- *Corresponding Author:
- K. Mangathayaru
Sri Ramachandra College of Pharmacy, Sri Ramachandra Medical College and Research Institute (DU), Porur, Chennai-600 116, India.
E-mail: kvmanga@yahoo.com
| Date of Submission | 4 January 2005 |
| Date of Revision | 28 March 2005 |
| Date of Acceptance | 15 February 2006 |
| Indian J Pharm Sci,2006, 68 (1): 88-90 |
Abstract
A liquid alkaloid has been isolated from the aerial parts of Leucas aspera (Willd) link and identified as nicotine, based on physicochemical, TLC, HPTLC and RP-HPLC analysis.
Introduction
Leucas aspera (Labiatae), commonly called chota halkusa, grows as a weed on wastelands and roadsides all over India. Entire plant is used as an insecticide and indicated in traditional medicine for coughs, colds, painful swellings and chronic skin eruptions [1]. It possesses wound healing property and is used in cobra venom poisoning [2]. The plant has been scientifically investigated for antiinflammatory [3], analgesic activity [4], and for its protective effect against cobra-venom poisoning in mice [5]. Compounds isolated from the plant include long-chain aliphatic compounds [6-8], triterpenes [9,10], sterols-sitosterol, stigmasterol, campesterol and a novel phenolic compound [8].
With the exception of a positive chemical test in a preliminary phytochemical study conducted on the shoots of Leucas aspera way back in 1947 [11], there have been no reports so far on the isolation of alkaloids from this plant. This work was envisaged to isolate and identify the alkaloids present in this plant.
The herb in full bloom was collected from our University Medicinal Farm, authenticated at Botany Field Research Laboratory, Madras University, Chennai, and a voucher specimen deposited in the pharmacognosy laboratory for future reference.
TLC was done on silica gel G (E Merck, Mumbai), which was activated at 110° for 30 min. Methanol (analytical grade) used was from S. D. Fine chemicals, Mumbai, and all other organic solvents used were of laboratory grade. UV spectrum was recorded on a Systronics model 117 UV/VIS spectrophotometer with matched quartz cells. HPTLC studies were done on Camag Linomat IV model, using Camag automatic sampler III. Development was in Camag twin trough chamber and scanning was done using Camag TLC scanner 2 in the absorbance mode. Data interpretation was done using CATS 4 software. Silica gel 60 F254 TLC plates (20×20 cm, layer thickness 0.2 mm, E Merck, Germany) were used as stationary phase. Reverse phase HPLC was performed on Shimadzu, SPDA system using a Rheodyne injector of 20 μl volume. RP analytical column C18 ODS (250×4.6 mm) of 5 μl hypersil packing was used. HPLC grade methanol (E Merck, Mumbai) was used as mobile phase at a flow rate of 1 ml/ min and effluent monitored at 260 nm. The system was equipped with software class VP series, version 5.02. Nicotine was obtained from Sigma Chemical Company (St. Louis, USA).
The shade-dried plant material (1 kg) devoid of roots was reduced to a fine powder. Methanol extract (ME, yield- 18% w/w of dried plant material) was prepared by cold maceration with methanol, followed by concentration in a rotary vacuum evaporator. Two different methods were adopted for the isolation of alkaloids. ME (50 g) was subjected to separation of alkaloids by reported standard procedure [12] with slight modification. The crude alkaloidal residue, upon further purification, yielded an oily residue (L-OR, 0.04% w/w of dried plant material) with a strikingly characteristic odour recalling tobacco.
Liquid nature of L-OR and problems with purification in method 1 prompted us to follow conventional distillation used for the separation of volatile alkaloids [12]. Extraction of the distillate with diethyl ether followed by evaporation yielded a colourless oily liquid (LAA, 0.016% w/w of the dried plant material) which gave a single spot on TLC.
L-OR was a light yellow coloured oily liquid with an intense acrid burning taste and characteristic tobacco odour. With alkaloidal reagents, it gave characteristic coloured precipitates confirming its alkaloid nature. L-OR gave a positive Sanchez test [13] for nicotine. TLC of L-OR in methanol:ammonia (200:3 v/v) – solvent system A (SSA), and toluene:ethyl acetate:diethyl amine (70:20:10 v/v) – solvent system B (SSB) gave in each a bright orange spot with Dragendroff’s reagent. However there were additional spots.
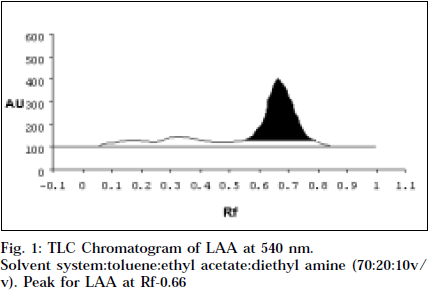
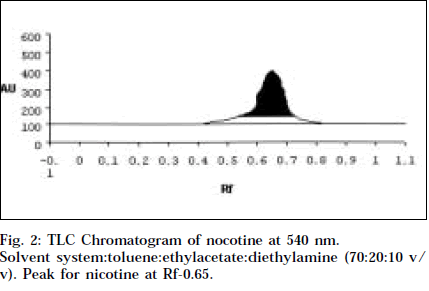
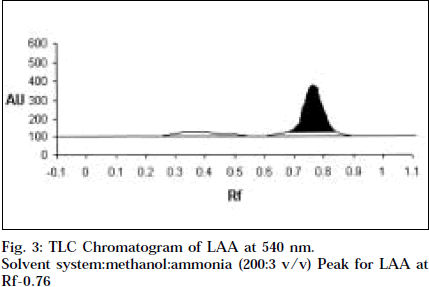
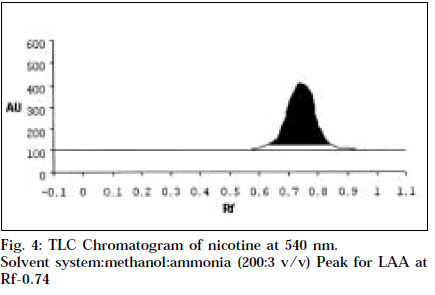
Co-TLC of LAA with nicotine in SSA and SSB gave a clean orange spot with perfectly matching Rf 0.76 and 0.66 respectively. Also the colour began to fade quickly, which is typical of nicotine [14].
UV spectrum of LAA in 0.1 M sulphuric acid gave two absorbance maxima at 235 and 260 nm, comparing favourably with nicotine [15].
Comparitive HPTLC of nicotine and LAA was done to confirm the identity of LAA. Nicotine and LAA (10 μl each) was applied undiluted and developed in SSA and SSB. The spots were scanned at 254 and 366 nm, and at 544 nm after derivitisation with Dragendroff’s. Both the tracks showed a single major spot at Rf 0.76 and 0.66 respectively, and a single peak at the same Rf when scanned at 254 nm and 540 nm (Figs.1-4). Variable wavelength scan of these spots on the two tracks showed them to have the same wavelength of maximum absorbance, i.e., 260 nm.
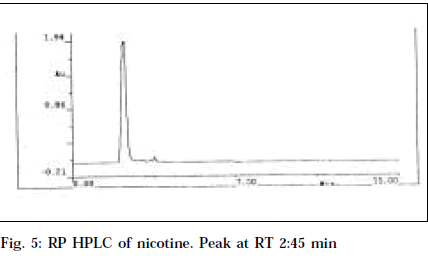
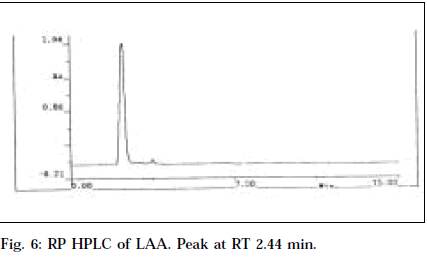
RP HPLC of nicotine and LAA, applied by separate injections in dilution of 100 μg/ml using methanol as mobile phase with effluent monitoring at 260 nm, resulted in similar retention time of 2.45 and 2.44 min, respectively (Figs. 5 and 6). Also LAA was shown to be 93% pure from % peak area calculations. This confirms the purity of LAA and identifies it as nicotine.
This is the first report of an alkaloid from Leucas aspera. It has been isolated in a pure form. Its liquid nature, bitter acrid taste, strikingly characteristic tobacco-like odour, matching Rf with nicotine (0.66) in TLC and HPTLC, common λmax (260 nm) in UV and HPTLC studies, same retention time (2.44 and 2.45 min) in HPLC studies positively confirm the identity of LAA as nicotine.
The insecticidal property of the herb could be due to the presence of nicotine. Presence of alkaloid in Leucas aspera is all the more significant from the chemotaxonomic point of view. This is because Leucas aspera belongs to Labiatae, which is under the order Tubiflorae. Of the 26 families in this order, only 4 families are well represented for alkaloids [16]. A few pyridine and pyrrolidine alkaloids have been reported in Labiatae. Presence of nicotine, a pyridine pyrrolidine alkaloid, in Leucas aspera is further supported and reinforced by this fact.
References
- Chopra R. N., Nayar S.L. and Chopra I.C., In; Glossary of Indian Medicinal Plants, NISCAIR, CSIR, New Delhi, 2002,153.
- Anonymous, In; Wealth of India (Raw Materials), Vol. 4, Publications and Information Directorate, CSIR, New Delhi, 2001, 36.
- Reddy, K.M., Viswanathan, S., Thirugnanasambantham, D., Santa, R and Lalitha,K., Ancient Science of Life, 1986, 3, 168.
- Reddy, K.M., Viswanathan, S., Thirugnanasambantham,D., Santa, R and Lalitha,K., Fitoterapia, 1993,64, 151.
- Reddy, K.M., Viswanathan, S., Thirugnanasambantham,D., Santa, R and Lalitha,K., Fitoterapia, 1993, 64, 442.
- Misra, T. N., Singh, R. S., Pandey, H. S and Singh. S., Phytochemistry, 1992, 31, 1809.
- Misra, T. N., Singh R. S., Prasad, C. and Singh, S., Indian J.Chem., 1993, 32, 199.
- Misra, T. N., Singh, R. S., Pandey, H. S and Singh. S., Indian J.Chem., 1995, 34, 1108.
- Choudhury, A.N. and Ghosh, D., J. Indian Chem. Soc., 1969, 46,95.
- Pradhan, B. P., Chakraborty, D.K. and Subba, G. C., Phytochemistry, 1990, 29, 1693.
- Shirazi, A.M.J., Indian J. Pharm., 1947, 9,116.
- Trease, G. E. and Evans, W.C., In; Pharmacognosy, 13thEdn.,BalliereTindall, London, 1989, 546.
- Cromwell, B. T., In; Paech and Tracey, M.V., Modern Methods of Plant analysis, Vol. 4, Narosa Publishing House, New Delhi, 1979, 490.
- Wagner, H. and Bladt. S., In; Plant Drug Analysis. A TLC Atlas, 2ndEdn., Springer Verlag, Berlin, 1996, 22.
- Purvis J., J. Chem. Soc, 1910, 97, 1035.
- Trease, G. E. and Evans, W.C., In; Pharmacognosy, 13thEdn.,BalliereTindall, London, 1989,217.