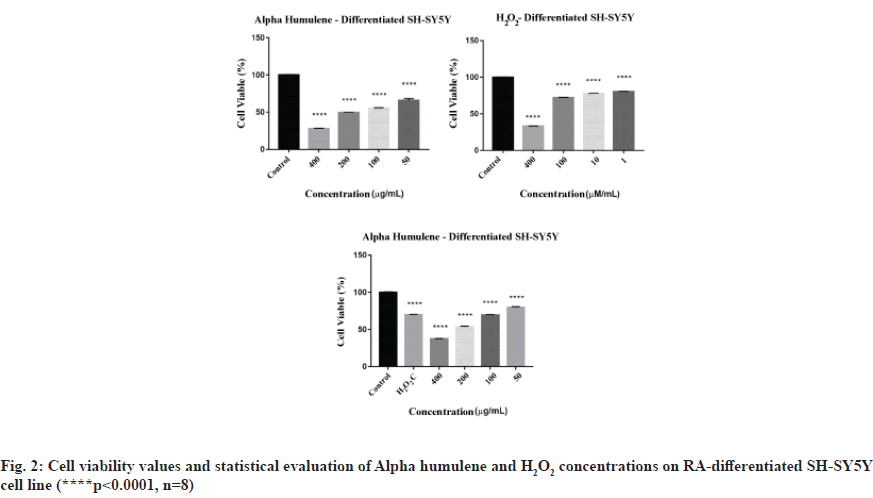
MTT results for Alpha humulene of SH-SY5Y cells were read in Cytation 3 cell imaging multimode reader at 540 nm wavelength, 8 replicates for each concentration. The average of the absorbance values of the control group was accepted as 100 % and the % viability values of other concentrations were calculated. Graphs generated from the data that obtained as a result of 24 h incubation of undifferentiated SH-SY5Y cells and RA-differentiated SH-SY5Y cells are shown in fig. 1 and fig. 2.
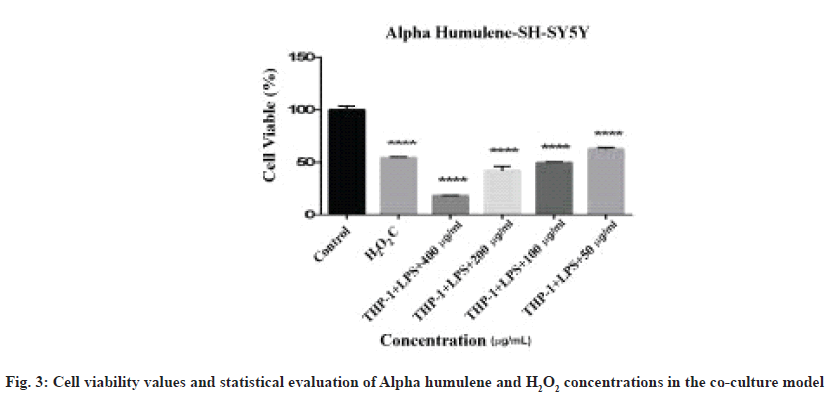
The graph of the neuroprotective effect of Alpha humulene, formed from the data obtained as a result of 24 h incubation in a co-culture model created with SH-SY5Y cell line RA-differentiated into neuronal phenotype and stimulated with E. coli LPS, is shown in fig. 3.
Determination of IC50 Value of Alpha humulene and H2O2 for undifferentiated and RA-differentiated SHSY5Y Using xCELLigence real-time cell analysis system
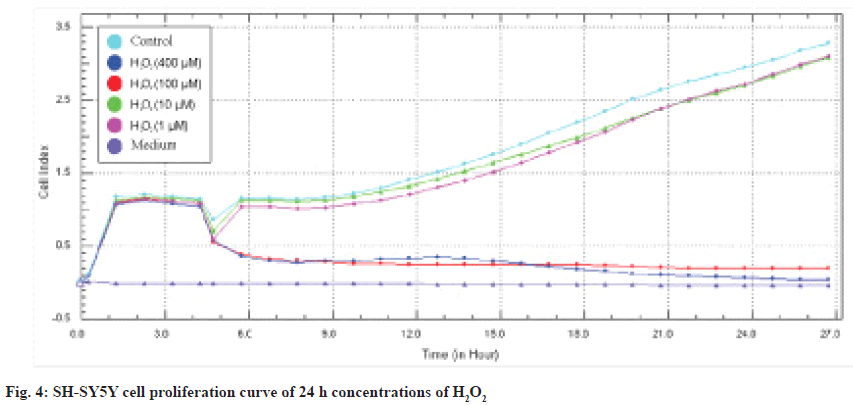
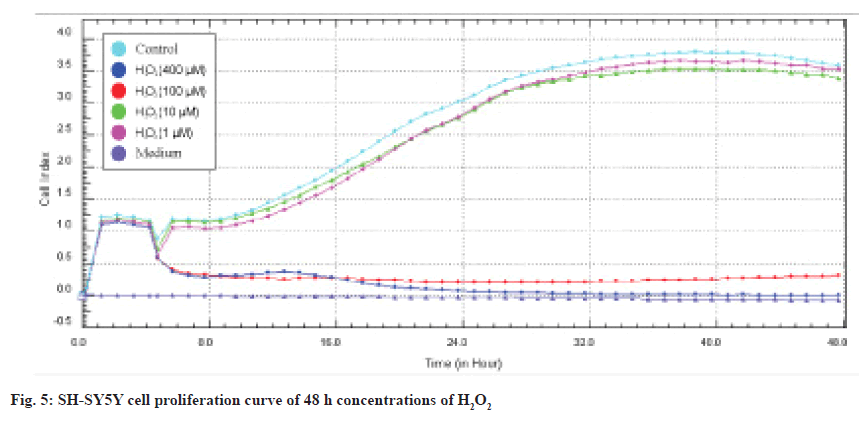
As a result of the screenings performed with the MTT cytotoxicity test, the concentration ranges of Alpha humulene (400-50 μM), H2O2 (400-1 μM) cell line was determined and applied in the RTCA DP system. The device was stopped 48 h after the application of the concentrations (72 h in total) and the IC50 values were automatically calculated by the RTCA DP Software 1.2.1 program. In SH-SY5Y cells, IC50 values at 24 and 48 h calculated according to the real-time cell index data of H2O2 were determined as 42 μM and 38 μM, respectively and are shown in fig. 4 and fig. 5.
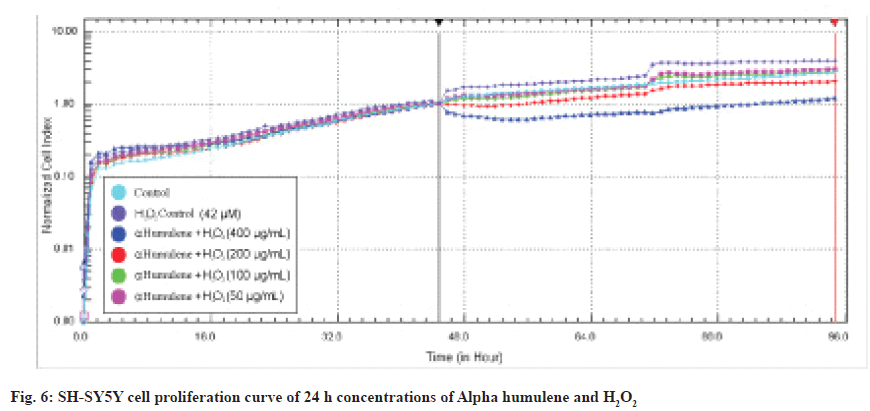
IC50 values were calculated in the RTCA-DP system with 50, 100, 200 and 400 μg/mL Alpha humulene on SH-SY5Y cells and H2O2 concentrations with an IC50 value of 42 μM according to the slope graph. The calculated IC50 values are 221 μg/ mL at 24 h and 209 μg/ml at 48 h. The 24 h cell proliferation curves of the concentrations of 400-50 μg/ml Alpha humulene and 42 μM H2O2 in SH-SY5Y cells with the RTCA-DP analysis system are shown in fig. 6.
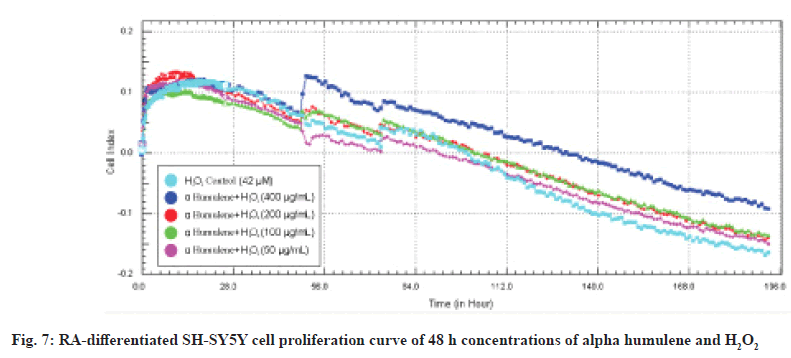
When its neuroprotective effects on RA-differentiated SH-SY5Y cells are evaluated in real time, the neuroprotective effect of Alpha humulene on RAdifferentiated SH-SY5Y cells is >400 μg/ml at 48 h calculated based on real-time cell index data. The 48 h cell proliferation curves of the concentrations of 50, 100, 200 and 400 μg/ml Alpha humulene and 42 μM of H2O2 in RA-differentiated SH-SY5Y cells with the RTCA-DP analysis system are shown in fig. 7.
First of all, concentration scanning was performed for Alpha humulene and H2O2 in high concentration ranges by MTT study and cytotoxicity analysis was performed in the xCELLigence system and IC50 value was determined according to 24 h cell index values. With the obtained IC50 values, the non-cytotoxic concentration to be used in the next experiments was determined.
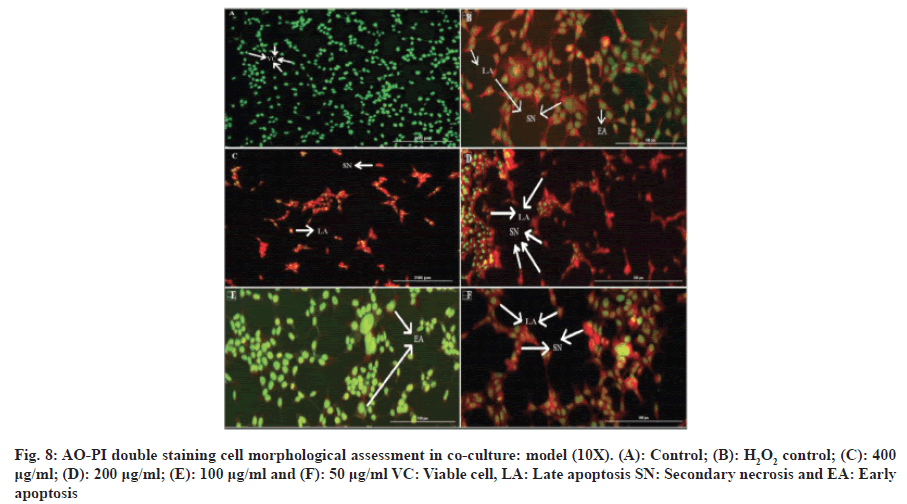
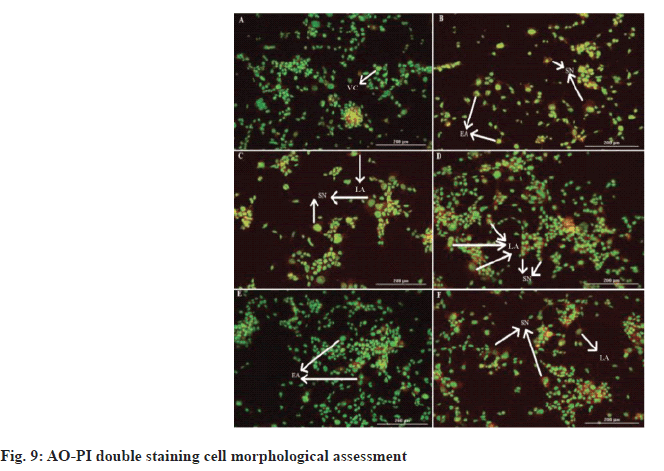
Fluorescence microscopy was used to observe the morphological changes and apoptotic properties of undifferentiated and RA-differentiated SH-SY5Y cells that underwent the AO/PI staining procedure. AO stains cell nuclei bright green to indicate viable cells, while PI stains nuclei red to indicate damaged and necrotic cells[8]. Control cells that were not treated with Alpha humulene and H2O2 were observed to have bright green and intact nuclei. In H2O2 control cells treated with 42 μM of H2O2 for 2 h, green nuclei indicate viable cells, while those stained red indicate apoptosis in H2O2 induced cytotoxicity as shown in fig. 8 and fig. 9.
After cell treatment with alpha humulene at a concentration of 400 μg/ml, necrosis cells were visualized in red. When the cells were treated with 200 μg/ml concentration of alpha humulene, it was determined that the cells in orange color increased significantly and accordingly, it caused late apoptosis. Early apoptosis features including chromatin condensation were observed after cell treatment with 100 μg/ml of Alpha humulene. Alpha humulene was found to increase apoptosis with red-orange colored cells after cell treatment with a concentration of 50 μg/ml. It was determined that the cells that were not treated with Alpha humulene and H2O2 had normally show round and green nuclei. Membrane bubbling and chromatin condensation were detected in SH-SY5Y cells treated with 100 μg/ml of Alpha humulene for 24 h and 42 μM H2O2 for 2 h, which are signs of early apoptosis.
Presence of reddish-orange color was detected in cells treated with the highest and lowest concentrations of alpha humulene (400 and 200 and 50 μg/ml), which was determined as the late stage of apoptosis or necrosis.
Neurodegenerative disorders are characterized by many diseases with macro and micro changes occurring in different parts of the brain[9]. AD, PD, HD, ALS, and SAD are common neurodegenerative diseases. AD is a neurodegenerative disease characterized by the gradual loss of behaviour, memory, cognition and functionality, which significantly reduces the quality of daily life[10]. Although age is considered to be the primary risk factor in AD, the pathology of the disease originates from free radicals[11]. The histopathological changes in AD result from the accumulation of extracellular Amyloid Beta (Aβ) plaques and intracellular tau Neurofibrillary Tangles (NFY), and its pathology is thought to be associated with oxidative stress together with the neurotoxicity of H2O2 and Aβ peptide[12]. PD, which is the second most common neurodegenerative disease, is characterized by the loss of dopaminergic neurons in the pars compacta part of the substantia nigra of the brain[13]. Pathological mechanisms resulting from degeneration of ROS and dopaminergic neurons are due to excessive production of ROS or other free radicals in the brain, mitochondrial dysfunction or inflammation. It has been determined that the increase in ROS causes oxidative stress and ROS plays an important role in the development of PH by causing α-synuclein accumulation and proteasomal degradation[14-16]. HD, another neurodegenerative disease, is an inherited neurodegenerative disease that occurs as a result of mitochondrial dysfunction and neurodegeneration with ROS-induced oxidative stress and apoptosis. HD is characterized by involuntary movements, cognitive impairment, neuropsychiatric symptoms, and premature death[17-19]. ALS, another neurodegenerative disease, is a disease that progresses with mitochondrial dysfunction with increased production of ROS, characterized by loss of motor neurons[20,21].
It has been accepted in the literature that cannabinoids are a useful therapeutic agent due to their low toxicity and suitable for use in the treatment of neurological diseases[4,22]. In this study, we investigated the antiproliferative, antiapoptotic and neuroprotective effects of alpha humulene, the characteristic terpene of Humulus lupulus, in an in vitro co-culture model. As a result of the literature review, although similar studies were found in human neuroblastoma cell lines SH-SY5Y and THP-1 monocyte cell lines, in vitro co-culture studies with Alpha humulene were not found.
First of all, the MTT assay was used to investigate the cytotoxic effects of alpha humulene on SHSY5Y cells. MTT assay was performed to determine the concentration range for SH-SY5Y cells. We determined the IC50 values obtained from the cytotoxicity results using the xCELLigence real-time cell analysis system RTCA-DP. In the xCELLigence real-time cell analysis system, SH-SY5Y cells were RA-differentiated in the instrument for 5 d. After being treated with concentration groups at the end of the differentiation process, cell damage was performed with H2O2 before the experiment. According to the MTT results we obtained, significant results were obtained in the range of 400-50 μg/ml of Alpha humulene in cell damage induced by H2O2 on SH-SY5Y cells. H2O2 was studied in the range of 400-1 μM in RTCA-DP. With H2O2 induced cell damage, H2O2 was studied in the concentration of 42 μM and Alpha humulene in the range of 400-50 μg/ml. The results obtained with RTCA-DP were calculated by the RTCA-DP Software 1.2.1 program. The IC50 for H2O2 on the SH-SY5Y cell line is 42 μM at 24 h and 38 μM at 48 h. The IC50 of Alpha humulene for the neuroprotective effect on the undifferentiated SHSY5Y cell line is 221 μg/ml at 24 h and 209 μg/ml at 48 h. The IC50 of Alpha humulene for neuroprotective effect on the SH-SY5Y cell line RA-differentiated into neuronal phenotype was calculated as >400 μg/ml.
Our study is in parallel with a study that investigated the cytotoxic effect of Alpha humulene on various human tumor cell lines (MCF-7, DLD-1, L-929) by MTT method. According to the results obtained, the IC50 value of alpha humulen for the MCF-7 cell line was 10 mg/ml, the IC50 value for the DLD1 cell line was >40 μg/ml, and the IC50 value for the L-929 cell line was >40 mg/ml[2].
In the literature, there are neuroprotective effect studies of microglia activated by an inducer such as LPS, in which an in vitro co-culture model was established with the SH-SY5Y human neuroblastoma cell line[23-25].
We also evaluated the apoptotic effects of Alpha humulene, whose IC50 values we found in our study, on SH-SY5Y cells. In this context, independent images were taken using Cytation 3 Multi-Mode Reader to determine the apoptosis for neuroprotective effect in the co-culture model created with SH-SY5Y cell line RA-differentiated by AO/PI staining method and THP-1 cells stimulated with E. coli LPS.
In one of the previous studies, the neuroprotective effect of ethyl acetate extracts of germinated brown rice on H2O2 induced cell damage on the SH-SY5Y cell line was investigated. The anti-apoptotic effects of the extracts were evaluated by AO/PI staining method at 1-30 ppm concentrations and 250 μM H2O2. As a result, it was reported that ethyl acetate extracts of germinated brown rice increased cell viability, showed anti-apoptotic effect of cells, and cells tended to less apoptosis[8].
Lastly, according to the results obtained from fluorescence measurements, H2O2 IC50 42 μM, Alpha humulene 50, 100, 200, 400 μg/ml+42 μM of H2O2 (p<0.0001****) concentration groups were found to be significant. In one of the previous studies, the cytotoxic effect of 7-Geranyloxycinnamic at concentrations of 1.04-16.67 μM was examined, 5.97 μM of curcumin and 300 μM of H2O2 were incubated for 4 h. According to the results obtained by applying the AO/PI staining procedure, they reported that the protective effect of 7-Geranyloxycinnamic and curcumin may be related to its ability to prevent apoptosis[26].
As a result, in the light of the information obtained from the literature review for this study, it was determined that Alpha humulene, which has antimicrobial, anticancer and local anesthetic properties, has neuroprotective properties against H2O2 induced cell damage. In the in vitro co-culture model created with THP-1 cells stimulated with E. coli LPS, the apoptotic stages of the effects of Alpha humulene occurring with different concentration ranges were morphologically observed.
Appreciation:
We would like to thank Anadolu University Scientific Research Projects Coordination Unit for the financial support it has provided to carry out these studies within the scope of the scientific research project no. 1905S070.
Conflict of interests:
The authors declared no conflict of interests.
References
- Neuenschwander U, Czarniecki B, Hermans I. Origin of regioselectivity in α-humulene functionalization. J Orgn Chem 2012;77(6):2865-9.
[Crossref] [Google Scholar] [PubMed]
- Legault J, Pichette A. Potentiating effect of β?caryophyllene on anticancer activity of α?humulene, isocaryophyllene and paclitaxel. J Pharm Pharmacol 2007;59(12):1643-7.
[Crossref] [Google Scholar] [PubMed]
- Gitler AD, Dhillon P, Shorter J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis Model Mech 2017;10(5):499-502.
- Iuvone T, Esposito G, De Filippis D, Scuderi C, Steardo L. Cannabidiol: A promising drug for neurodegenerative disorders. CNS Neurosci Ther 2009;15(1):65-75.
[Crossref] [Google Scholar] [PubMed]
- Mechoulam R, Parker LA, Gallily R. Cannabidiol: An overview of some pharmacological aspects. J Clin Pharmacol 2002;42(S1):11S-9S.
[Crossref] [Google Scholar] [PubMed]
- Kluger B, Triolo P, Jones W, Jankovic J. The therapeutic potential of cannabinoids for movement disorders. Movement Dis 2015;30(3):313-27.
[Crossref] [Google Scholar] [PubMed]
- Lastres-Becker I, Molina-Holgado F, Ramos JA, Mechoulam R, Fernández-Ruiz J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: Relevance to Parkinson's disease. Neurobiol Dis 2005;19(1-2):96-107.
[Crossref] [Google Scholar] [PubMed]
- Azmi NH, Ismail N, Imam MU, Ismail M. Ethyl acetate extract of germinated brown rice attenuates hydrogen peroxide-induced oxidative stress in human SH-SY5Y neuroblastoma cells: role of anti-apoptotic, pro-survival and antioxidant genes. BMC Complement Altern Med 2013;13(1):177.
[Crossref] [Google Scholar] [PubMed]
- Fratiglioni L, Qiu C. Prevention of common neurodegenerative disorders in the elderly. Exp Gerontol 2009;44(1-2):46-50.
[Crossref] [Google Scholar] [PubMed]
- Sutin AR, Stephan Y, Terracciano A. Verbal fluency and risk of dementia. Int J Geriatr Psychiatry 2019;34(6):863-7.
[Crossref] [Google Scholar] [PubMed]
- Clark TA, Lee HP, Rolston RK, Zhu X, Marlatt MW, Castellani RJ, et al. Oxidative stress and its implications for future treatments and management of Alzheimer disease. Int J Biomed Sci 2010;6(3):225.
[Google Scholar] [PubMed]
- Gella A, Durany N. Oxidative stress in Alzheimer disease. Cell Adhesion Migration 2009;3(1):88-93.
- Eriksen JL, Wszolek Z, Petrucelli L. Molecular pathogenesis of Parkinson disease. Arch Neurol 2005;62(3):353-7.
[Crossref] [Google Scholar] [PubMed]
- Cookson MR. Parkinsonism due to mutations in PINK1, parkin, and DJ-1 and oxidative stress and mitochondrial pathways. Cold Spring Harbor Perspect Med 2012;2(9):a009415.
[Crossref] [Google Scholar] [PubMed]
- Ganguly G, Chakrabarti S, Chatterjee U, Saso L. Proteinopathy, oxidative stress and mitochondrial dysfunction: cross talk in Alzheimer’s disease and Parkinson’s disease. Drug Des Devel Ther 2017:797-810.
[Crossref] [Google Scholar] [PubMed]
- Guo JD, Zhao X, Li Y, Li GR, Liu XL. Damage to dopaminergic neurons by oxidative stress in Parkinson's disease. Int J Mol Med 2018;41(4):1817-25.
[Crossref] [Google Scholar] [PubMed]
- Browne SE, Ferrante RJ, Beal MF. Oxidative stress in Huntington's disease. Brain Pathol 1999;9(1):147-63.
[Crossref] [Google Scholar] [PubMed]
- Fisher ER, Hayden MR. Multisource ascertainment of Huntington disease in Canada: Prevalence and population at risk. Movement Disord 2014;29(1):105-14.
[Crossref] [Google Scholar] [PubMed]
- Shirendeb U, Reddy AP, Manczak M, Calkins MJ, Mao P, Tagle DA, et al. Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington's disease: Implications for selective neuronal damage. Hum Mol Genet 2011;20(7):1438-55.
[Crossref] [Google Scholar] [PubMed]
- Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Amyotrophic lateral sclerosis. Lancet.2011;377(9769):942-55.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Bao J, Khatibi NH, Chen L, Wang H, Duan Y, et al. Olfactory ensheathing cell transplantation into spinal cord prolongs the survival of mutant sod1g93a ALS rats through neuroprotection and Remyelination. Anat Rec 2011;294(5):847-57.
[Crossref] [Google Scholar] [PubMed]
- Petrosino S, Verde R, Vaia M, Allarà M, Iuvone T, Di Marzo V. Anti-inflammatory properties of cannabidiol, a nonpsychotropic cannabinoid, in experimental allergic contact dermatitis. J Pharmacol Exp Ther 2018;365(3):652-63.
[Crossref] [Google Scholar] [PubMed]
- DaSilva NA, Nahar PP, Ma H, Eid A, Wei Z, Meschwitz S, et al. Pomegranate ellagitannin-gut microbial-derived metabolites, urolithins, inhibit neuroinflammation in vitro. Nutr Neurosci 2019;22(3):185-95.
[Crossref] [Google Scholar] [PubMed]
- Shih YT, Chen IJ, Wu YC, Lo YC. San?Huang?Xie?Xin?Tang protects against activated microglia?and 6?OHDA?induced toxicity in neuronal SH?SY5Y cells. Evid Based Complement Alternat Med 2011;2011(1):429384.
[Crossref] [Google Scholar] [PubMed]
- Ma K, Wu HY, Zhang B, He X, Li BX. Neurotoxicity effects of atrazine-induced SH-SY5Y human dopaminergic neuroblastoma cells via microglial activation. Mol Biosyst 2015;11(11):2915-24.
[Crossref] [Google Scholar] [PubMed]
- Abdulwanis Mohamed Z, Mohamed Eliaser E, Jaafaru MS, Nordin N, Ioannides C, Abdull Razis AF. Neuroprotective effects of 7-geranyloxycinnamic acid from melicope Lunu ankenda leaves. Molecules 2020;25(16):3724.
[Crossref] [Google Scholar] [PubMed]