- Corresponding Author:
- S. Y. GABHE E-mail: sy_gabhe@yahoo.com
| Date of Accepted | 16-Jan-2011 |
| Date of Revised | 26-Oct-2010 |
| Date of Received | 9-Apr-2009 |
Abstract
A simple isocratic reversed phase high performance liquid chromatography was used to separate three impurities present in the sample of 8-chlorotheophylline. LC-MS was used for the characterization of impurities. Based on mass spectral data, the structures of these impurities were characterized as 3,7-dihydro-1,3-dimethyl-1H-purine-2,6-dione (impurity I), 3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione (impurity II) and isomer of 8-chloro- 1,3-dimethyl-2,6(3H,1H)-purinedione (impurity III).
Keywords
8-Chlorotheophylline, impurity, LC-MS, reversed phase HPLC, theophylline
Introduction
The bulk drug industry forms base of all pharmaceutical industries, as it is the source of active pharmaceutical ingredients (APIs) of specified quality. The major challenge for bulk drug industries is to produce the final drug of required quality and purity, economically. The purity of APIs depends on several factors such as raw materials, their methods of manufacture and type of crystallization or purification process. However, it is almost impossible to obtain absolutely pure materials, as impurities get incorporated into them either during manufacture, purification or storage[1]. An impurity is any component of a drug substance (excluding water) that is not chemical entity defined as drug substance[2]. For the drug substance produced by chemical synthesis, ICH classifies impurities into three categories such as organic impurities, inorganic impurities and residual solvents[3]. The impurities present in the substance may be toxic, may change physical or chemical properties of the substance, thus making it medicinally useless. Impurities may lower the shelf life of the product and may cause difficulties in the formulation[1]. Therefore, not only control of impurities but also the qualification of impurities is a critical issue to the bulk drug industries.
Antihistamines such as dimenhydrinate and promethazine theoclate are widely used drugs in the treatment of motion sickness. 8-Chlorotheophylline, chemically 8-chloro-1,3-dimethyl-2,6(1H, 3H)- purinedione, is an intermediate which is used in the preparation of salt form of these drugs[4,5]. It is essential to ensure purity and safety of dimenhydrinate and promethazine theoclate. In order to achieve this, 8-chlorotheophylline must be obtained with the highest purity and with a known impurity profile.
From literature survey it is found that Wadke et al. studied the interactions of 9-methylisoalloxazine and 3,9-dimethylisoalloxazine with 8-chlorotheophylline[6]. 8-chlorotheophylline was used as internal standard for determination of urate by HPLC method[7]. It was also determined potentiometrically[8]. Simultaneous determination of chlorphenoxamine hydrochloride, 8–chlorotheophylline and caffeine in multicomponent dosage form was conducted by a thin layer chromatography–densitometric method[9]. Gil et al. investigated the electroanalytical behavior of 8-chlorotheophylline by cyclic voltammetry and by differential pulse polarography and determined its content in pharmaceutical preparations by differential pulse polarography[10]. Stability indicating RP-HPLC method was developed and validated for 8-chlorotheophylline along with diphenhydramine and caffeine[11]. Ratio-spectra zero-crossing first-derivative spectrophotometric and chemometric methods were also developed for simultaneous determination of caffeine, 8-chlorotheophylline and chlorphenoxamine hydrochloride in ternary mixtures[12]. So far no chromatographic and spectroscopic method has been reported for separation and characterization of impurities present in 8-chlorotheophylline. Therefore the present work was undertaken with aim to isolate and characterize impurities present in 8-chlorotheophylline using modern analytical techniques.
8-Chlorotheophylline was a gift sample from Kores (India) Ltd., Thane. All the other chemicals and reagents were procured from S. D. Fine Chemicals Ltd (Mumbai, India). The solvents used for TLC and preparative TLC studies were of analytical grade and those used for HPLC studies were of HPLC grade. Sodium acetate trihydrate of AR grade was used for preparation of buffer.
Initially TLC studies were carried out in order to know the number of impurities present in the sample. Precoated TLC plates of Silica gel 60GF254 (Merck) were used as stationary phase. The sample was dissolved in minimum amount of ethyl acetate and this solution was used for spotting the TLC plates. Various mobile phases were tried. Ethyl acetate:toluene:glacial acetic acid (10:0.3:0.5 v/v/v) showed better separation as compared to other mobile phases. Four components were separated from the sample of 8-chlorotheophylline using TLC with the Rf values of 0.029, 0.132, 0.198 and 0.852, respectively. 8-Chlorotheophylline had an Rf of 0.852.
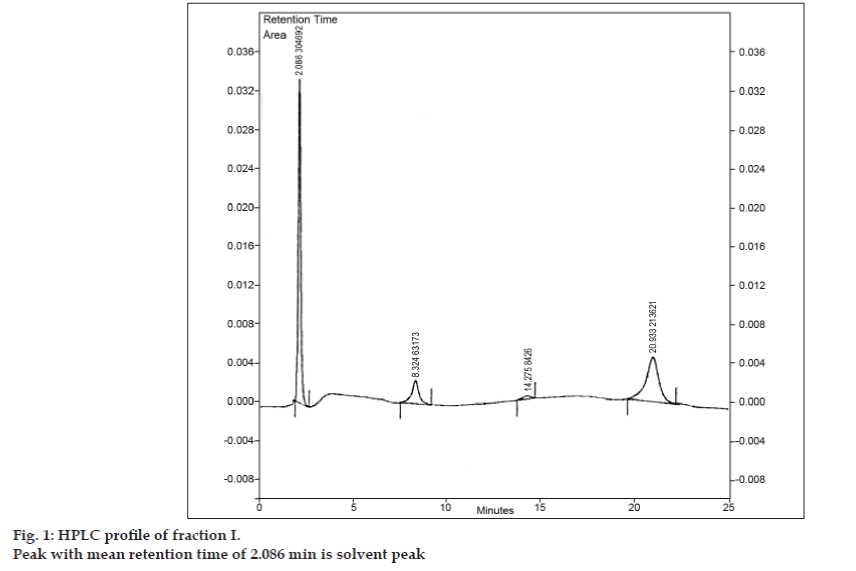
Once the mobile phase for TLC was developed, an attempt was made to separate the mixture using preparative TLC. Sample was dissolved in minimum amount of ethyl acetate and spotted in the form of band. All three impurities were separated from 8-chlorotheophylline by preparative TLC using the same chromatographic conditions used in TLC studies. The different bands were collected separately and extracted using ethyl acetate. Since quantities of each isolated impurity I, II and III were very less, it was decided to isolate these impurities collectively. Impurity I, II and III were collectively designated as fraction I. Fraction I was isolated from 8-chlorotheophylline by preparative TLC. As each impurity was not isolated separately, different identification techniques such as IR, NMR could not be undertaken. It was decided to carry out further investigation using LC-MS, which allows simultaneous separation and characterization. Prior to LC-MS analysis, HPLC profile for fraction I was developed. A Tosoh high performance liquid chromatograph equipped with reciprocating pump CCPM, a pump controller PX8010 and UV detector was used for HPLC studies. A loop of 20 µl capacity was fitted to the injection valve unit. Fraction I was dissolved in acetonitrile and was subjected to reversed phase HPLC analysis with mobile phase comprising of acetonitrile: sodium acetate trihydrate (pH 3.57; 0.01 M) (5:95 v/v). The column selected was Phenomenex ODS (250×4.6 mm I.D.; particle size 5 µm). The flow rate was 1.5 ml/min and the detection was monitored at a wavelength of 280 nm. The mobile phase was filtered through G5 sintered glass filter under vacuum prior to use and sonicated to remove air bubbles. HPLC analysis of fraction I also revealed three peaks with the mean retention times of 8.324 min, 14.275 min and 20.933 min, respectively (fig. 1). Peak with mean retention time of 2.086 min was the solvent peak.
Fraction I was then subjected to LC-MS analysis for characterization of impurities. LC–MS studies were carried out on a system in which LC part consisted of 1100 series HPLC (Agilent Technologies, USA) comprising of a vacuum degasser (G1322A), quaternary pump (G1311A), an auto sampler (G1313A) and a UV/visible detector (G1314A) and MS part consisted of triple quadrupole mass spectrometer Quattro II (Micromass UK Ltd., UK).
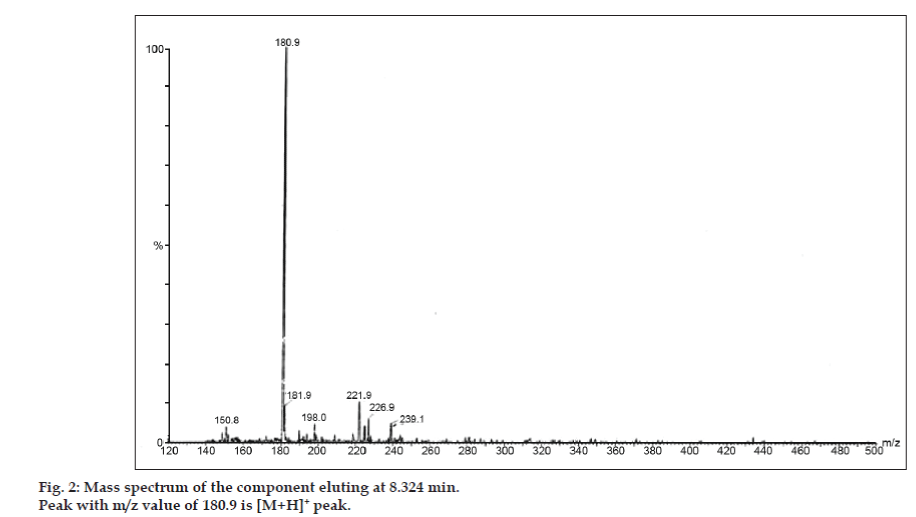
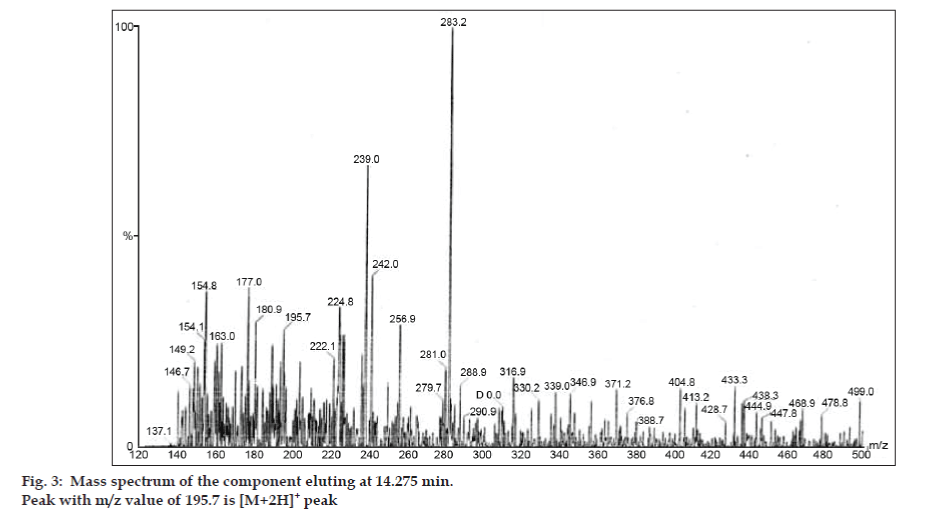
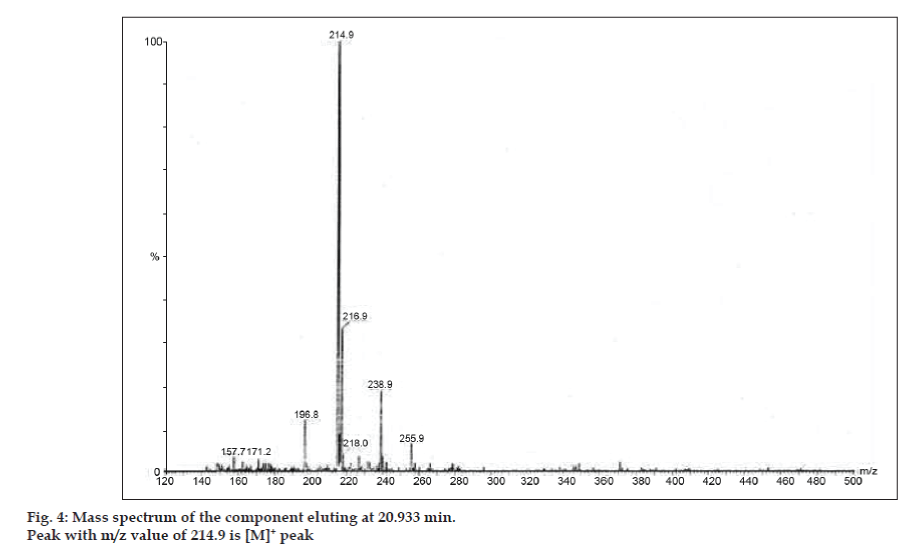
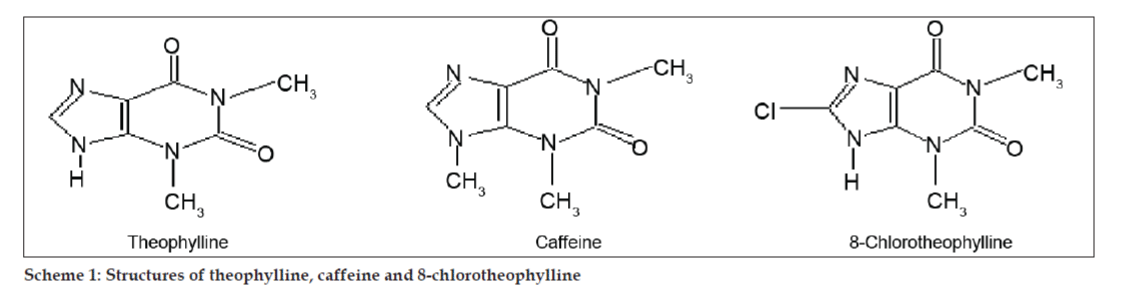
In LC-MS studies, Liquid chromatographic separations were achieved by Phenomenex C18 column (250×4.6 mm, 5 µm) at room temperature with the mobile phase of acetonitrile: sodium acetate buffer (5:95, v/v) at a flow rate of 1.5 ml/min. The mass spectrometer was run in positive electron spray ionization (ESI) mode with mass/charge (m/z) ratio in the range of 120–500 m/z. Nitrogen was used as nebulizing gas. Data were acquired and processed by Masslynx software. Mass spectral data of the impurities were obtained (figs. 2, 3 and 4). Fragmentation pathway for three peaks is characterized by loss of methyl group and/or carbonyl group. Peaks with m/z values of 180.9, 195.7 and 214.9 are corresponding to [M+H]+, [M+2H]+ and [M]+ peaks respectively. According to the MS data obtained, the impurity eluting at 8.324 min, 14.275 min and 20.933 min were theophylline (mol. wt. 180), caffeine (mol. wt. 194) and an isomer of 8-chlorotheophylline (mol. wt. 214.5), respectively (Table 1). Thus, three impurities were separated and their structures were elucidated based on mass spectral data (Scheme 1).
| Peak no. |
Retention time (min) |
Fragment ions (m/z) | Identification |
|---|---|---|---|
| 1. | 8.324 | 181.9 [M+2H]+, 180.9 [M+H]+, 150.8 [M+H-CH3-CH3]+ | Theophylline |
| 2. | 14.275 | 195.7 [M+2H]+, 180.9 [M+2H-CH3]+,149.2 [M-CH3-CH3-CH3]+, 137.1[M+H-CH3-CH3-CO]+ | Caffeine |
| 3. | 20.933 | 216.9 [M+2H]+, 214.9 [M]+, 171.2 [M-CH3-CO]+, 157.7 [M+H-CH3-CH3-CO]+ | Isomer of 8- Chlorotheophylline |
TABLE 1: HPLC-MS IDENTIFICATION OF FRACTION I
Acknowledgements
Authors are thankful to Kores (India) Ltd., Thane for providing gift sample of 8-chlorotheophylline.
References
- Kasture AV, Wadodkar SG, Mahadik KR, More HV. Pharmaceutical Analysis. Vol. 1. Pune: NiraliPrakashan; 1997. p. 12-4.
- United State Pharmacopoeia, Vol. 26. Rockville, MD: United States Pharmacopoeia Convention, Inc.; 1999. p. 2049-59.
- Ahuja S, Alsante KM. Handbook of isolation and characterization of impurities in pharmaceuticals. California: Academic Press; 2003. p. 6.
- Foye WO, Lemke TL, Williams DA. Principles of Medicinal Chemistry. 4th ed. New Delhi: B. I. Waverly Pvt Ltd; 1995. p. 419.
- Reynolds JE. Martindale-The Extra Pharmacopoeia. 29th ed. London: Pharmaceutical Press; 1989. p. 451,459.
- Wadke DA, Guttman DE. Complex formation influence on reaction rate III. Interaction of some isoalloxazines with 8-chlorotheophylline as determined by spectral, solubility, and kinetic methods. J Pharm Sci 1965;54:1293.
- Bennett MJ, Patchett BP, Worthy E. A simple HPLC method for the determination of urate in serum and urine using 8-chlorotheophylline as internal standard. Med Lab Sci 1984;41:108-11.
- Nikolic K, Medenica M. Potentiometric determination of 8-chlorotheophylline. Microchimica Acta 1986;88:5.
- Bebawy LI, El-Kousy NM. Simultaneous determination of some multicomponent dosage forms by quantitative thin layer chromatography densitometric method. J Pharm Biomed Anal 1999;20:663-70.
- Gil EP, Blazquez LC, Garcia-MoncoCarra RM, Misiego AS. Polarographic Behavior of 8-Chlorotheophylline and its Determination in Dosage Forms. Electroanalysis 1993;5:343.
- Barbas C, Garcia A, Saavedra L, Castro M. Optimization and validation of a method for the determination of caffeine, 8-chlorotheophylline and diphenhydramine by isocratic high-performance liquid chromatography Stress test for stability evaluation. J ChromatogrA 2000;870:97-103.
- Kelani KM. Simultaneous determination of caffeine, 8- chlorotheophylline, and chlorphenoxamine hydrochloride in ternary mixtures by ratio-spectra zero-crossing first-derivative spectrophotometric and chemometric methods. J AOAC Int 2005;88:1126-34.