- Corresponding Author:
- M. Gandhimathi
Department of Pharmaceutical Analysis, College of Pharmacy, Sri Ramakrishna Institute of Paramedical Sciences, 395-Sarojini Naidu Road, New Siddhapudur, Coimbatore-641 044, India. E-mail: gands72@yahoo.co.in
| Date of Submission | 12 June 2009 |
| Date of Revision | 6 February 2009 |
| Date of Acceptance | 12 June 2009 |
| Indian J Pharm Sci, 2009, 71 (3): 311-313 |
Abstract
A simple, precise and rapid HPLC method has been developed and validated for the estimation of quinapril and hydrochlorothiazide simultaneously in combined dosage form. The mobile phase used was a mixture of 0.1% v/v triethylamine (pH 3.5), containing 1 mM of hexane sulphonic acid: acetonitrile (30:70% v/v). The detection of quinapril and hydrochlorothiazide was carried out on photo diode array detector at 220 nm. Results of the analysis were validated statistically and by recovery studies. The proposed method can be successfully used to determine the drug contents of marketed formulation.
Keywords
Quinapril, hydrochlorothiazide, ion pair-HPLC, validation
Hydrochlorothiazide (HZ) [1] is a benzothiazidine derivative used as diuretic agent. Quinapril [1] (QP) is an isoquinoline carboxylic acid derivative used in treatment of hypertension. Literature survey [2-11] revealed that only a few analytical methods including LC-MS, HPLC methods were reported for the determination of quinapril and its metabolite, in dosage form and plasma. HPLC and LC-MS methods have been reported for the estimation of hydrochlorothiazide alone and also in combination with other drugs. A derivative spectroscopy and chemometric methods have been reported for the simultaneous estimation of quinapril and hydrochlorothiazide. However, so far no HPLC method has been reported for simultaneous estimation of quinapril and hydrochlorothiazide in combination. Hence, this paper reports a simple, precise and accurate HPLC method for the simultaneous estimation of quinapril and hydrochlorothiazide from combined dosage form.
Separation was carried out on an isocratic HPLC system, Shimadzu LC-Class 10AT VP pump with PDA detector, Class LC 10 software and RP-C18 Gemini (150×4.5 mm, 5 μ). The chromatographic estimation was performed using the following conditions: the mobile phase used was of 0.1% v/v triethylamine (pH 3.5), containing 1 mM of hexane sulphonic acid: acetonitrile (30:70 %v/v). The run time and flow rate were 6 min and 1 ml/min, respectively. Detection wavelength was set at 220 nm.
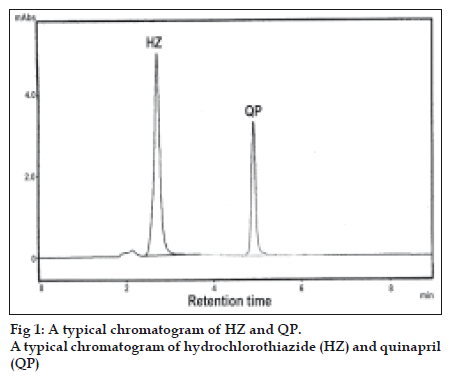
Standard stock solutions 1 mg/ml of QP and 1.2 mg/ml of HZ were prepared in acetonitrile. For construction of calibration graph, working standard solution were prepared by further diluting the stock solution with mobile phase to get a concentration of 30-150 μg/ml of QP and 40-200 μg/ml of HZ. This method was applied to determine QP and HZ in combined formulation of market samples. For the analysis of tablet formulation an accurately weighed tablet powder equivalent to 10 mg of QP and 12.5 mg of HZ were taken in a volumetric flask. The powder was dissolved in acetonitrile, shaken thoroughly and made upto the volume with acetonitrile, Then the solution was filtered through Whatman filter paper No. 41 and further diluted with mobile phase to get the concentration of 90 μg/ml and 120 μg/ml of QP and HZ, respectively. These solutions were injected and the chromatograms were recorded. A typical chromatogram of HZ and QP is shown in fig 1.
The method was validated in terms of linearity, accuracy, inter-day and intra-day precision, reproducibility and specificity. The limit of detection and limit of quantification was also determined. The accuracy of the method was evaluated by carrying out recovery studies. For that, known concentrations of standard were added to the pre-analyzed sample solution and the recovery was calculated. The precision was determined by analyzing standard solutions in the linearity range of calibration curve in triplicate on the same day (intra day precision) and different days (inter day precision). The corresponding relative standard deviation were determined and found to be < 2.5. The validated data is furnished in Table 1.
| System suitability Parameters | Results | |
|---|---|---|
| Quinapril | Hydrochlorthiazide | |
| Theoretical plates | 8837 | 6551 |
| Resolution | - | 3.31 |
| Linearity (μg/ml) | 30-150 | 40-200 |
| Correlation coefficient | 0.9946 | 0.9953 |
| % Recovery | 98.78 | 99.38 |
| LOD (μg/ml) | 0.05 | 0.02 |
| LOQ (μg/ml) | 0.4 | 0.1 |
| Tailing factor | 1.13 | 1.09 |
LOD: Limit of detection and LOQ: limit of quantitation
Table 1: System suitability test parameters for quinapril and hydrochlorothiazide by prpposed method
Both QP and HZ are soluble in acetonitrile, therefore it was selected as solvent. The mixture of 0.1% v/v triethylamine (pH 3.5), containing 1 mM of hexane sulphonic acid:acetonitrile (30:70% v/v) could resolve QP and HZ with better resolution. The retention times of QP and HZ are 4.5 min and 1.8 min, respectively. Linearity range for QP and HZ were 30-150 μg/ ml (r = 0.9946) and 40-200 μg/ml (r = 0.9953), respectively. The linear regression equations are Y= 348 +52.9X for QP and Y= 19.299+173.209X for HZ.
The high percentage of recovery of the drugs indicates that the method is highly accurate and reliable (Table 2). The content and the percentage of drugs in market samples (Table 3) indicate that the proposed method is simple, rapid, precise and accurate for the estimation of quinapril and hydrochlorothiazide in its pharmaceutical formulation.
| Drug | Amount of standard added (mg) | Amount recovered (mg) | % Recovery |
|---|---|---|---|
| Quinapril | 5 | 5.11 | 102.20 |
| 10 | 10.25 | 102.50 | |
| Hydrochlorothiazide | 6 | 5.98 | 99.67 |
| 12 | 11.96 | 99.66 |
Table 2:Recovery study of proposed method
| Drug | Amount(mg/tablet) %Label | %RSD* | ||
|---|---|---|---|---|
| Labeled | Found* | claim* | ||
| Quinapril | 20 | 19.94 | 99.70 | 1.3733 |
| Hydrochlorothiazide | 25 | 24.97 | 99.89 | 0.7767 |
An average value±relative standard deviation of 6 observations
Table 3: Results of analysis for quinapril and hydrochlororthiazide in formulation
Acknowledgements
The authors thank M/S SNR Sons charitable trust for providing facilities to carry out the work.
References
- Budavari S, editors. The Merck index. 13th ed. Whitehouse Station, NJ:Merck and Co., Inc.; 2001.
- Freerd AL, Steven B, Stilbering SB, Kolodsick KJ, Rossi DT, Mahjour M, et al. Improving the detection of degradants and impurities in pharmaceutical drug products by applying mass spectral and chromatographic searching. Int J Pharm 2005;304:135-44.
- Abbara GC, Aymad G, Hinh S, Diquett B. Simultaneous determination of quinapril and its active metabolite quinaprilat in human plasma using high performance liquid chromatography with ultraviolet detection. JChromatogr Anal Technol Biomed Life Sci 2002;76:199-207.
- Dinc E, Balenu D. Continuous wavelet transform and chemometric methods for quantitative resolution of a binary mixture of quinapril and hydrochlorothiazide in tablets. J BrazChemSoc 2007;18:962-8.
- Kowalczuk D, Hopkala H. Application of derivative spectrophotometry for simultaneous determination of quinapril and hydrochlorothiazide in the combination tablets. J AOAC Int 2004;87:847-51.
- Freed AL, Kale U, Ando H, Rossi DT, Kingsmill CA. The development and stability assessment of extemporaneous pediatric formulations of accupril. J Pharm Biomed Anal 2004;35:727-38.
- Takubo T, Okada H, Ishii M, Hara K, Ishii Y. Sensitive and selective liquid chromatography-electro spray ionization tandem mass spectrometry analysis of hydrohlorothiazide in rat plasma. J ChromatogrBAnalytTechnol Biomed Life Sci 2004;806:199-203.
- Ramakrishna NVS, Vishwottam KN, Manoj S, Koteswara M, Wishu S, Varma DP. Sensitive liquid chromatography-tandem mass spectrometry method for quantification of hydrohlorothiazide in human plasma. Biomed Chromatogr 2005;19:751-60.
- Wankhede SB, Tajne MR, Gupta KR, Wadodkar SG. RPHPLC Methodfor simultaneous estimation of telmisartan and hydrochlorothiazide intablet dosage form. Indian J Pharm Sci 2007;69:298-300.
- Erk N. Simultaneous determination of irbesartan and hydrohlorothiazide in human plasma by liquid chromatography. J Chromatogr B AnalytTechnol Biomed Life Sci 2003;784:195-201.
- Erk N. Simultaneous analysis of can desartan cilexetil and hydrohlorothiazide in human plasma and dosage forms using HPLC with photo diode array detector. J Liquid ChromatogrRelat Tech 2003;26:2581-91.