- *Corresponding Author:
- Pramilla Sah
Desert Plant Analysis Laboratory, Department of Chemistry, J.N.V. University, Jodhpur-342 005, India
E-mail: pramilla_s@yahoo.co.uk
| Date of Submission | 16 March 2005 |
| Date of Revision | 22 March 2006 |
| Date of Acceptance | 13 December 2006 |
| Indian J Pharm Sci, 2006, 68 (6): 768-771 |
Abstract
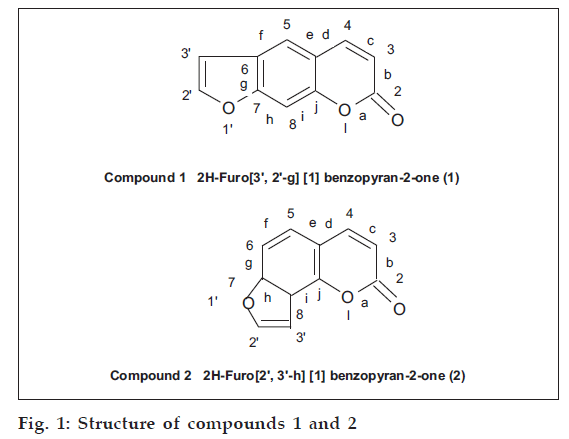
Isolation of isomeric furocoumarins from the seeds of Psoralea corylifolia Linn. (Family Leguminosae) is being reported for the first time from the variety found in western Rajasthan. On the basis of physical and spectral parameters, i.e., UV, IR, NMR, mass and chemical reactions such as hydrolysis, alkali fusion and oxidation, they have been identified as 2H-furo[3',2'-g][1] benzopyran-2-one (1) and 2H-furo[2',3'-h] [1-] benzopyran-2-one (2).
Psoralea corylifolia L. is an annual weed growing throughout the plains of India especially in the semi-arid regions of Rajasthan and the eastern districts of Punjab adjoining Uttar Pradesh. The plant is of immense biological importance and it has been widely exploited since ages for its magical effect against several skin diseases like psoriasis, leucoderma and leprosy [1-3]. Broad spectrum of biological activities, i.e., cytotoxicity [4], antimutagenic [5], bone calcification [6], antiinflammatory [7], antibacterial [8], antifungal [9], antifeedant [10] and antirepellant [11], were reported.
The present communication reports the isolation and identification of isomeric furocoumarins together for the first time from the seeds of this plant found in the desert region of Rajasthan. Most of the workers, however have tried to isolate these compounds separately from the deposit that separated from the oil and purified these only by fractional crystallization [12-14].
Materials and Methods
Melting points were determined using Neolab simple electrical apparatus. All the spectral analysis was done using AR grade solvents. The UV spectra were taken on a Shimadzu–1601 spectrometer. FTIR analysis was performed on a Shimadzu–810 A spectrometer using KBr pellets. 1HNMR analysis was performed on a Brucker DP 200 model (200 MHz, FTNMR) while mass spectrum was obtained on a Jeol D-300 model with electron impact gun.Spots were identified by UV lamp (365 nm) and by spraying with a 10% solution of potassium hydroxide in methanol or 10% antimony chloride in chloroform.
Extraction and isolation
About 1 kg of Psoralea corylifolia procured from the local market was sown. The plants, which were reproduced (its identity was established by the Department of Botany, Jai Narain Vyas University, Jodhpur). Seeds of this plant were used for the present chemical investigation.
They were cleaned dried prior to use. The air-dried seeds were crushed and Soxhlet extracted with 2l of hexane. The defatted crushed seeds were further extracted 5-6 times with 2l of chloroform. The chloroform extract were combined together, solvent was removed by distillation giving a dark reddish brown extract. Small amounts of this extract were applied on top of a silica gel (60-120 mesh size, Glaxo Laboratories, Mumbai) column (2×40 cm) in the form of a narrow band. Loading was done by making dry slurry of the extract. The column was eluted at the rate of 50 ml/h using pure hexane and hexane-chloroform mixture 95:5 and 90:10. Twenty five millilitre fractions were collected and examined by TLC. First 50 fractions (25 ml each) which were eluted with hexane were mixed together on the basis of TLC examination. A small amount of an oily residue was isolated which could not be purified. The column was then eluted with 95:5 hexane-chloroform mixture and 24 fractions of 25 ml each were mixed together on the basis of TLC. Distillation of the solvent and recrystallisation with methanol gave compound (1), (fig. 1).
The column was further eluted with hexane-chloroform mixture 90:10. First 10 fractions were mixed on the basis of TLC. The residue showed the presence of three compounds. As the amount obtained was less it could not be purified. Next 25 ml fractions, which were eluted with the same solvent system were then mixed on the basis of TLC and showed the presence of only one compound in majority. This was compound (2) (fig. 1), which was purified and recrystallized with methanol.
2H–Furo [3’,2’-g] [1] benzopyran-2-one (1)
Obtained as creamish white crystalline needles, recrystallized from methanol, m.p. 158°, Rf 0.655, benzene:methanol (95:5). UV λmax (EtOH) nm (log ε):329 (0.906), 292 (1.487), 243.5 (3.135) and 209.5 (2.443). UV λmax (EtOH + NaOH) nm (log ε): 342 (0.892), 246 (1.138).I.R.V max (KBr): 3170, 3120, 3055, 1720, 1556, 1545, 1505,1280, 1140, 1025, 898, 825 and 750 cm-1. 1H–NMR (CDCl3) : 6.35 (d, 1H, C–3 J ~ 9.5 Hz), 6.86 (d, 1H, C–3’, J = 2.5 Hz), 7.43 (d, 1H, C–2’, J = 2.5 Hz), 7.73 (s, 2H, C–5 and C–8), 7.86 (d, 1H, C–4, J ~ 9.5 Hz). [M+] 186 (C11H6O3) (100) m/z, 51 (22.4), 52 (20.0), 74 (8.6), 75 (8.0), 76 (9.8), 102 (28.0), 129 (9.5), 130 (10.3), 131 (1.6), 158 (40.8), 159 (8.9). Found C = 70.91, H = 3.38; calcd. C = 70.97, H = 3.25 for C11H6O3.
Alkaline hydrolysis of compound (1) gave rectangular prism shaped crystals identified as α-hydroxy cinnamic acid, m.p. 225-227°. Structure was confirmed by further reaction with methanol and 20% NaOH mixture in presence of dimethyl sulphate converting it to its methyl ether. The derivative on recrystallization from ethanol, gave rhombic prisms, m.p. 223°. Alkali fusion gave 4,6-dihydroxy-1,3-benzene dicarboxylic acid, m.p. 326°. Oxidation with chromic acid gave creamish coloured needles on recrystallisation with methanol. The compound was characterized as 6-formyl-7hydroxy-coumarin, m.p. 260°.
With 30% solution of H2O2 in 5% alcoholic KOH compound (1) gave a solid, identified as furan dicarboxylic acid. Recrystallisation was done with acetone, m.p. 226°. KMnO4 oxidation gave an acid which was identified by esterification as 6-carboxy-7-hydroxy coumarin, obtained as a crystalline residue, m.p. 196°. A 2-nitro derivative of compound (1) was prepared. Recrystallisation with ethanol, gave brownish-yellow crystals. m.p. 278° (decomposed).
2H-Furo[2’,3’-h] [1] benzopyran-2-one (2)
This was obtained as white crystalline flakes. Recrystallisation was done using methanol, m.p. 138°, Rf 0.625, benzene:methanol (95 : 5). UV λ max EtOH) nm (log ε): 300 (2.081), 240 (3.913), 216 (3.010). UV λ max (EtOH + NaOH) nm (log ε) : 340 (1.042), 245 (3.913), 278 (0.762). IR νmax (KBr): 3080, 3038, 1720, 1620, 1557, 1510, 1268, 1125, 1045, 835 and 745 cm-1.1H–NMR (CDCl3) : 6.39 (d, 1H, C–3, J = 9.5Hz), 7.09 (d, 1H, C–3’, J = 2.5Hz) 7.37 (2d, 2H, C–5 and C–6, J = 2.5Hz), 7.68 (d, 1H, C–2’, J = 2.5Hz), 7.80 (d’, 1H, C–4, J = 9.5 Hz). [M+] 186 (C11H6O3) (100), m/z, 50 (12.0), 51 (34.8), 63 (10.0), 74 (9.8),75 (16.9), 76 (18.6), 79 (7.7), 93 (12.0), 102 (36.9), 103 (2.4), 113 (2.4), 120 (1.9), 129 (8.1), 130 (41.5), 131 (3.3), 138 (1.8), 157 (4.0), 158 (92.5), 160 (2.0), 187 (22.0).
Alkaline hydrolysis of compound (2) gave colourless rectangular plates, m.p. 173°, identified as a substituted hydroxy cinnamic acid derivative. Structure was confirmed by synthesizing its methyl ether. Recrystallisation with pyridine gave rhombic plates, m.p. 213°. Colourless needles were obtained on recrystallisation from aqueous solution, m.p. 185° which were characterized as 2,4-dihydroxy-benzene-1,3-dicarboxylic acid. Oxidation with acidified KMnO4, gave 8-carboxy-7-hydroxy coumarin, m.p. 212°. Compound (2) (100 mg) was dissolved in 4 ml of 10% NaOH solution. To this was added 0.5%, solution of potassium persulphate. The solid obtained was filtered dried and recrystallized using methanol, m.p. 253°.
Results and Discussion
Both the compounds isolated gave characteristic fluorescence as shown by coumarins. Compound (1) showed blue while compound (2) showed purple fluorescence when both these compounds were irradiated under UV lamp. The color of these compounds was intensified on spraying their chromatogram with 10% KOH in methanol or 10% antimony chloride in chloroform. The presence of an unsubstituted furocoumarin nucleus in (1) and (2) is clearly indicated by their alkaline hydrolysis, alkali fusion and oxidation products. Formation of α-hydroxy cinnamic acid derivatives indicate the presence of a lactone group in a coumarin nuclei. Alkaline hydrogen peroxide oxidation gave furan 1,2-dicarboxylic acid indicating the presence of a disubstituted furan ring. The association of furan ring on ‘g’ face of compound (1) and on ‘h’ face in compound (2) was ascertained by the KMnO4 oxidation products, i.e., 6-carboxyl-7-hydroxy coumarin and 8-carboxy-7-hydroxycoumarin, respectively. The linear fusion of furan ring with coumarin in compound (1) and angular fusion in compound (2) has also been shown by the alkali fusion/ degradation products. Compound (1) gave 4,6-dihydroxy- benzene-1,3-dicarboxylic acid while compound (2) gave 2,4-dihydroxy-benzene-1,3-dicarboxylic acid.
The UV spectra also support the linear and angular nature. Both these compounds showed peaks in the region 300-329, 240-243.5 and 209.5-216 nm. However, an additional peak at 292 nm was observed in compound (1).
Another interesting fact was that compound (2) showed three absorption peaks instead of two as reported in the literature. Both these compounds were clearly differentiated by their UV spectra. A less intense peak at 329 nm in compound (1) and an intense peak at 300 nm for compound (2) were also visible. A bathochromic shift in the alkaline media with peaks around 340-342 nm and 245-246 nm is another characteristic feature of coumarins showing the lactonic functional group. The IR spectra also supported their furocoumarin nature. The presence of aromatic, conjugated carbonyl group was indicated by the presence of a strong band at 1720 cm-1. This is also a distinct peak of coumarins and α-pyrones. The peak due to C-H stretching was indicated by weak bands at 3055, 3120, 3170 cm-1 in compound (1) and at 3038 and 3080 cm–1 in compound (2). Peak due to (C-O) stretching was observed at 1280 and 1140 cm-1 in compound (1) and at 1268 and 1125 cm–1 in compound (2). The C-H in plane and out of plane bending vibrations in furan and the benzene rings were supported by bands at 825, 750 cm–1 in compound (1) and at 835 and 745 cm-1 in compound (2). Peaks at 1025 cm-1 and 1045 cm-1 in these compounds further confirmed the presence of furan ring.
The un-substituted nature of furocoumarin was displayed by the 1H-NMR of these compounds. Observation of a pair of doublets centred at δ 6.35 and δ 7.86 due to one aromatic proton each at C-3 and C-4 carbon atoms of the pyrone ring in compound (1). Another pair of doublets centered at δ 7.43 due to C-2’ proton and at δ 6.86 due to C-3’ proton of the furan ring was visible. A singlet at δ 7.73 for two protons present at C-5 and C-8 carbon atoms indicated the uncoupled protons of the benzene ring and thus supporting the presence of a linear unsubstituted furanocoumarin nucleus. On this basis compound (1) was characterized as 2H-furo[3’,2’-g] [1] benzopyran-2-one.
Compound (2) gave a pair of doublets at δ 6.39 and at δ 7.80 due to a single proton each at C-3 and C-4 carbon atoms, indicating the unsubstituted nature of its pyrone ring. Two doublets at δ 7.09 and δ 7.68 indicated the strongly coupled protons at C-3’ and C-2’ carbon atoms, respectively, of the furan ring, indicating the unsubstituted nature. Appearance of two doublets at δ 7.37 was characterized for the two ortho protons of the benzene ring unlike a singlet at δ 7.73 due to the para positioned aromatic protons of compound (1).
This clearly supported that the furan ring was fused with the benzene ring angularly at C–7 and C–8 carbon atoms, leaving behind the unsubstituted protons at C–5 and C–6 carbon atoms, which was confirmed by the strong ortho coupling of the protons in the NMR spectrum of this compound. Hence, compound (2) was identified as 2H-furo [2’,3’-h]–[1]–benzopyran–2–one.
The structure of both these compounds was further confirmed by their mass spectra. The mass spectra appeared to be similar, indicating their isomeric nature. In both these compounds, the molecular ion peaks were the base peaks at M+ 186. Further fragmentation in both these compounds was shown by the elimination of CO molecules, i.e., (M-28) fragments. It was indicated by the presence of peaks at m/z 158 (M-28), m/z (M-28-28)), m/z 102 (M-28-28-28). The only characteristic feature in the mass spectra of compound (2) was the presence of more intense m/z 158 peak (M-CO), i.e., almost 92% of the base peak. This indicated that the (M-CO) fragment of angular furanocoumarin was much more stable than the fragment of the linear furanocoumarin.
Acknowledgements
The authors wish to thank the Director RISC, CDRI, Lucknow for the spectral analysis and to the Head of Department, Department of Chemistry for providing the laboratory facilities.
References
- Kiritikar, K.R. and Basu, B.D., In; Indian Medicinal Plants 2ndEdn., Vol. 1, 1990, 536.
- Siddiqui, A.A. and Ansari, S.H., HamdardMedicus, 1995, 38, 109.
- Rangari, V.D. and Agarwal, S.R., Indian Drugs, 1992, 29, 662.
- Yang, Y.M., Hyun, J.W., Sung, M.S., Chung, H.S., Kim, B.K., Paik,W.H. and Kang, S.S., Planta Med., 1996, 62, 353.
- Wall, M.E., Wani, M.C., Mani Kumar, G., Abraham, P., Taylor, H., Hughes, T.J., Warner, J. and McGivney, R., J. Nat. Prod., 1988, 51, 1084.
- Miura, H., Nishida, H. and Linuma, M., Planta Med., 1996, 62, 150.
- Gaind, K.N., Dhar, R.N., Chopra, B.M. and Kaul, R.N., Indian J.Pharm., 1965, 27, 198.
- Chintalwar, G.J., Ramakrishnan, V., Luthria, D.L. and Banerji, A.,Indian J. Exp. Biol., 1978, 16, 1216.
- Mukherji, B., J. Sci. Ind. Res., 1956, 15A, 1.
- Chintalwar, G.J., Ramakrishnan, V., Luthria, D.L. and Banerji, A.,Indian J. Exp. Biol., 1992, 30, 858.
- Qureshi, S.A., Mohiuddin, S. and Qureshi, R.A., Pak. J. Zoo., 1988,20, 201.
- Jois, H.S., Manjunath, B.L. and VenkataRao, S., J. Indian Chem.Soc., 1933, 10, 44.
- Sheshadri, T.R. and VenkataRao, C., Proceedings Indian Acad.Sci., 1937, 5, 351.
- Khanna, P.L. and Seshadri, T.R., Curr. Sci. (India), 1976, 40, 505.