- *Corresponding Author:
- Fei Ma
Department of Otorhinolaryngology, The Seventh People’s Hospital of Zhengzhou, Zhengzhou, Henan Province 450000, China
E-mail: mafei846@126.com
| Date of Received | 10 February 2023 |
| Date of Revision | 05 August 2023 |
| Date of Acceptance | 22 January 2024 |
| Indian J Pharm Sci 2024;86(1):308-313 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Isoliquiritin has anti-tumor effects in a variety of cancers. circ_0023028 has been confirmed to enhance laryngeal squamous cell carcinoma progression. However, whether isoliquiritin affects laryngeal squamous cell carcinoma progression by regulating circ_0023028 is unknown. TU177 cells were divided into 8 groups; control group, isoliquiritin 25 μmol/l group, isoliquiritin 50 μmol/l group, isoliquiritin 100 μmol/l group, si-NC group, si-circ_0023028 group, isoliquiritin+plasmid cloning deoxyribonucleic acid group and isoliquiritin+plasmid cloning deoxyribonucleic acid-circ_0023028 group. Cell functions were examined by 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide assay, colony formation assay, flow cytometry, transwell assay and wound healing assay. circ_0023028 expression was examined by quantitative real-time polymerase chain reaction, and cleaved caspase-3 level was evaluated using Western blot. circ_0023028 expression was higher in laryngeal squamous cell carcinoma tissue. Isoliquiritin increased cell inhibition rate, apoptosis rate and cleaved caspase-3 level, while reduced scratch healing rate, colony cell number, migrated cell number, and circ_0023028 expression in TU177 cells. Silencing of circ_0023028 enhanced cell inhibition rate, apoptosis rate and cleaved caspase-3 level, while suppressed scratch healing rate, colony cell number and migrated cell number in TU177 cells. Besides, overexpression of circ_0023028 reversed the effect of isoliquiritin on laryngeal squamous cell carcinoma cell proliferation, apoptosis and migration. Isoliquiritin inhibited laryngeal squamous cell carcinoma cell growth and migration via reducing circ_0023028 expression.
Keywords
Isoliquiritin, circ_0023028, laryngeal squamous cell carcinoma, caspase-3, metastasis
Laryngeal Squamous Cell Carcinoma (LSCC) is one of the common malignant tumors of the head and neck worldwide, in which malignant metastasis and recurrence are the main reasons for LSCC patient’s poor survival[1,2]. Patients with mid to late-stage LSCC are treated with manual excision of the diseased tissue in combination with chemotherapy or radiotherapy[3,4]. But with the prolongation of treatment, the appearance of drug resistance limits the effectiveness of LSCC treatment in some patients[5,6]. Therefore, it is necessary to clarify molecular mechanism affecting LSCC process to provide new way for LSCC treatment.
Isoliquiritin (ISL) is a phenolic flavonoid compound extracted from within the traditional Chinese medicine Glycyrrhiza glabra[7]. ISL has been shown to have significant anti-tumor effects[8,9]. Recent findings revealed that ISL could induce lung cancer cell apoptosis[10]. Also, ISL promoted ferroptosis to improve the doxorubicin resistance in breast cancer[11]. In this, we found that ISL had an inhibition on LSCC cell apoptosis. However, ISL roles in LSCC progression have not been revealed.
Circular Ribonuclic Acid (circRNA), a special non-coding RNA, can be acted as target for cancer treatment[12]. It was reported that circ_0023028 was upregulated in laryngeal cancer tissues, and it’s silencing restrained cancer malignant progression[13]. Besides, circ_0023028 knockdown was confirmed to inhibit LSCC proliferation and metastasis[14]. Therefore, circ_0023028 has the potential to be a molecular target of LSCC treatment. In this, we found that ISL reduced circ_0023028 expression. However, whether ISL regulates circ_0023028 expression to exert anti-tumor effect in LSCC is unclear.
Here, we aimed to reveal ISL roles in LSCC progression and explore its underlying molecular mechanisms. Based on the above, we proposed the following hypothesis; ISL inhibited LSCC progression by suppressing circ_0023028 expression.
Materials and Methods
Sample collection:
The study was approved by the Seventh People’s Hospital of Zhengzhou. All participants signed an informed consent form. LSCC tissues and paracancerous tissues of 73 cases were taken from 73 individuals diagnosed with LSCC (age 35 y-72 y, 44 males and 29 females) in the Seventh People’s Hospital of Zhengzhou.
Cell culture and grouping:
TU177 cells (Biovector, Beijing, China) were grown in Roswell Park Memorial Institute (RPMI)-1640 medium containing 10 % Fetal Bovine Serum (FBS). Cells were inoculated in 24-well plates and treated with different concentrations of ISL (Meilune, Dalian, China), and labelled as; ISL 25 μmol/l group, ISL 50 μmol/l group, and ISL 100 μmol/l group. Normal cultured cells were used as control group. TU177 cells were transfected with si-NC/si-circ_0023028 or plasmid cloning Deoxyribonucleic Acid (pcDNA)/pcDNA-circ_0023028, and then treated with 100 μmol/l ISL, and labelled as; si-NC group, si-circ_0023028 group, ISL+pcDNA group and ISL+pcDNA-circ_0023028 group.
Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR):
Total RNAs were extracted by TRIzol reagent (Invitrogen, Carlsbad, California, United States of America (USA)). Extracted RNA was reverse-transcribed into complementary DNA (cDNA) and then used for PCR amplification. circ_0023028 expression was analyzed by 2−ΔΔCt method.
Cell proliferation assay:
3-[4,5-Dimethylthiazol-2-yl]-2,5 Diphenyl Tetrazolium Bromide (MTT) assay: TU177 cells in 96-well plates were cultured for 48 h. Cells were treated with MTT reagent (Beyotime, Shanghai, China) and then incubated with Dimethyl Sulfoxide (DMSO) reagent. After that, cell inhibition rate was evaluated using a microplate reader.
Colony formation assay: TU177 cells in 6-well plates were cultured for 14 d. After fixed using 4 % paraformaldehyde and stained with crystal violet, colony cell numbers were counted under the microscope.
Flow cytometry:
TU177 cells were incubated with Annexin V-Fluorescein Isothiocyanate (FITC) and Propidium Iodide (PI) solution (Beyotime). Apoptosis rate was analyzed under flow cytometry.
Western blot:
Protein was extracted, separated and transferred to Polyvinylidene Difluoride (PVDF) membranes. Membrane was incubated with anti-cleaved caspase-3 (ab2302, 1:1000), anti-Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (ab9485, 1:2500) and secondary antibody (ab205718, 1:50000). Afterwards, membrane was incubated with Beyo Electrochemiluminescence (ECL) plus solution (Beyotime) to detect protein signals.
Transwell assay:
TU177 cells were inoculated in the upper of transwell chamber, and complete medium was filled into lower chamber. After 24 h, cells were fixed and stained, and then migrated cell numbers were counted under a microscope.
Wound healing assay:
TU177 cells were inoculated in 24-well plates, and cell layer was created a wound using a 10 μl pipette. After 24 h, cell wound area was photographed to count the scratch healing rate.
Statistical analysis:
Data were presented as mean±Standard Deviation (SD) using Graph Pad Prism 7.0 software. Results were compared using Student’s t-test or Analysis of Variance (ANOVA). p<0.05 was considered as significant.
Results and Discussion
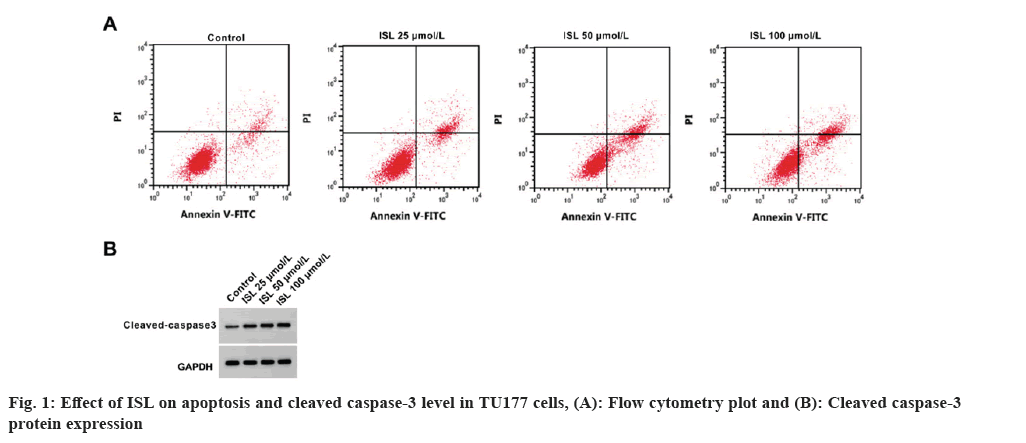
As shown in Table 1, circ_0023028 expression was remarkably increased in LSCC tissues. The inhibition rate, apoptosis rate and cleaved caspase-3 level were significantly increased, while the colony cell numbers were markedly decreased by ISL in a dose-depend manner (fig. 1 and Table 2). The scratch healing rate and migrated cell numbers of TU177 cells were reduced by ISL in a dose-dependent manner (Table 3). Circ_0023028 expression was gradually decreased in TU77 cells with the increasing of ISL treatment concentrations (Table 4).
| Group | n | circ_0023028 |
|---|---|---|
| Paracancerous tissues | 73 | 0.78±0.14 |
| LSCC tissues | 73 | 2.40±0.28* |
| t | 44.214 | |
| p | 0.000 |
Note: *p<0.05, considered as significant
Table 1: circ_0023028 expression In LSCC tissues.
| Group | Percentage | Cloned cell numbers | Cleaved caspase-3 | |
|---|---|---|---|---|
| Inhibition | Apoptosis | |||
| Control | 0.00±0.00 | 6.67±0.36 | 134.89±6.94 | 0.15±0.02 |
| 25 μmol/l ISL | 18.56±1.01* | 11.90±0.78* | 101.11±4.43* | 0.32±0.02* |
| 50 μmol/l ISL | 32.18±2.28*# | 16.60±1.05*# | 76.00±2.54*# | 0.52±0.04*# |
| 100 μmol/l ISL | 46.46±4.59*#△ | 21.53±1.11*#△ | 50.22±2.78*#△ | 0.74±0.06*#△ |
| F | 517.438 | 474.428 | 573.258 | 389.350 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *#△p<0.05, considered as significant
Table 2: Effect of ISL on TU177 cell proliferation and apoptosis.
| Group | Scratch healing rate | Migrated cell numbers |
|---|---|---|
| Control | 64.48±2.54 | 180.89±11.37 |
| 25 μmol/l ISL | 52.24±2.06* | 153.33±5.96* |
| 50 μmol/l ISL | 40.76±1.99*# | 111.00±6.83*# |
| 100 μmol/l ISL | 30.63±1.47*#△ | 62.33±2.00*#△ |
| F | 456.645 | 447.565 |
| p | 0.000 | 0.000 |
Note: *#△p<0.05, considered as significant
Table 3: Effect of ISL on migration of TU177 cells.
| Group | circ_0023028 |
|---|---|
| Control | 1.00±0.00 |
| 25 μmol/l ISL | 0.74±0.05* |
| 50 μmol/l ISL | 0.44±0.04*# |
| 100 μmol/l ISL | 0.22±0.02*#△ |
| F | 912.000 |
| p | 0.000 |
Note: *#△p<0.05, considered as significant
Table 4: Effect of ISL on circ_0023028 expression in TU177 cells.
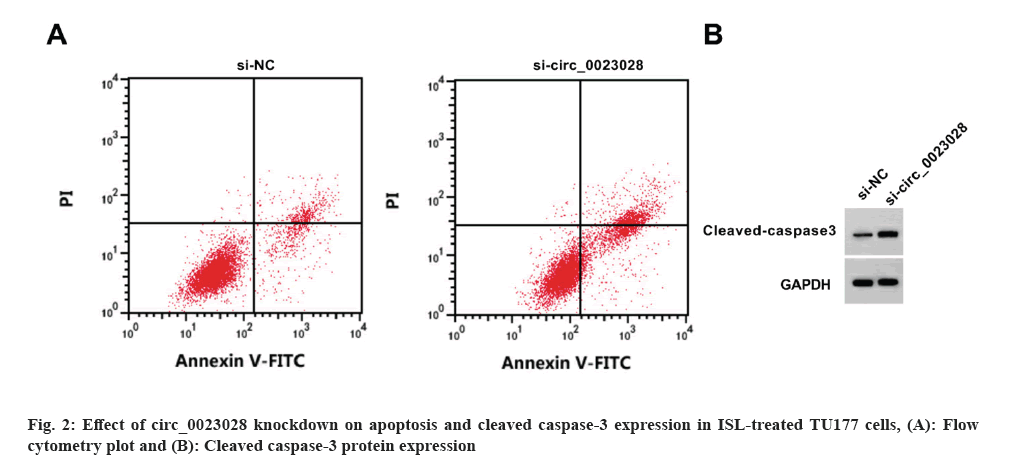
Circ_0023028 expression was reduced by the transfection of si-circ_0023028. Circ_0023028 silencing increased the inhibition rate, apoptosis rate, and cleaved caspase-3 level, whereas decreased the scratch healing rate, colony cell number, and migrated cell number in TU177 cells (fig. 2 and Table 5).
| Group | circ_0023028 | Percentage | Numbers | Cleaved caspase-3 | |||
|---|---|---|---|---|---|---|---|
| Inhibition | Apoptosis | Scratch healing | Colony cell | Migrated cell | |||
| si-NC | 1.00±0.00 | 0.00±0.00 | 6.69±0.43 | 64.57±2.61 | 133.22±8.66 | 179.78±10.95 | 0.17±0.02 |
| si-circ_0023028 | 0.27±0.03* | 54.08±2.45* | 23.39±1.51* | 24.61±1.60* | 43.00±2.00* | 52.78±1.81* | 0.83±0.05* |
| t | 73.000 | 66.220 | 31.910 | 39.159 | 30.452 | 34.329 | 36.768 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05, considered as significant
Table 5: Effect of circ_0023028 knockdown on proliferation, apoptosis and migration in ISL-treated TU177 cells.
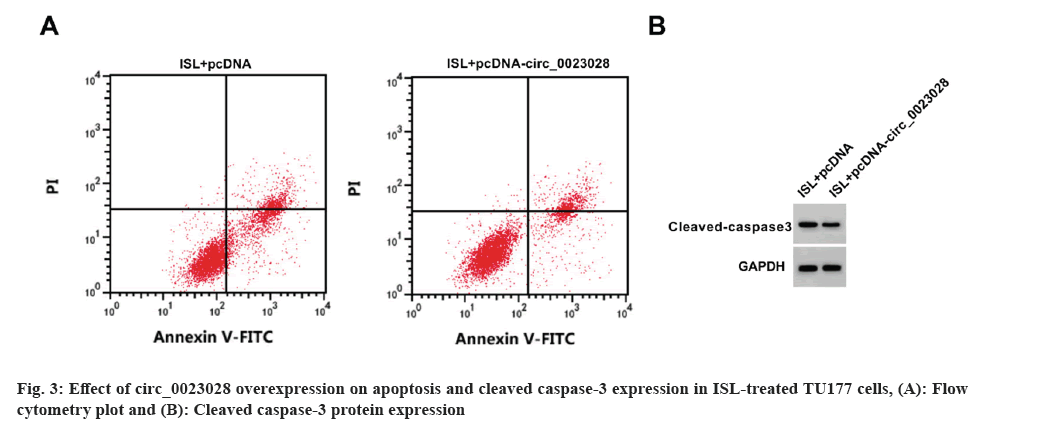
Circ_0023028 expression was elevated by circ_0023028 overexpression vector. The inhibition rate, apoptosis rate, and cleaved caspase-3 level were enhanced, while the scratch healing rate, colony cell number and migrated cell number were suppressed in ISL+pcDNA-circ_0023028 group (fig. 3 and Table 6).
| Group | circ_0023028 | Percentage | Cells number | Cleaved caspase-3 | |||
|---|---|---|---|---|---|---|---|
| Inhibition | Apoptosis | Scratch healing | Cloned | migrated | |||
| ISL+pcDNA | 1.00±0.00 | 46.92±3.97 | 21.67±1.28 | 29.98±1.99 | 52.00±1.63 | 60.44±3.13 | 0.74±0.05 |
| ISL+pcDNA-circ_0023028 | 3.44±0.11* | 20.18±1.04* | 13.49±0.93* | 47.07±2.42* | 94.33±2.91* | 136.22±6.25* | 0.35±0.03* |
| t | 66.545 | 19.547 | 15.510 | 16.364 | 38.073 | 32.524 | 20.065 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05, considered as significant
Table 6: Effect of circ_0023028 overexpression on proliferation, apoptosis and migration in ISL-treated TU177 cells.
Glycyrrhiza glabra, a leguminous plant with a long history of medicinal use, and is rich in chemical components such as triterpenoids, flavonoids, alkaloids and polysaccharides[15]. Modern pharmacological experiments show that it has immune regulation, hypoglycemic and anti-tumor effects[16,17]. ISL is extracted from Glycyrrhiza glabra[18]. Previous studies had shown that ISL promoted gastric cancer cell apoptosis in dose-dependent manner[19]. Also, ISL decreased cell viability and increased apoptosis in breast cancer[11]. The above findings confirmed that ISL could resistant to tumorigenesis. Here, we found that ISL treatment promoted apoptosis, while suppressed proliferation and migration, suggesting that ISL inhibited cell growth and migration to hinder LSCC malignant progression.
With the discovery and reporting of more and more circRNAs, much attention has been paid to exploring the biological functions of circRNAs[20,21]. CircRNAs, vary widely in their expression abundance, are usually tissue-specific and disease-specific[22]. Wang et al.[23] found that circRNA_100290 downregulation inhibited LSCC cell growth and metastasis. Circ-CCND1 facilitated LSCC cell proliferation by regulating miR-646 expression[24]. Previous study had revealed that circ_0023028 contributed to LSCC cell proliferation and migration. Similar to this reports, our study confirmed the high circ_0023028 expression in LSCC tissues. Circ_0023028 knockdown enhanced apoptosis, while suppressed proliferation and migration in LSCC cells, verifying that circ_0023028 might promote LSCC progression. Also, circ_0023028 expression was reduced in ISL-treated LSCC cells, and its overexpression reversed ISL-mediated the inhibition on LSCC cell growth and migration, showing that ISL affected LSCC progression via reducing circ_0023028 expression.
In summary, ISL inhibited LSCC cell growth and migration by inhibiting circ_0023028 expression. The results of this study provided new ideas for the clinical management of LSCC.
Conflict of interests:
The authors declared no conflict of interests.
References
- Kim DH, Kim SW, Han JS, Kim GJ, Basurrah MA, Hwang SH. The prognostic utilities of various risk factors for laryngeal squamous cell carcinoma: A systematic review and meta-analysis. Med 2023;59(3):497.
[Crossref] [Google Scholar] [PubMed]
- Prasad A, Carey RM, Panara K, Rajasekaran K, Cannady SB, Newman JG, et al. Nodal metastasis in surgically treated laryngeal squamous cell carcinoma. Head Neck 2023;45(9):2303-12.
[Crossref] [Google Scholar] [PubMed]
- Haddad RI, Posner M, Hitt R, Cohen EE, Schulten J, Lefebvre JL, et al. Induction chemotherapy in locally advanced squamous cell carcinoma of the head and neck: Role, controversy, and future directions. Ann Oncol 2018;29(5):1130-40.
[Crossref] [Google Scholar] [PubMed]
- Nie J, Li L, Tan F, Wang H, Wang H, Zou L, et al. Effect of CADM1 on TPF-induced chemotherapy in laryngeal squamous cell carcinoma. J Int Med Res 2023;51(4):3000605231168017.
[Crossref] [Google Scholar] [PubMed]
- Gao W, Guo H, Niu M, Zheng X, Zhang Y, Xue X, et al. circPARD3 drives malignant progression and chemo resistance of laryngeal squamous cell carcinoma by inhibiting autophagy through the PRKCI-Akt-mTOR pathway. Mol Cancer 2020;19(1):166.
[Crossref] [Google Scholar] [PubMed]
- Jiang Q, Liu S, Hou L, Guan Y, Yang S, Luo Z. The implication of lncRNA MALAT1 in promoting chemo-resistance of laryngeal squamous cell carcinoma cells. J Clin Lab Anal 2020;34(4):e23116.
- Li Y, Song W, Tong Y, Zhang X, Zhao J, Gao X, et al. Isoliquiritin ameliorates depression by suppressing NLRP3-mediated pyroptosis via miRNA-27a/SYK/NF-κB axis. J Neuroinflammation 2021;18(1):1-23.
[Crossref] [Google Scholar] [PubMed]
- Hasan MK, Ara I, Mondal MS, Kabir Y. Phytochemistry, pharmacological activity, and potential health benefits of Glycyrrhiza glabra. Heliyon 2021;7(6):e07240.
[Crossref] [Google Scholar] [PubMed]
- Ohno H, Araho D, Uesawa Y, Kagaya H, Ishihara M, Sakagami H, et al. Evaluation of cytotoxiciy and tumor-specificity of licorice flavonoids based on chemical structure. Anticancer Res 2013;33(8):3061-8.
[Google Scholar] [PubMed]
- Zhou Y, Ho WS. Combination of liquiritin, isoliquiritin and isoliquirigenin induce apoptotic cell death through upregulating p53 and p21 in the A549 non-small cell lung cancer cells. Oncol Rep 2014;31(1):298-304.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Li Y, Zhang J, Luo C. Isoliquiritin modulates ferroptosis via NF-κB signaling inhibition and alleviates doxorubicin resistance in breast cancer. Immunopharmacol Immunotoxicol 2023;45(4):443-54.
[Crossref] [Google Scholar] [PubMed]
- Chen L, Shan G. CircRNA in cancer: Fundamental mechanism and clinical potential. Cancer Lett 2021;505:49-57.
[Crossref] [Google Scholar] [PubMed]
- Chen X, Su X, Zhu C, Zhou J. Knockdown of hsa_circ_0023028 inhibits cell proliferation, migration, and invasion in laryngeal cancer by sponging miR-194-5p. Biosci Rep 2019;39(6):BSR20190177.
[Crossref] [Google Scholar] [PubMed]
- Zheng Y, Duan L, Yang Y, Luo D, Yan B. Circ_0023028 contributes to the progression of laryngeal squamous cell carcinoma by upregulating LASP1 through miR-486-3p. Mol Cell Biochem 2021;476(8):2951-61.
[Crossref] [Google Scholar] [PubMed]
- Pastorino G, Cornara L, Soares S, Rodrigues F, Oliveira MB. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother Res 2018;32(12):2323-39.
[Crossref] [Google Scholar] [PubMed]
- Song W, Wang Y, Li G, Xue S, Zhang G, Dang Y, et al. Modulating the gut microbiota is involved in the effect of low-molecular-weight Glycyrrhiza polysaccharide on immune function. Gut Microbes 2023;15(2):2276814.
[Crossref] [Google Scholar] [PubMed]
- Kang MH, Jang GY, Ji YJ, Lee JH, Choi SJ, Hyun TK, et al. Antioxidant and anti-melanogenic activities of heat-treated licorice (Wongam, Glycyrrhiza glabra×G. uralensis) extract. Curr Issues Mol Biol 2021;43(2):1171-87.
[Crossref] [Google Scholar] [PubMed]
- Miao Z, Gu M, Raza F, Zafar H, Huang J, Yang Y, et al. Isoliquiritin ameliorates ulcerative colitis in rats through caspase 3/HMGB1/TLR4 dependent signaling pathway. Curr Gene Ther 2024;24(1):73-92.
[Crossref] [Google Scholar] [PubMed]
- Shi X, Zou M, He J, Xie H, Li X. Studies on the identification of constituents in ethanol extract of radix glycyrrhizae and their anticancer activity. Afr J Tradit Complement Altern Med 2014;11(2):334-8.
[Crossref] [Google Scholar] [PubMed]
- Chen L, Wang C, Sun H, Wang J, Liang Y, Wang Y, et al. The bioinformatics toolbox for circRNA discovery and analysis. Brief Bioinform 2021;22(2):1706-28.
[Crossref] [Google Scholar] [PubMed]
- Ju J, Song YN, Chen XZ, Wang T, Liu CY, Wang K. circRNA is a potential target for cardiovascular diseases treatment. Mol Cell Biochem 2022;477(2):417-30.
[Crossref] [Google Scholar] [PubMed]
- Zhou WY, Cai ZR, Liu J, Wang DS, Ju HQ, Xu RH. Circular RNA: Metabolism, functions and interactions with proteins. Mol Cancer 2020;19(1):172.
- Wang Z, Huang C, Zhang A, Lu C, Liu L. Overexpression of circRNA_100290 promotes the progression of laryngeal squamous cell carcinoma through the miR-136-5p/RAP2C axis. Biomed Pharmacother 2020;125:109874.
[Crossref] [Google Scholar] [PubMed]
- Zang Y, Li J, Wan B, Tai Y. circRNA circ-CCND1 promotes the proliferation of laryngeal squamous cell carcinoma through elevating CCND1 expression via interacting with HuR and miR-646. J Cell Mol Med 2020;24(4):2423-33.
[Crossref] [Google Scholar] [PubMed]