- *Corresponding Author:
- O. Goshain
Department of Pharmaceutical Chemistry, College of Pharmacy, Teerthanker Mahaveer University, Moradabad 244001, India
E-mail: omprakashgoshain@gmail.com
| Date of Received | 02 July 2021 |
| Date of Revision | 17 September 2021 |
| Date of Acceptance | 08 July 2022 |
| Indian J Pharm Sci 2022;84(4):969-978 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study was designed to investigate the role of estrogen receptor and KATP channel in cardioprotective effect of remote aortic preconditioning in rat heart. Remote preconditioning is a phenomenon in which brief episodes of ischemia and reperfusion to remote organs protect the target organ against sustained ischemia/ reperfusion induced injury. Protective effects of remote aortic preconditioning are well established in the heart, but their mechanisms still remain to be elucidated. Remote aortic preconditioning in rats was produced by introducing four episodes of ischemia and reperfusion, each comprising of 5 min occlusion and 5 min reperfusion, through abdominal aorta. Perfused rat heart was isolated and subjected to global ischemia for 30 min followed by reperfusion for 120 min. The degree of cardiac injury was assessed by analysing the level of lactate dehydrogenase, creatin kinase, reactive oxygen species and nitrite in coronary effluent. Myocardial infarct size was also estimated macroscopically using tetrazolium tri chloride staining. Ethylinestradiol (10 μg/kg, orally) as estrogen receptor agonist was noted to reproduce remote aortic preconditioning mediated cardioprotection in ovariectomized rat. However, administration of glibenclamide along with or prior to ethylinestradiol abolished cardioprotection. Moreover, glibenclamide a KATP channel blocker, abolish cardioprotective effect of remote aortic preconditioning with daidzein a caveolin inhibitor (0.2 mg/kg subcutaneous). Ethylinestradiol significantly increased the release of nitric oxide and restored the attenuated cardioprotective effect of remote aortic preconditioning. These data provide the evidence that estrogen receptor activation involved in cardioprotective effect of remote aortic preconditioning-mediated trough opening of KATP channels.
Keywords
Cardioprotection, ischemic preconditioning, ischemia/reperfusion injury, remote aortic preconditioning, estrogen receptor, KATP channel
Coronary Heart Disease (CHD) is the leading cause of mortality in adult women[1]. The risk of cardiovascular disease increases sharply after middle age in women, especially after menopause[2]. Coronary artery disease is among the most important of these diseases[3]. The incidence of CHD in females increases with age. Ovarian hormones are believed to protect female hearts from arteriosclerosis and CHD[1]. The causes of cardiovascular disease are diverse but atherosclerosis and/or hypertension are the most common, particularly atherosclerosis which is associated with endothelial dysfunction[4]. Clinical investigations of the effect of Hormone Replacement Therapy (HRT), such as the Women’s Health Initiative and the Heart and Estrogen- Progestin Replacement Study II[5] have failed to demonstrate the usefulness of HRT in preventing the development of CHD and in reducing the mortality in post-menopausal patients with CHD. Collectively, these clinical investigations indicate that HRT should not be used for the protection of the cardiovascular system[6]. At least in part, the failure of these investigations reflects the fact that our understanding of the cardiovascular effects of menopause and HRT is still limited. The most powerful cardioprotective phenomenon identified till date is Ischemic Preconditioning (IPC), an innate response that renders the heart relatively resistant to Ischemia/Reperfusion (I/R) injury.
IPC is defined as the cardioprotection conferred by multiple and brief episodes of occlusion/reperfusion (intermittent hypoperfusion) against the development of irreversible damage following a subsequent more severe ischemic episode[7]. The drawback of this therapeutic strategy is the requirement for the intervention to be applied prior to the index ischemic event, which is impossible to predict in the case of an acute myocardial infarction[8]. In this regard, remote ischemic conditioning may provide a non-invasive endogenous therapeutic strategy for protecting the heart against acute I/R Injury (IRI)[9]. Remote Aortic Preconditioning (RAPC) is well-documented in various animal models, but the molecular mechanism involved in remote preconditioning is still not defined[10]. The occlusion of circumflex artery has produced protection of myocardium supplied by left anterior descending coronary artery and this phenomenon is termed as intracardiac preconditioning[11]. Short occlusion of renal[12] abdominal aorta or mesenteric artery[13] has been documented to prevent myocardium against ischemia and reperfusion-induced injury. This phenomenon has been termed as “remote preconditioning” or intra organ preconditioning or preconditioning at distant site[14]. The aim of this study was to determine the influence of oestrogen withdrawal on the development of IPC and RAPC along with involvement of Estrogen Receptor (ER) and mitochondrial KATP channels. To accomplish these goals, we evaluated whether estrogen replacement can restore the RAPC effect in ovariectomized female rats. Ovariectomy reduces the estrogen level and abolishes cardioprotective effect of RAPC. Further administration of estrogen restores the attenuated effect of RAPC via opening of mitochondrial KATP channel through caveolin protein. We have shown that estrogen deficiency primarily affected Nitric Oxide Synthase (NOS3) activity through a mechanism involving protein-protein interaction especially with caveolin-1. Glibenclamide is the potassium channel blocker and diadzein is caveolin inhibitor that diminished the restored cardioprotective effect of estrogen on RAPC.
Materials and Methods
Experimental animals:
Healthy female Wistar albino rats weighing 200-250 g were procured from the central animal house facility of Bharat Institute of Technology, School of Pharmacy. All animals were housed at an ambient temperature (22°±1°) and relative humidity (55 %±5 %) with standard diet and water ad libitium. Diadzein, sodium nitroprusside and phenylephrine were purchased from Sigma Aldrich Pvt. Ltd., Mumbai, India. Folin-Ciocalteu’s phenol reagent [5,5,Dithiobis (2-Nitro Benzoic acid) (DTNB), reduced glutathione, bovine serum albumin, sulfanilamide, N-Naphthylethylenediamine and thiobarbituric acid], chlorogenic acid, DTNB, thiobarbituric acid, nitrobluetetrazolium, Tetrazolium Tri Chloride (TTC) were purchased from Sisco Research Lab, Mumbai, India. Ethinylestradiol and glibenclamide were obtained as gift sample from Cipla India. All reagents used in this study were of analytical grade and freshly prepared before use. The protocol was approved by the Institutional Animal Ethics Committee as per the guidance of the Committee for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Environment and Forests (Animal Welfare Division), Government of India. The work was divided into 3 protocols; normal perse (36 rats), RAPC (36 rats) and RAPC with ovariectomy (36 rats). The rats were divided into six groups (n=6): sham control, disease control and treatment group of diadzein (0.2 mg/kg, s.c daily), glibenclamide (10 mg/kg, p.o daily), Ethinylestradiol (10 and 20 µg/kg, p.o daily) for all of the studies.
Induction of RAPC:
Induction of RAPC was carried out according to the method reported by Taliyan et al.[15]. In brief, each rat was anesthetized with thiopental sodium (40 mg/kg, i.p.). A 2 cm long incision was given on the abdomen. Lower portion of abdominal aorta was isolated below the point of origin of renal artery and a silken suture (numbered 5/0) was used to make a shoelace knot to occlude the abdominal aorta and knot was untied for reperfusion. Four episodes of ischemia and reperfusion, each comprising of 5 min occlusion and 5 min reperfusion, were used to produce RAPC.
Isolated perfused rat heart:
Heart was rapidly excised and immediately mounted on Langendorff’s apparatus. Isolated heart was retrogradely perfused at constant pressure of 80 mm Hg with Kreb’s Henseleit solution maintained at 37° bubbled with 95 % oxygen and 5 % carbon dioxide. Flow rate was maintained at 7-9 ml/min using Hoffman’s screw. The heart was enclosed in a double wall jacket and the temperature was maintained by circulating water heated to 37°. Global ischemia was produced for 30 min by blocking the inflow of Kreb’s Henseleit solution. It was followed by reperfusion for 120 min.
Estimation of biochemical parameters: Coronary effluent was collected immediately 30 min after reperfusion for estimation of Lactate Dehydrogenase (LDH)[16], nitrite[17] and 5 min after reperfusion for estimation of Creatine Kinase-MB (CK-MB)[18]. The optical density at 550 nm was measured using spectrophotometer (Ultraviolet-1700 Spectrophotometer, Shimadzu, Japan).
Assessment of myocardial infarct size:
Infarct size was measured by macroscopic method using TTC-staining dye and the infracted area reported as the percentage of total ventricular area[19]. Heart was removed from the Langendorff’s apparatus, both the auricles and the root of the aorta were excised and the ventricles were frozen. Ventricles were then sliced into uniform sections of 2-3 mm thickness and incubated in 1 % TTC, at 37° in 0.2 M Tris buffer (pH 7.4) for 20 min. TTC was converted to red formazone pigment because of reduced Nicotinamide Adenine Dinucleotide (NADH) in the viable cells while the infracted cells remained unstained or dull yellow. The ventricular slices were placed between two glass plates and a transparent plastic grid with 100 squares in 1 cm2 was placed above it. The average area of each slice was calculated by counting the number of squares on either side. The stained and unstained or dull yellow area was counted similarly. The infarcted area was expressed as a percentage of the total ventricular area[15].
Statistical analysis:
Statistical analysis was done using Sigmastat version 3.5. All results were expressed as mean±Standard Deviation (SD). Data was statistically analysed using one-way analysis of variance followed by Tukey’s multiple range test; p>0.005 was considered to be statistically significant.
Results and Discussion
In the present study, I/R injury in the heart significantly raised the level of LDH, CK-MB and reduced level of nitrite in coronary effluent. RAPC and estrogen pharmacological preconditioning has significantly attenuated the I/R induced cardiac injury. Ovariectomy, in female rat significantly impaired the RAPC induced protection which was significantly restored by the administration of estrogen (ethinylestradiol 10 µg/kg p.o). The effect of ER in I/R and RAPC with or without ovariectomy were attenuated by blocking the KATP channel through glibenclamide and caveolin inhibitor diadzein.
Global ischemia for 30 min followed by 120 min reperfusion significantly increased the level of LDH, CK-MB and myocardial infract size. The peak level of LDH was observed at a 0 min and 30 min while the level of CK-MB was observed at 5 min. These observation were consistent with previous reports (Langendroff,s preparation and working heart preparation) and thus are hemodynamically comparable to investigate the effect of pharmacological agent on ischemia reperfusion induced myocardial injury.
The protective effect of estrogen treatment has been demonstrated previously. The modulation of ER has been reported to produce ischemia preconditioning like cardioprotective effect[20]. Remote Ischemic Preconditioning (RIPC) reduces I/R injury. The underlying mechanism involves with the RIPC was opening of sarcolemmal and mitochondrial KATP channels[21]. Moreover, short occlusion followed by reperfusion of arteries in the anatomical region such as abdominal aorta, limb and renal occlusion has been demonstrated to produce cardioprotection against global ischemia. In this study, four episodes of abdominal aortic preconditioning have significantly attenuated ischemia and reperfusion induced increase in myocardial infract size and level of LDH and CK-MB. Ovariectomy reportedly reduces cardiac function and increases myocardial infract size in response to I/R[22]. Till date the role of RIPC in ovariectomized rat heart has not been demonstrated. We have hypothesized the role of estrogen pharmacological preconditioning in ovariectomized rat. The plots shown below clearly demonstrated that there was no significant change in the level of LDH (fig. 1), CK-MB (fig. 2), Nitric oxide (NO) (fig. 3) and myocardial infarct size (fig. 4) in sham control, ovariectomy control as well as drug and vehicle perse group.
Fig. 1: Level of LDH in the coronary effluent in normal groups
Note: Sham control, (CM): Carboxy Methyl cellulose; (DM): Dimethyl sulfoxide; (ED): Ethinylestradiol Dose; (G): Glibenclamide; (Di): Diadzein and (OC): Ovariectomy Control. Values were expressed as mean±SD for each group (n=6). No significant differences were observed in all control group and perse group
Fig. 2: Level of CK-MB in the coronary effluent in normal groups
Note: (DM): Dimethyl sulfoxide; (CM): Carboxy Methyl cellulose; (ED): Ethinylestradiol Dose (10 μg/kg); (G): Glibenclamide; (OC): Ovariectomy Control and (Di): Diadzein. Values were expressed as mean±SD for each group (n=6). No significant differences were observed in all control group, drug and vehicle perse groups
Fig. 3: Level of nitrite in the coronary effluent in normal groups
Note: (DM): Dimethyl sulfoxide; (CM): Carboxy Methyl cellulose; (ED): Ethinylestradiol Dose (10 μg/kg); (G): Glibenclamide; (OC): Ovariectomy Control and (Di): Diadzein. Values were expressed as mean±SD for each group (n=6). No significant differences were observed in all control group, drug and vehicle perse groups
Fig. 4: Myocardial infarct size in normal groups
Note: (DM): Dimethyl sulfoxide; (CM): Carboxy Methyl cellulose; (ED): Ethinylestradiol Dose (10 μg/kg); (G): Glibenclamide; (OC): Ovariectomy Control and (Di): Diadzein. Values were expressed as mean±SD for each group (n=6). No significant differences were observed in all control group, drug and vehicle perse groups
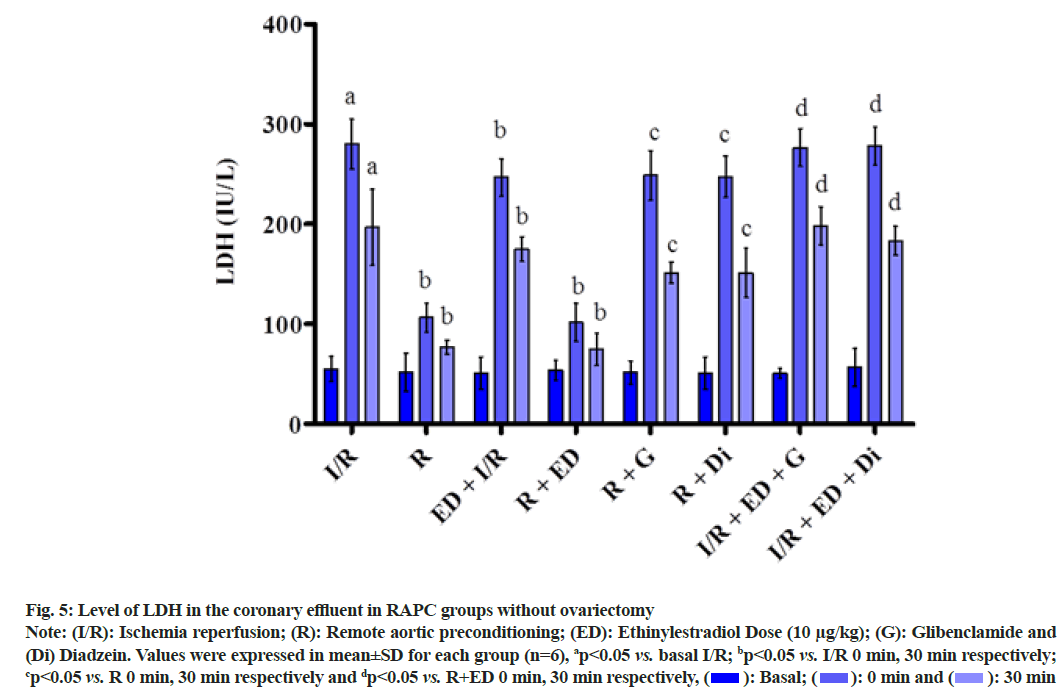
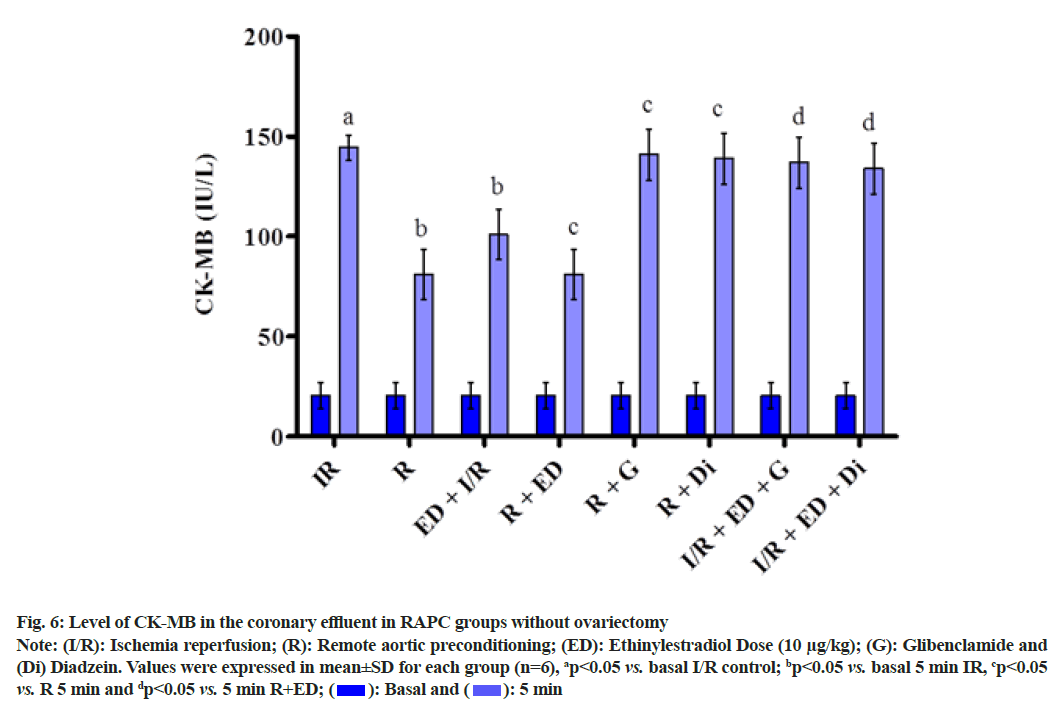
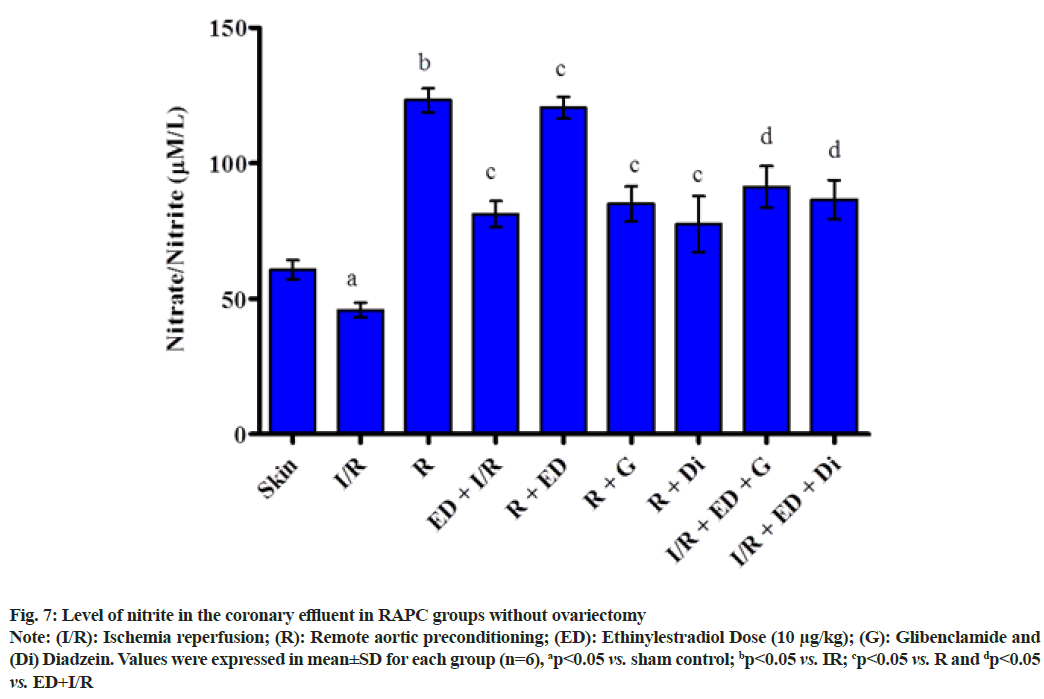
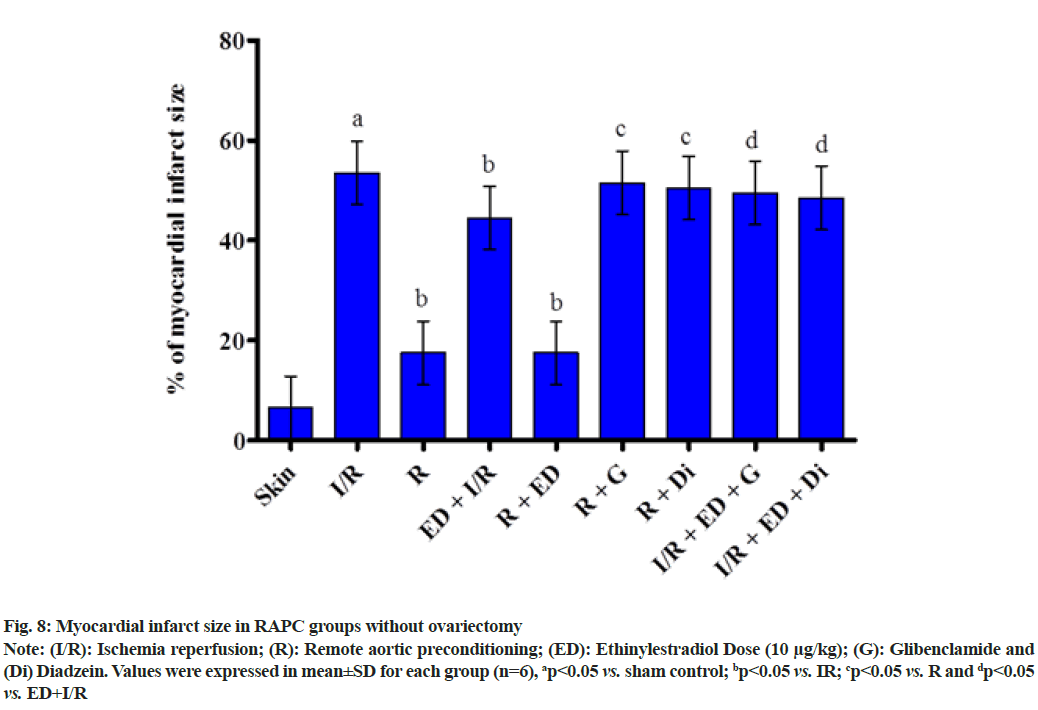
The plot showed below demonstrated the effect of RAPC on the level of LDH (fig. 5), CK-MB (fig. 6), NO (fig. 7) in the coronary effluent and myocardial infarct size in the rat without overiectomy. It is clear from the plots obtained that RAPC significantly reduces the level of LDH, CK-MB and myocardial infarct size while increases the level of nitrites (fig. 8). Administration of estrogen in I/R group and in RAPC group did not bring significant changes in comparison to I/R and RAPC respectively. Administration of glibenclamide and diazdein attenuated the cardioprotective effect of RAPC which establish the role of KATP channels and caveolin protein in ER mediated cardioprotective effect of RAPC as glibenclamide blocks KATP channels and diadzein acts as caveolin protein inhibitors.
Fig. 5: Level of LDH in the coronary effluent in RAPC groups without ovariectomy
Note: (I/R): Ischemia reperfusion; (R): Remote aortic preconditioning; (ED): Ethinylestradiol Dose (10 μg/kg); (G): Glibenclamide and (Di) Diadzein. Values were expressed in mean±SD for each group (n=6), ap<0.05 vs. basal I/R; bp<0.05 vs. I/R 0 min, 30 min respectively; cp<0.05 vs. R 0 min, 30 min respectively and dp<0.05 vs. R+ED 0 min, 30 min respectively,  30 min
30 min
Fig. 6: Level of CK-MB in the coronary effluent in RAPC groups without ovariectomy
Note: (I/R): Ischemia reperfusion; (R): Remote aortic preconditioning; (ED): Ethinylestradiol Dose (10 μg/kg); (G): Glibenclamide and (Di) Diadzein. Values were expressed in mean±SD for each group (n=6), ap<0.05 vs. basal I/R control; bp<0.05 vs. basal 5 min IR, cp<0.05 vs. R 5 min and dp<0.05 vs. 5 min R+ED; 5 min
5 min
Fig. 7: Level of nitrite in the coronary effluent in RAPC groups without ovariectomy
Note: (I/R): Ischemia reperfusion; (R): Remote aortic preconditioning; (ED): Ethinylestradiol Dose (10 μg/kg); (G): Glibenclamide and (Di) Diadzein. Values were expressed in mean±SD for each group (n=6), ap<0.05 vs. sham control; bp<0.05 vs. IR; cp<0.05 vs. R and dp<0.05 vs. ED+I/R
Fig. 8: Myocardial infarct size in RAPC groups without ovariectomy
Note: (I/R): Ischemia reperfusion; (R): Remote aortic preconditioning; (ED): Ethinylestradiol Dose (10 μg/kg); (G): Glibenclamide and (Di) Diadzein. Values were expressed in mean±SD for each group (n=6), ap<0.05 vs. sham control; bp<0.05 vs. IR; cp<0.05 vs. R and dp<0.05 vs. ED+I/R
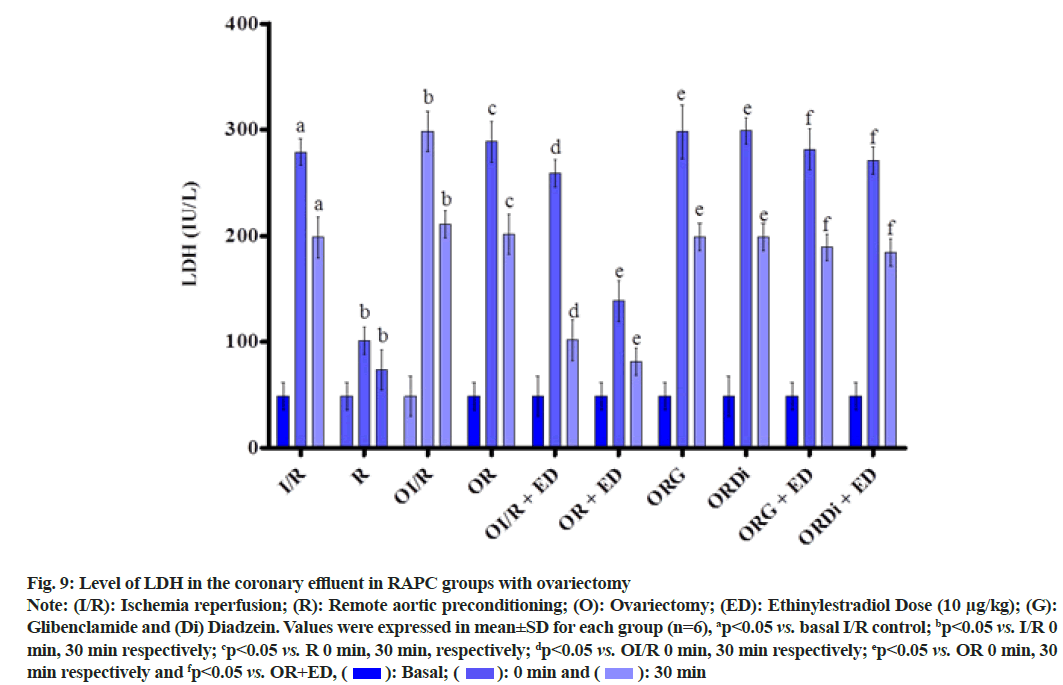
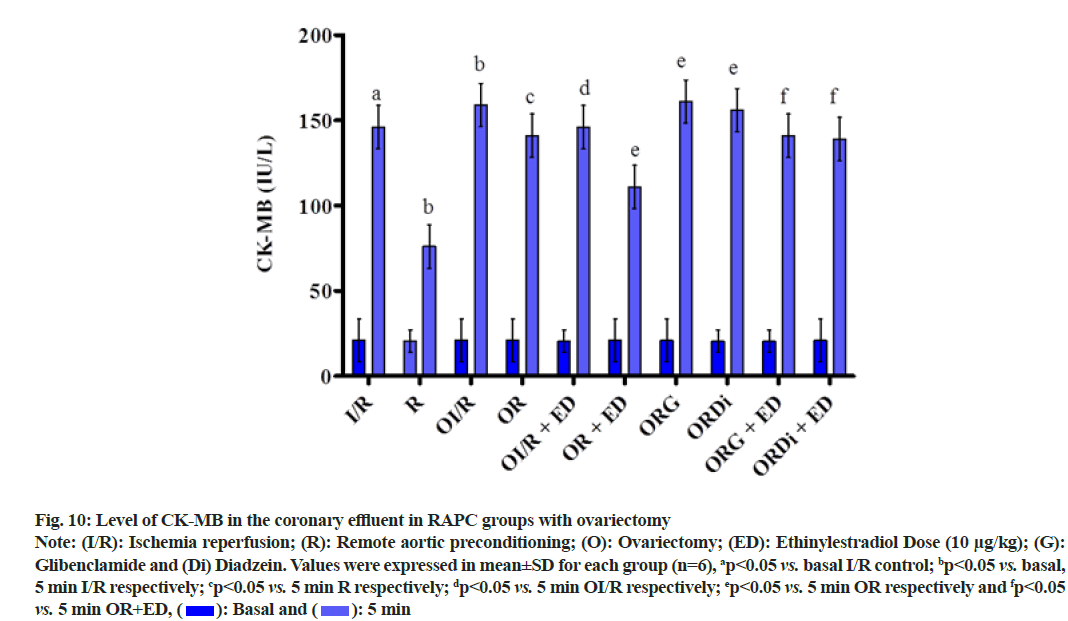
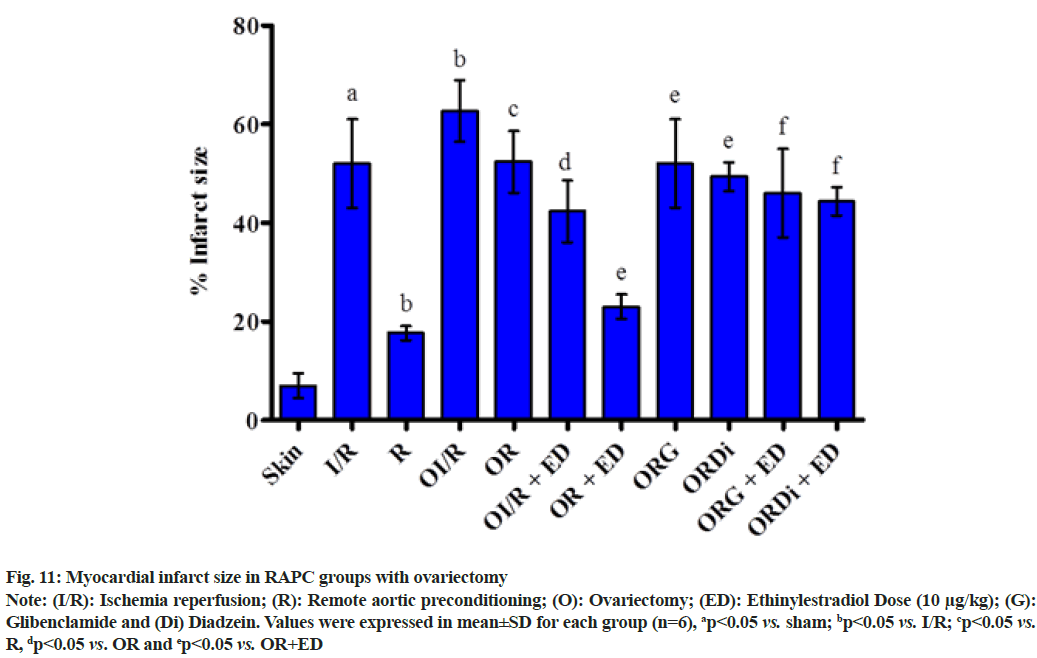
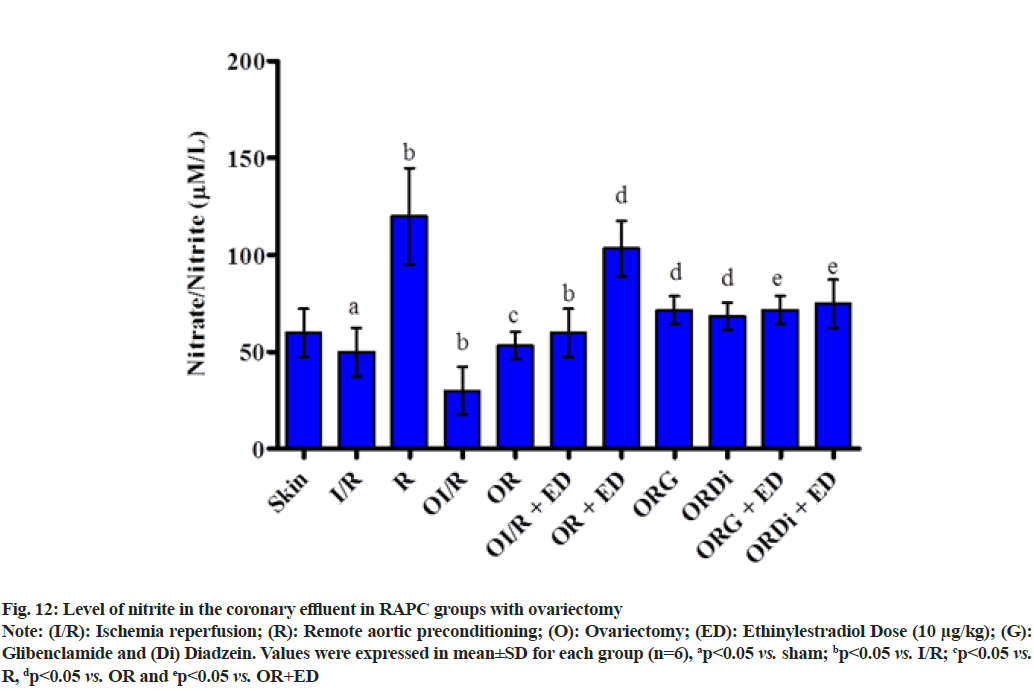
The plot shown below demonstrated the effect of RAPC in ovariectomized rats on the level of LDH, CK-MB and NO in the coronary effluent and on myocardial infarct size. Administration of estrogen in ovariectomized rats significantly decreased the level of LDH (fig. 9), CK-MB (fig. 10) and myocardial infarct size (fig. 11) while increased the level of nitrate (fig. 12).
Fig. 9: Level of LDH in the coronary effluent in RAPC groups with ovariectomy
Note: (I/R): Ischemia reperfusion; (R): Remote aortic preconditioning; (O): Ovariectomy; (ED): Ethinylestradiol Dose (10 μg/kg); (G): Glibenclamide and (Di) Diadzein. Values were expressed in mean±SD for each group (n=6), ap<0.05 vs. basal I/R control; bp<0.05 vs. I/R 0 min, 30 min respectively; cp<0.05 vs. R 0 min, 30 min, respectively; dp<0.05 vs. OI/R 0 min, 30 min respectively; ep<0.05 vs. OR 0 min, 30 min respectively and fp<0.05 vs. OR+ED,  30 min
30 min
Fig. 10: Level of CK-MB in the coronary effluent in RAPC groups with ovariectomy
Note: (I/R): Ischemia reperfusion; (R): Remote aortic preconditioning; (O): Ovariectomy; (ED): Ethinylestradiol Dose (10 μg/kg); (G): Glibenclamide and (Di) Diadzein. Values were expressed in mean±SD for each group (n=6), ap<0.05 vs. basal I/R control; bp<0.05 vs. basal, 5 min I/R respectively; cp<0.05 vs. 5 min R respectively; dp<0.05 vs. 5 min OI/R respectively; ep<0.05 vs. 5 min OR respectively and fp<0.05 vs. 5 min OR+ED,  5 min
5 min
Fig. 11: Myocardial infarct size in RAPC groups with ovariectomy
Note: (I/R): Ischemia reperfusion; (R): Remote aortic preconditioning; (O): Ovariectomy; (ED): Ethinylestradiol Dose (10 μg/kg); (G): Glibenclamide and (Di) Diadzein. Values were expressed in mean±SD for each group (n=6), ap<0.05 vs. sham; bp<0.05 vs. I/R; cp<0.05 vs.R, dp<0.05 vs. OR and ep<0.05 vs. OR+ED
Fig. 12: Level of nitrite in the coronary effluent in RAPC groups with ovariectomy
Note: (I/R): Ischemia reperfusion; (R): Remote aortic preconditioning; (O): Ovariectomy; (ED): Ethinylestradiol Dose (10 μg/kg); (G): Glibenclamide and (Di) Diadzein. Values were expressed in mean±SD for each group (n=6), ap<0.05 vs. sham; bp<0.05 vs. I/R; cp<0.05 vs. R, dp<0.05 vs. OR and ep<0.05 vs. OR+ED
It may be concluded that attenuation of cardioprotective effect of RAPC in ovariectomized rat heart is due to deficiency of estrogen and some defect in caveolin-endothelial NOS (eNOS) complex which leads to decrease in the availability of NO and consequently decreased activation of mitochondrial KATP channels. Estrogen regulates the expression of caveolin protein in the normal female rat and gives the protection to the heart from the ischemic attacks. But in the case of ovariectomized rats, cardioprotection is attenuated due to the deficiency of estrogen that abolishes the effect of RAPC. The RAPC along with estrogen induced changes in eNOS and NO restores the cardioprotective effect that closely mimic those produced by RAPC in the non-diseased heart. The cardioprotective effect is further abolished in the daidzein or glibenclamide pre-treated ovariectomized rat heart.
Acknowledgements:
The financial assistance from Bharat Institute of Technology, Meerut is gratefully acknowledged.
Conflict of interests:
The authors declare no conflict of interest.
References
- Shinmura K, Nagai M, Tamaki K, Bolli R. Loss of ischaemic preconditioning in ovariectomized rat hearts: Possible involvement of impaired protein kinase C ε phosphorylation. Cardiovasc Res 2008;79(3):387-94.
[Crossref] [Google Scholar] [PubMed]
- Abdulnour J, Doucet E, Brochu M, Lavoie JM, Strychar I, Rabasa-Lhoret R, et al. The effect of the menopausal transition on body composition and cardiometabolic risk factors: A Montreal-Ottawa New Emerging Team group study. Menopause 2012;19(7):760-7.
[Crossref] [Google Scholar] [PubMed]
- Babaee S, Shafiei Z, Sadeghi MM, Nik AY, Valiani M. Effectiveness of massage therapy on the mood of patients after open-heart surgery. Iran J Nurs Midwifery Res 2012;17(2):S120.
[Google Scholar] [PubMed]
- Lee DS, Steinbaugh GE, Quarrie R, Yang F, Talukder MH, Zweier JL, et al. Ischemic postconditioning does not provide cardioprotection from long-term ischemic injury in isolated male or female rat hearts. J Surg Res 2010;164(2):175-81.
[Crossref] [Google Scholar] [PubMed]
- Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, et al. Inflammatory biomarkers, hormone replacement therapy and incident coronary heart disease: Prospective analysis from the Women's Health Initiative observational study. JAMA 2002;288(8):980-7.
[Crossref] [Google Scholar] [PubMed]
- Mosca L, Appel LJ, Benjamin EJ, Berra K, Chandra-Strobos N, Fabunmi RP, et al. Evidence-based guidelines for cardiovascular disease prevention in women. Arterioscler Thromb Vasc Biol 2004;109(5):672-93.
[Crossref] [Google Scholar] [PubMed]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986;74(5):1124-36.
[Crossref] [Google Scholar] [PubMed]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 2003;285(2):H579-88.
[Crossref] [Google Scholar] [PubMed]
- Lim SY, Hausenloy DJ. Remote ischemic conditioning: From bench to bedside. Front Physiol 2012;3:27.
[Crossref] [Google Scholar] [PubMed]
- Taliyan R, Sharma PL. Possible mechanism of protective effect of thalidomide in STZ-induced-neuropathic pain behavior in rats. Inflammopharmacology 2012;20(2):89-97.
[Crossref] [Google Scholar] [PubMed]
- Przyklenk K, Darling CE, Dickson EW, Whittaker P. Cardioprotection ‘Outside the Box’. Basic Res Cardiol 2003;98(3):149-57.
[Crossref] [Google Scholar] [PubMed]
- Pell TJ, Baxter GF, Yellon DM, Drew GM. Renal ischemia preconditions myocardium: Role of adenosine receptors and ATP-sensitive potassium channels. Am J Physiol 1998;275(5):H1542-7.
[Crossref] [Google Scholar] [PubMed]
- Singh D, Chopra K. Evidence of the role of angiotensin AT. Methods Find Exp Clin Pharmacol 2004;26(2):117-22.
[Crossref] [Google Scholar] [PubMed]
- Weinbrenner C, Schulze F, Sárváry L, Strasser RH. Remote preconditioning by infrarenal aortic occlusion is operative via δ1-opioid receptors and free radicals in vivo in the rat heart. Cardiovasc Res 2004;61(3):591-9.
[Crossref] [Google Scholar] [PubMed]
- Taliyan R, Singh M, Sharma PL, Yadav HN, Sidhu KS. Possible involvement of α1-adrenergic receptor and KATP channels in cardioprotective effect of remote aortic preconditioning in isolated rat heart. J Cardiovasc Dis Res 2010;1(3):145-51.
[Crossref] [Google Scholar] [PubMed]
- King J. A routine method for the estimation of lactic dehydrogenase activity. J Med Lab Technol 1959;16:265-72.
[Google Scholar] [PubMed]
- Parikh V, Singh M. Possible role of cardiac mast cell degranulation and preservation of nitric oxide release in isolated rat heart subjected to ischaemic preconditioning. Mol Cell Biochem 1999;199(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Hughes BP. A method for the estimation of serum creatine kinase and its use in comparing creatine kinase and aldolase activity in normal and pathological sera. Clin Chim Acta 1962;7(5):597-603.
[Crossref] [Google Scholar] [PubMed]
- Fishbein MC, Meerbaum S, Rit J, Lando U, Kanmatsuse K, Mercier JC, et al. Early phase acute myocardial infarct size quantification: Validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J 1981;101(5):593-600.
[Crossref] [Google Scholar] [PubMed]
- Juggi JS, Hoteit LJ, Babiker FA, Joseph S, Mustafa AS. Protective role of normothermic, hyperthermic and estrogen preconditioning and pretreatment on tumour necrosis factor-alpha-induced damage. Exp Clin Cardiol 2011;16(2):e5.
[Google Scholar] [PubMed]
- Kristiansen SB, Henning O, Kharbanda RK, Nielsen-Kudsk JE, Schmidt MR, Redington AN, et al. Remote preconditioning reduces ischemic injury in the explanted heart by a KATP channel-dependent mechanism. Am J Physiol Heart Circ Physiol 2005;288(3):H1252-6.
[Crossref] [Google Scholar] [PubMed]
- Demerouti E, Andreadou I, Aggeli IK, Farmakis D, Zoga A, Gaitanaki C, et al. Ovariectomy reinstates the infarct size-limiting effect of postconditioning in female rabbits. Cell Biochem Biophys 2013;65(3):373-80.
[Crossref] [Google Scholar] [PubMed]