- *Corresponding Author:
- Kirti Agarwal
Department of Chemistry, B. M. S. College of Engineering, Basavanagudi, Bengaluru, Karnataka 560019, India
E-mail: kirti07.agarwal@gmail.com
| Date of Received | 21 July 2021 |
| Date of Revision | 26 September 2021 |
| Date of Acceptance | 01 July 2022 |
| Indian J Pharm Sci 2022;84(4):797-811 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
After extensive work spanning more than half a century, liposomes have crossed the chasm and emerged as a prominent candidate in mainstream drug delivery. Post the historic launch of the first liposome-based drug, it is in the last three decades when liposome technology has slowly transformed from a research apparatus to a versatile drug carrier system influencing many biomedical areas. Liposomes have the ability to deliver a drug to a target site with the least amount of systemic toxicity by overcoming hurdles of cellular and tissue uptake, stabilizing therapeutic compounds, and improving biodistribution. They have been investigated for diverse applications such as treatment of cancer, delivery of gene and vaccine, treatment of lung and skin diseases, treatment of tumours, and imaging tumours at the site of infection. They are leading present-day smart delivery systems due to their flexible biophysical and physicochemical properties, which permit easy control to address different delivery concerns. This review will discuss various advances and updates in liposome-assisted drug delivery and the current clinical use of liposomes for biomedical applications.
Keywords
Liposome, drug delivery, stealth liposomes, ethosome, nebulised liposomes
The first description for liposomes was given by British haematologist Alec Bangham and his colleagues in 1965[1] as swollen phospholipids system. This concept resulted in the development of model membrane systems and ever since liposomes have been studied at length as nanocarriers for the delivery of drugs and pharmaceuticals[2-4].
Liposomes are phospholipid vesicles consisting of one or more concentric lipid bilayers and have a structural resemblance to cellular membranes. They can be designed to retain their physical properties at body temperature, through proper lipid composition using phospholipids with high phase transition temperature[5]. Besides composition, properties of liposomes are governed by several other factors which include their method of preparation, size, surface charge, firmness of bilayer and surface functionalization[6].
It has been well established that liposomes have an internal volume and can entrap a drug. The ability to incorporate both hydrophilic and hydrophobic drugs in them makes them a valuable drug delivery system. Liposome assisted drug delivery is associated with several advantages including improving the solubility of a drug[7], releasing a drug at the target site in a sustainable manner[8], providing targeted delivery[9], providing protection against drug degradation, reducing toxic side effects of the drug to normal cells[8], delivering drugs to multidrug resistance tissues by combination therapy[10] and improving the therapeutic index of drugs[11].
An important milestone in liposomal drug delivery was the development of the remote drug loading process based on ammonium sulphate gradient in the 1990s. When remote drug loading was applied to Doxorubicin (DOX), accumulation in the aqueous phase of the liposomes reached a record of 100-fold concentration in the remote loading medium[12]. Other major developments include the introduction of Polyethylene Glycol (PEG)ylated liposomes with enhanced circulation times and reduced reticuloendothelial uptake, which were useful in the treatment of cancers/ tumours. With the advent of clinical translation of liposome-based drugs, it was highly desirable to develop a programmable, automated delivery system to control the physicochemical characteristics of liposomes. A number of methods were developed for automated production of liposomes, amongst them it was a robust platform-microfluidic technology, which provided control over size, lamellarity, membrane composition and internal contents[13]. Significant progress was made in the area of targeting liposomes to specific cells or organelles by active/passive targeting[14,15,36]. Lipoplexes, which are lipid-based assemblies of non-covalently associated Deoxyribonucleic Acid (DNA) by chargecharge interactions, are a promising alternative in gene therapy[16].
Liposomes as a nanomedicine device and their ability to enhance the bioavailability of drugs are extensively reviewed[17,18]. It is also worth mentioning that though liposomes are used to reduce the systemic toxicity of the entrapped drug, they can be toxic to normal cells and can trigger immune responses[19].
This is one among some limitations of liposome assisted drug delivery, however, it is not discussed in detail in this review. Nonetheless, significant advances in recent years have led to an exponential growth in liposomal delivery systems and resulted in several new medical applications to treat various diseases. Currently, many liposome assisted drugs are in clinical use to treat cancer and various other diseases, while many others are awaiting clinical trial results[17].
Liposomes, as extraneous substances, are subjected to many obstacles including detection, deactivation and elimination processes from the defense system once injected into the body. To become a successful drug carrier, liposomes should have the ability to overcome all such obstacles and deliver drugs to the target site. This can be achieved by designing liposomes with desirable properties but not restricted to: Targeting ability to various specific organelles, improved circulation times, enhanced skin penetration (ethosomes) and the capacity for pulmonary delivery of drugs (nebulised liposomes).
This review will discuss the advances in liposome assisted drug delivery, including developments in the design of liposomes for controlling rapid clearance, remote drug loading and drug release. The steps forward towards improved circulation times through stealth liposomes are deliberated. It will cover the progress in dermal delivery of drugs through ethosomes and pulmonary delivery by nebulised liposomes.
Further, it will discuss the targeting of liposomes to specific organelles, an overview of Nucleic Acid Therapeutics (NATs) and wrap up with a section on the current clinical use of liposomes for biomedical applications. As this review is written in the middle of a global pandemic caused by Severe Acute Respiratory Syndrome Coronavirus 2019 (SARS-CoV-19), a section on its vaccines has also been added.
Designing Liposomes with Optimal Properties
Liposome design to overcome rapid clearance:
It quickly became evident that there were many problems linked with the in vivo use of classical liposomes. Prominently, there were problems of uptake into the cells of the Mononuclear Phagocyte System (MPS) present in the liver and spleen[3] and retaining the entrapped molecules inside the liposome[21]. At the same time, it was shown that strong physical interactions of liposome membranes with serum proteins results in drug release[22]. Many solutions have been implemented to tackle these challenges. One such solution involved changing the membrane environment by incorporating cholesterol in it, which reduced the fluidity of the membrane and resulted in lesser leakage of entrapped solutes[23]. It was also demonstrated that the introduction of sphingomyelin resulted in transforming the fluid phase bilayer into a solid-phase bilayer with reduced leakage of content[24].
Another approach to developing liposomes with desirable properties was by changing the chemical structure of phospholipid predominantly for Phosphatidylcholine (PC). The phospholipid molecule can be divided into three major parts, the polar head group, glycerol backbone and fatty acyl chains. Efforts have been made by several research teams to modify each of the three parts, by introducing additional groups or by changing the chemical nature of phosphatidylcholine[22,25,26]. The polar head group region was changed by introducing ligands or functional groups so that it can recognize and bind target receptor/antigen to the surface of small unilamellar liposomes[27]. Modifications in carbonyl ester bonds at both sn-1 and sn-2 with ether and carbamyl esters resulted in improvement in stability and circulation times in vivo[28]. Further, promising systems for drug delivery can be attained through innovation in design strategies, where a variety of liposomal components can be used as building blocks for control over targeting and drug release[29]. Every component plays a very definite role and can be replaced if a different therapeutic effect is desired[29].
A methodology to increase the half-life of liposomes by grafting PEG on their surface was developed in the 1970s. Soon after, several papers reported a significant decrease in rapid clearance of PEG-coated liposomes from circulation. These liposomes are addressed as stealth liposomes later in this review.
Remote drug loading:
Drug characteristics are important to engineer liposomes for the retention of the entrapped drug. Analogous to biological membranes, liposomes have low permeability to hydrophilic drugs and high permeability to hydrophobic drugs. Irrespective of the nature of the drugs, they can be loaded into the liposome, but their retention capacities may differ. For a long time, drug encapsulation in the liposome was carried out at the time of liposome manufacture. In the year 1993, Haran et al. performed a study that resulted in the historic achievement of the remote drug loading concept which made possible the ability to load a drug into liposomes after manufacture, independent of site and time of liposome preparation. Subsequently, there were reports on remote loading of the drug through numerous approaches.
One such approach was based on transmembrane pH gradient generated against the internal acidic buffer or salts such as ammonium persulfate which on dissociation generate protons[20]. By this method, loading of DOX was effective up to 98 % in large unilamellar vesicles composed of egg phosphatidylcholine/cholesterol (7/3 mol/mol) with a transmembrane phosphate gradient[12]. Doxil®, the first nano-drug approved by Food and Drug Administration (FDA) uses the same technology[30]. At first, remote drug loading was used for drugs that were weak bases, but this approach allowed for loading hydrophobic weak acids as well[31].
The addition of water-miscible solvent to the liposomal loading mixture demonstrated active loading of poorly soluble drugs in the liposomal core. This method improved the encapsulation efficiency and formulation stability of the liposomes and gave rise to Solvent Assisted Loading Technology (SALT)[32].
Another approach invalidated the long-held requirement that aqueous solubility and the presence of weakly basic groups on the surface of the drug were the essential requirements for remote loading. Since very few chemotherapeutic agents possess these properties, cyclodextrin sugars with hydrophilic surfaces were utilised to solubilize hydrophobic drugs in their cavities for remote loading of the drug[33]. At present, a unique method of remote drug loading is required for each drug depending on its properties and a universal method that can be applied to all types of drugs is awaited.
Drug release:
A number of crucial aspects need to play together for optimal therapeutic activity at a target site. The drug needs to be delivered to the site for a sufficient period and at the required rate to reach the optimal therapeutic activity. Drug release is an important characteristic of the therapeutic activity of a drug as the drug entrapped in the liposome is not bioavailable till it is released[3]. The drug release rate denotes the ability of the accumulated liposomes to enhance the bioavailability and therapeutic index of the drug.
The optimal drug release is visualized to improve the efficacy of the drug. Several modalities for the sitespecific release of drugs have been developed that rely on creating defects in the liposome membrane by internal and external triggers. External triggers include heat, light or ultrasound as energy sources. Internal triggers utilize the abnormalities of the diseased cells/ tissues in the biological system such as enzymes, pH etc. Andresen et al. established the presence of some enzymes present in certain tumours can break the liposomal lipids and cause drug release. Enzyme based drug release works irrespective of tumour localization and emerges as a major advantage[15].
Serum proteins are known to interact with liposomes and cause drug release. However, the introduction of cholesterol decreases membrane fluidity and drug release. It would be appropriate to design liposomes by carefully selecting the drug lipid ratio, lipid composition, etc. to achieve optimal drug release rate[34]. The role played by drug lipid ratio was found to be of great significance in the case of liposomal vincristine formulations in controlling the drug release rate and antitumour efficacy[34]. In a pharmacokinetics study for the treatment of cerebral ischemia/reperfusion injury, release properties of fasudil were found to vary with lipid composition and internal phase of the liposomes[35]. In fact, the maximum therapeutic index among different formulations was observed with a mid-level release rate of fasudil from liposomes. Advancements in liposome technology have facilitated the design of liposomes with different drug release rates as desired by the therapeutic application.
Targeting of Liposomes
For liposomes to be successful as drug carriers, they have to be selective in delivering drugs to various tumours/cancer cells/organelles. Certain targeting strategies to various types of tumours and cancer cells are summarized below, however many other categories like targeting desired organelles and receptors in the cytoplasm/nucleus are not in the scope of this review.
Passive targeting:
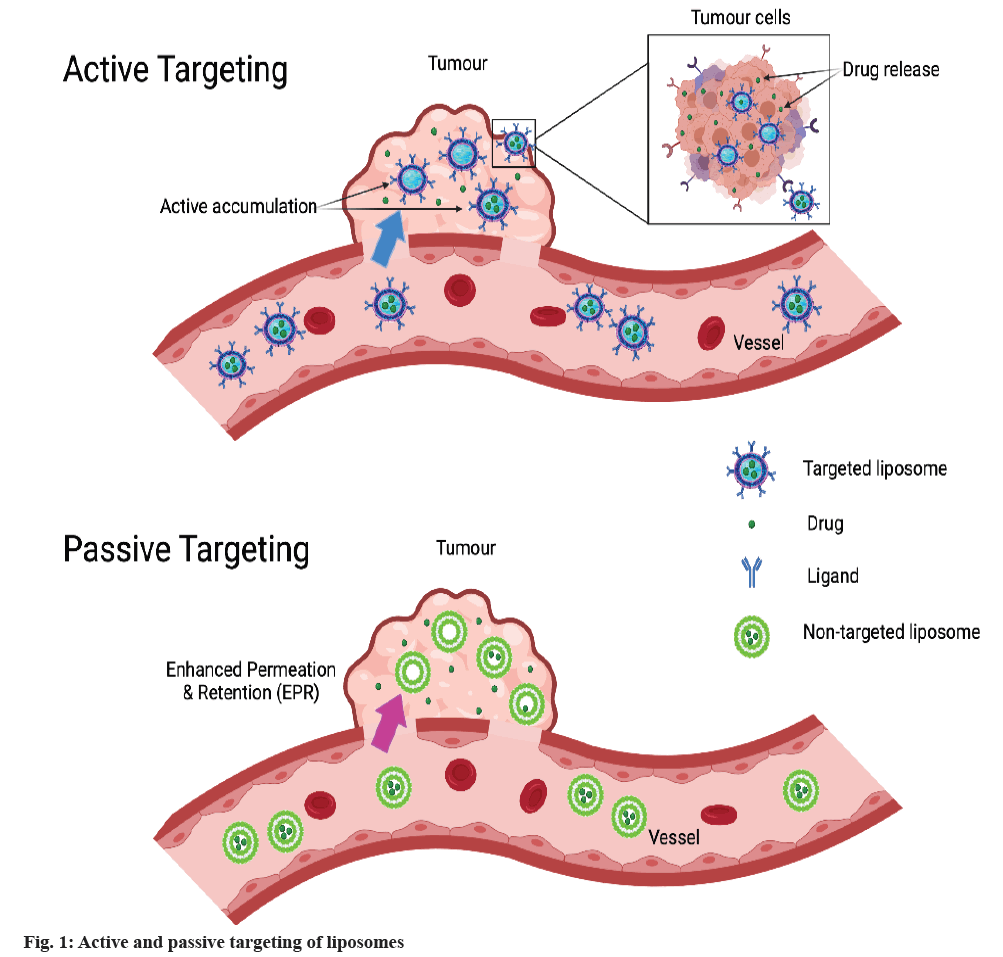
When liposomes get targeted by the natural distribution system to certain organs (liver and spleen) and tissues (tumour and cancer cells), the phenomenon is known as passive targeting[36]. In the cases of tumours, drug-loaded liposomes are known to accumulate in the tumour area due to the leaky vasculature Enhanced Permeability and Retention (EPR) effect (non-targeted liposomes) which makes the basis for cancer therapy by passive targeting, as shown in fig. 1[36]. This effect can be used to differentiate between normal and cancerous tissues as the capillaries in the tumour area possess increased permeability. In addition, besides permeability, there are several features including porosity and pore size of tumour which vary with the type and stage of the tumour and influence the passive targeting effect[36].
Active targeting with surface functionalization:
The strategy used here is to target the drug to cancer cells or tumour tissues with minimum accumulation in healthy cells or tissues, in turn causing higher efficacy and minimum toxicity. Active targeting is achieved by grafting specific ligands/antigens on the surface of the liposome. The choice of targeted ligands is based on the overexpressed/regulated receptor on tumour tissue. The ligand bearing liposomes require specific interactions of ligands with receptors present on the target cells, followed by the uptake of encapsulated cargo by receptor-mediated endocytosis[37], as shown in fig. 1 which is made with biorender.com. In some reports, the ligand targeted liposomes showed enhanced efficacy.
However, in some other experimental reports, no such improvements were observed. The field of active targeting has been researched at length and several methodologies have been developed for attaching antibodies or other adjuvants to the liposome surface[38,39].
Liposome surface structure can be modified either by adding specific proteins/antibodies/ immunoglobulins[40,41] or by changing the charge on the membrane[42]. In addition, a large number of other small molecules such as vitamins[43], peptides[44], aptamers[45] and affibodies[46] have been scrutinised by several researchers to improve the targeting of liposomes. Amongst these, immunoliposomes, which are antibody coupled liposomes, have been most explored in the past few decades as nanotechnology platforms[47]. It has been established in experimental research that antibody coupled liposomes could improve the selective toxicity of anti-cancer drugs[38,39,48]. However, these findings had limited scope as antibody-targeted liposomes cleared rapidly from circulation and their distribution was restricted to non-MPS. In a comparative study, immunoliposomes and non-targeted liposomes showed no difference in their bio-distribution[50].
Targeting of overexpressed receptors on cancer cells:
Unlike normal cells, cancer cells have many receptors which are overexpressed. These receptors can be targeted to result in higher uptake of anticancer agents into cancer cells at the cancer site. Epidermal Growth Factor Receptor (EGFR) that is overexpressed in many cancers and can be utilised for targeting, has four receptors-Human Epidermal Growth Factor Receptor (HER) 1, HER2, HER3 and HER4. In one study, antibody-drug conjugate composed of a HER2-targeted antibody with liposomal DOX was found to increase the tumour localisation in case of HER2 overexpressing breast cancer[49]. Kirpotin et al. emphasized liposome internalisation into the target cells for liposomeencapsulated drug targeting, in contrast to sheer surface attachment[50]. The same was revealed from DOX-loaded immunoliposomes in the case of HER2, where higher antitumor activity was reported when internalising agents were used[50].
Cluster of Differentiation (CD) targeting:
In the case of many tumours such as breast, colon etc., CD44 a receptor protein is overexpressed. It was demonstrated in a recent report that CD44 antibody modified liposomes have much higher sustained drug release compared to free Timosaponin AIII (TAIII) in vitro. There was increased TAIII circulation time, tumour targeted accumulation and improved antitumour activity in CD44 cancer cases along with the absence of noticeable toxicity against Hep G2 cells in a mouse Xenograft model[48]. However, in some cases, it may also result in a high level of antigen expression in non-target cells and result in nonspecific toxicity.
Elsewhere, efforts were made to utilise the antibody driven liposomes for therapy, imaging and diagnostics[51]. It is essential to mention that, though many drugs are in different stages of trial[52], a clinical breakthrough for immunoliposomes is still awaited. Despite the research and advancements in this field, studies to understand the advantages of ligand targeted liposomes over passively targeted liposomes have been inconclusive and the concept of targeted liposomes to improve drug delivery has met with scepticism and challenges.
Stealth Liposome for Improved Circulation Times
It was established by several researchers that coating liposomes with inert hydrophilic polymers such as PEG resulted in longer circulating liposomes[53] because of reduced adsorption by blood proteins. Such liposomes are known as stealth liposomes. PEG is biocompatible, non-toxic and has very low antigenicity and immunogenicity[53]. The presence of PEG on the surface of liposomes reduces the MPS uptake and thus enhances the circulation time, provides long linear kinetics, enhances bioavailability and prevents their binding to opsonins[38,50,55]. Immordino et al. reported that PEG-coated liposomes offer strong inter-bilayer repulsion, which allows them to overcome attractive van der Waals forces, thereby evading aggregation and stabilising liposomes[53]. Stealth liposomes were demonstrated to have dose-independent pharmacokinetics dissimilar to classical liposomes[56,57].
One study used amino acid consensus sequences to form highly specific non-covalent bindings between proteins/ peptides and the surface of PEGylated liposomes. These were used to produce long-acting forms of FVIII and FVIIa, which normally require multiple injections because of their short half-lives and improve the efficacy of haemophilia treatment. The association of proteins with PEGylated liposomes enhanced their pharmacodynamics after administration[58]. Surface engineering of liposomes was used to develop stealth lipopolymers comprising non-phospholipids and PEGalternatives which demonstrated enhanced stability, lesser complement activation, ease of handling and low cost[59].
Stealth liposomes can be passively targeted to cancer tissues as they tend to accumulate in the interstitial spaces amid tumour cells. The active form of the drug from the liposome is released to the extracellular fluid of tumour cells and then diffuses to the cell interior[39]. The antitumor effect is dependent on the ability of liposomes to carry the anti-cancer drug to the tumour and its release to the extracellular fluid of the tumour cells. Recently pharmacokinetics and pharmacodynamics modeling and simulation techniques have emerged for the development and regulation of liposomal drugs[61]. In vivo research with tumour bearing mice revealed that stealth liposomes with slightly negative surface charge exhibit longer circulation time and have higher accumulation in tumours compared to stealth liposomes with more negative Zeta potential[63]. Elsewhere, people described the detachable PEG conjugate liposomes[64], in which detachment occurred due to thiolysis of dithiobenzyl urethane linkage, present between PEG and amino-containing phospholipid such as phosphoethanolamine. Once the liposomes reached the target site, enclosed contents were released with the loss of polymer coating[64]. Super Stealth Liposomes (SSLs), prepared with unique PEG-dendron-phospholipid demonstrated increased stability, low toxicity and high intracellular uptake in vivo and in vitro studies[65].
Another extensively researched area of interest is active targeting with PEG-coated liposomes. Ligands like peptides and monoclonal antibodies are used for active targeting[66]. The nucleosome specific monoclonal antibody 2C5 targeted PEGylated liposomes with encapsulated DOX showed 2.3 times higher cytotoxicity in murine Lewis lung carcinoma cell line and 1.6 times cytotoxicity in human mammary adenocarcinoma cell line compared to non-specific Immunoglobulin G (IgG) modified liposomes in vitro studies[38]. Sakurai et al. used a cyclic Arginylglycylaspartic acid (RGD) peptide KGGRAKD for targeting adipose vasculature by using prohibitin-targeted PEGylated nanoparticle encapsulating cytochrome C[66]. Such prohibitin targeted nanoparticles were successful in inducing antiobese effect, apoptosis and showed an unanticipated EPR effect similar to tumour tissue. Targeted liposomal formulations, HER2-targeted PEGylated antibodyliposomal DOX conjugate (MM-302) for breast cancer by Merrimack pharmaceuticals have cleared phase I trial results in 2015[67]. This is based on PEGylated liposome targeting using Erythroblastic Oncogene B 2 (ErbB2) (HER2) an antibody fragment as a ligand to deliver DOX[49]. Efforts are being made for further approval of the therapy.
Initially, PEG conjugated liposomes were considered non-immunogenic. Nevertheless, there are significant reports which demonstrated unpredicted immune responses in the form of Accelerated Blood Clearance (ABC) phenomena due to the production of antibodies against PEG and other components on repeated administration of such liposomes. They can also lead to a hypersensitivity immune response known as complement activation-related pseudo allergy[68]. PEGylation also influences the biological activity and bioavailability of the PEGylated product. PEG chains may shield the protein by surrounding it, at the same time alter its interaction capabilities and hence reduce the biological activities[69]. It can be deemed that these achievements in active targeting will permit the use of PEGylated liposomes to a great extent for nanomedicines.
Ethosomes for Improved Skin Penetration of Drugs
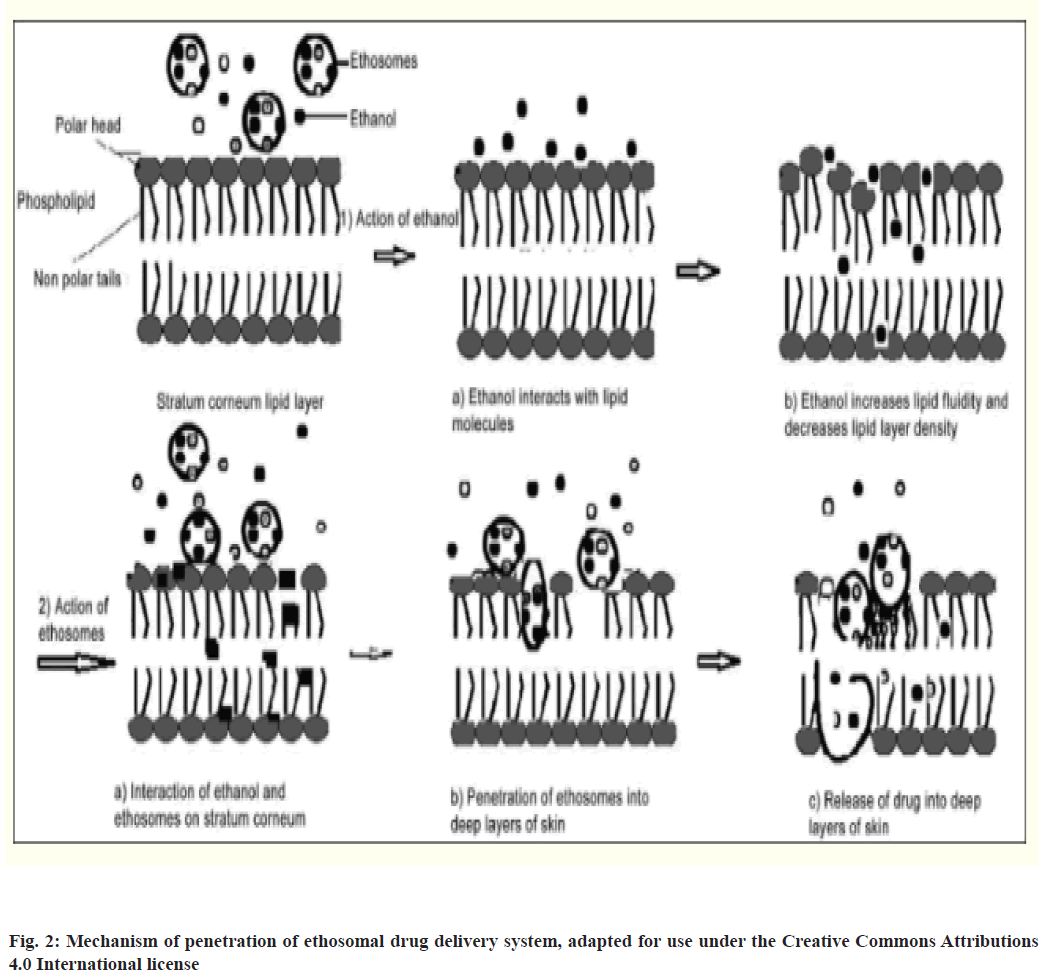
Ethosomes are modified liposomes primarily made up of phospholipids and a high concentration of ethanol and water. They are used for dermal and transdermal delivery of drugs. Classical liposomal systems are effective for local skin therapy as they form a drug reservoir in the upper layer of skin. However, permeation enhancing agents are needed to enable them to penetrate deeper for required levels of the therapeutic drug. The skin penetration property of liposomes was first studied and improved by Touitou et al.[70]. Their work involved introducing a high concentration of short-chain alcohol, like ethanol or isopropyl alcohol, in liposomes which effectively enhanced the dermal and transdermal delivery of drugs. Ethosomes with different physicochemical characteristics showed to facilitate the penetration of all probes as confirmed by high-intensity fluorescence when studies were carried out on transcellular delivery into mice 3T3 fibroblasts. The probes used were 4-(4-diethylamino)styryl-Nmethyl pyridinium iodide (D-289), Rhodamine Red dihexadecanoylglycerophosphoethanolamine (RR) and fluorescent phosphatidylcholine[71]. Moreover, almost no fluorescence was observed when probes were incorporated in hydroxyethanol solution or in liposomes[71].
Ethanol enhances the flexibility and fluidity of lipids by interacting with polar head group regions of lipid molecules which in turn increases membrane permeability which was confirmed by Phosphorus-31 Nuclear Magnetic Resonance (31P NMR) data[70]. After initial interaction, the ethosome undergoes fusion with skin lipids and releases the drug in skin layers[72,73]. It was also proposed by Varma and Pathak that the release of drug in deep layers of skin and its transdermal absorption is due to fusion of ethosome with skin lipids resulting in drug release at various points of penetration pathway, as shown in fig. 2[74].
Another study optimized ethosomal formulation by Box-Behnken experimental design, by using Rhodamine B to simulate a model lipophilic drug thereby act as a fluorescent tracer and 1-palmitoyl- 2-{12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino] dodecanoyl}-sn-glycero-3-phosphocholine as an ethosomal phospholipid and demonstrated percutaneous progression by confocal laser progression[75].
Ethosomes penetrated the skin via hair follicle and stratum corneum by breaking the vesicles, retaining the lipid in the epidermis and the fluorescent tracer penetrated gradually[75]. It was contrary to liposomebased drugs which were limited to the stratum corneum and could not pass the test compound through to deeper layers of skin.
In vitro permeation studies have been carried out to compare transdermal delivery of Salbutamol Sulfate (SS) a hydrophilic drug used as a bronchodilator from ethosomes and classical liposomes with different cholesterol and dicetyl phosphate concentration. The experimental results demonstrated higher efficiency of prepared gels when ethanol systems were used to deliver SS into mice skin both in quantity and depth compare to classical liposomes/aqueous/hydroalcoholic solution[76]. Studies indicate that lipid perturbation along with the elasticity of ethosome vesicles seems to be the main contributor to improved skin permeation[77]. Elsewhere Meloxicam (MX)-loaded liposomal formulations were prepared by varying surfactant charge, carbonchain length and surfactant content. Such formulations affected the physicochemical characteristics, stability and skin permeability of MX-loaded liposomes. One such formulation, with 29 % cationic surfactant with a C16 chain length demonstrated the most optimal skin permeation flux[78]. Moreover, another in vitro study on human Hypertrophic Scar (HS) and skin using Franz’s cells demonstrated that ethosomes are a highly effective carrier for 5-fluorouracil[79].
Highly diverse in vivo studies were carried out to study the ethosomal system for skin penetration, pharmacokinetics, pharmacodynamics, safety issues etc.[80]. Interestingly, anti-psoriasis, anti-fungal and skin cancer drugs were delivered successfully to deep skin layers and were reported in several studies[80-82]. Elsewhere, transdermal delivery of anti-diabetic drug repaglinide by ethosome demonstrated significantly higher permeation compared to the free drug[83]. Additional in vitro and in vivo studies, clinical findings with a special focus on adverse symptoms are needed for many more ethosome based formulations to find a place in clinical use.
Nebulised Liposomes for Pulmonary Delivery of Drugs
Treatment of respiratory diseases by inhaled liposomal antimicrobials is an important treatment method being used in the past few decades as an alternative to oral treatment. Through extensive development in liposome technology, many inhalable antifungal and antibiotic drugs are available for human use[84] and many others are in the clinical trial stage[85].
Pulmonary delivery of drugs with liposomes is of great interest due to their unique properties which include the ability to entrap the therapeutic drug and localize the drug effect after inhalation for extended periods[86]. This leads to a higher therapeutic index for the drug, enhanced retention at the target site, lowers the perspective of systemic adverse effects and lessens the dosing frequency[87,88]. In addition, liposomes can also be deposited inside alveolar macrophages through phagocytosis to improve the treatment of intracellular infection and also penetrate the biofilm produced by bacteria/fungi[89]. They prove that they are especially useful in the case of antibiotics with poor lung penetration and tissue distribution.
Monforte et al. demonstrated that concentrations of nebulised Amphotericin B (AmB) remained high for 14 d, for prophylaxis of Aspergillus infection. It did not affect the respiratory function and it was without any noteworthy systemic absorption of AmB[87]. Further, liposome-based drugs can facilitate the treatment of several respiratory infections including non-cystic bronchiectasis and cystic fibrosis, immunocompromised and mechanically ventilated patients[86,89]. Inhaled antimicrobials are a remarkable development and a beneficial approach to improve the management of respiratory infections.
A formulation of nebulised liposomes, which makes use of liposomal components Dipalmitoylphosphatidylcholine (DPPC) and cholesterol in a mole ratio of 7:3 and 7:4, together with nontoxic excipients sphingomyelin dimyristoyl phosphatidylcholine and/or ethylene glycol to prepare the liposomes has already been approved for clinical applications[84]. This can be used for controlled and sustained release of the drug in the lungs has found incredible success in therapy. Other preparations include the preparation of nebulised liposomal AmB for the treatment of Aspergillus infection in lung transplantation[87,90]. Nebulised liposomal drugs were also shown to be more effective in patients suffering from chronic infection of Pseudomonas aeruginosa with unmanageable cystic fibrosis caused by biofilm growth. When liposomal amikacin was given to these patients, it could penetrate the biofilm and perform targeted, sustained release of the drug. This establishes better in vivo efficacy of liposomal amikacin compared to the free drug[91].
Noteworthily, liposomal ciprofloxacin formulations are also in the developmental stage to treat lung infections. They are shown to provide high concentrations of antibiotics at the site of infection with lesser systemic exposure, thereby limiting side effects[90]. Another preclinical study on pharmacokinetics of liposomeencapsulated Hydroxycloroquine (HCQ) formulation was carried out by administering it through Intratracheal (IT) instillation in Sprague Dawley rats. Liposomal HCQ provided 30-fold higher lung exposure, 2.5 times more half-life compared to unformulated HCQ and demonstrated that inhalable liposomal HCQ could provide a notable clinical benefit and a potential treatment for Coronavirus Disease 2019 (COVID-19) [92]. The success of pharmacokinetic studies and clinical benefits on inhalable liposomal HCQ and other drugs in animal models can be a useful tool in treating pulmonary diseases in future.
Stimuli-Responsive Liposomes
Liposomes that trigger drug release when provided with an environmental cue are called stimuli-responsive liposomes and are promising candidates to provide sitespecific therapy. Stimuli-responsive liposomes can be put in two broad categories i.e., remotely controlled by physical factors like ultrasound/heat/light, or controlled by internal factors such as enzymes, pH, etc. Stimuliresponsive liposomes have been extensively researched and the ones which have progressed the most in clinical trials are thermosensitive liposomes[94].
Thermosensitive liposomes, initially developed in the 1980s, are useful as therapeutic agents[95,96]. Liposomes themselves have poor penetration and limited drug release factor in the tumour area. This can be improved by the application of heat through an external heat source to 40°-41° after administration. This increase in temperature results in compositional changes and release of encapsulated drugs, limiting the injury to the surrounding normal tissue. Another class of stimuli-responsive liposomes exploit light activatable chemicals i.e. photosensitizing agents to capture the light photons for release of cytotoxic molecular species[94,97]. Thermoresponsive liposomes developed for drugs such as DOX (with trade name ThermoDox®) have completed phase 3 clinical trials for hepatocellular carcinoma[98]. Other studies demonstrated delivery of both drugs and genes to the same cell, by incorporating DOX and Special AT-rich sequence-Binding protein-1 (SATB1) short hairpin Ribonucleic Acid (shRNA) vector in a co-delivery system[99]. The results established that the co-delivery system was associated with the required ability for targeted delivery. It also demonstrated the thermosensitive release of DOX and SATB1 gene silencing. Furthermore, co-delivery of DOX and SATB1 shRNA when loaded in nanoniosmes was demonstrated to inhibit cancer cell growth both in vitro and in vivo[99] and shows potential in combined chemotherapy and gene therapy in gastric cancer[100]. These stimuli can also be used for pulsatile drug release and can be standardized for the controlled release of drugs using hydrogels and micelles [101].
Delivery of Nucleic Acid, Therapeutics and Imaging
Basic nucleic acid polymers which comprise DNA or RNA molecules get degraded by biological fluids when injected into the body directly. Effective NATs can be achieved by encapsulating them in a protective liposome envelope[102]. NATs have been shown to enhance or eliminate specific gene expression and treat diseases like cancer as well as infectious and inflammatory diseases[103,104]. Early work in the 1980s by Juhel et al. demonstrated gene expression after intravenous injection through the use of recombinant plasmid encoding rat insulin encapsulated in large liposomes. In the following years, it was established that a positive charge, e.g., cationic lipid, is desired for the efficient association of nucleic acids with lipids[105,106]. This demonstration prompted several studies on cationic liposomes[107,108]. However, cationic liposomes were reported to show toxicity and immunoadjuvant activity towards the immune effect[109,110]. Elsewhere, Fusogenic Liposomes (FLs) were prepared by fusing conventional liposomes with inactivated viruses. FLs delivered the content directly to the cytoplasm without the drawback of cytotoxicity, hence emerged as a powerful tool for gene delivery systems[111,112].
However, it is recently shown that NATs have set foot in clinical applications after overcoming limitations of limited cellular uptake, low biological stability and offtarget effect.
Another interesting area of research is the use of Magnetic Nanoparticles (MNPs) as diagnostic tools because of their ability to penetrate deeper into tissues. When magnetic compounds (e.g. Ferric oxide (Fe2O3) or Fe3O4 or Gadolinium NPs) are introduced in liposomes, they can be recognized at the target site in an external magnetic field and extensively studied. Such liposomes are known as magnetically responsive liposomes. MNPs act as contrast agents in Magnetic Resonance Imaging (MRI)[113].
Liposome Based Vaccine for SARS-CoV
SARS-CoV is an infectious disease that occurred many times in the early 21st century. In 2020, SARSCoV- 19 caused a global pandemic and resulted in an unprecedented health crisis. Several teams of researchers began work on the development of a vaccine to combat it, however, in this review, the discussion will be limited to liposome/lipid nanoparticle based vaccines[114-116]. One such study conducted in 2009, identified four Human Leukocyte Antigen (HLA)-A*0201-restricted Cytotoxic T Lymphocyte (CTL) epitopes derived from SARS-CoV using HLA-A*0201 transgenic mice and recombinant adenovirus expressing predicted epitopes. These peptides were fastened to the surface of the liposomes and injected into the mice. One of the liposomal peptides cleared the vaccinia virus that expressed epitopes of SARS-CoV, indicating their possible use in vaccine manufacture[116]. Another group reported the formation of potent, safer and translatable nanovaccine by attaching the S1 subunit of the virus with two adjuvants. The adjuvants were “monophosphoryl lipid A” for Toll-like receptor 4 and “Cytosine-Guanine dinucleotide (CpG) oligodeoxynucleotide” for Toll-like receptor 9 in cationic liposomes. This nano-vaccine was shown to have a humoral immune response and significantly inhibit SARS-CoV-2 from infecting Vero cells[115]. It is important to understand the impact of the design of delivery systems in the field of vaccine development. One such study in this area showed that 1,2-Dioleoyl-3-Trimethylammonium Propane (DOTAP) and Dimethyldioctadecylammonium (DDA) liposomes were among the systems that induced the highest antigen expression in vitro[117].
At present two vaccines for SARS-CoV-19 by the pharmaceutical companies, Moderna and Pfizer utilize lipid nanoparticle systems for encapsulating messenger RNA (mRNA)-1273 to enhance their stability and performance[118]. Both have been approved (for emergency use) and are currently being used to vaccinate people in several countries[60,119]. It is essential to mention here that the mRNA molecules are extremely delicate and fragile and cannot withstand the enzymes both in laboratory conditions as well as in our bodies. The negative charge of mRNA electrostatically repulses the anionic cell membrane and hinders its entry into the cell and hence a delivery vehicle is needed. The development of these vaccines can be owed to the decades of development of liposomes/lipid nanoparticles research.
Liposomes in Clinical Use
AmB the first-ever liposomal preparation (L-AmB) by company Vestar under the brand name AmBisome, was introduced in Europe in the year 1990[122]. The drug was successfully marketed by Vester company. This was a pivotal step for liposomes in making the leap from experimental seclusion to clinical utility. However, Doxil®[30] was the first drug approved in the United States of America (USA) in the year 1995. Since then, several drugs have completed their clinical trials and are currently in use for the treatment of cancer, tumours, fungal infections etc., and many more are in different phases of clinical trials[35,121]. Table 1 provides details of the various drugs approved for clinical applications.
| Clinical products | Year of approval | Active agent | Lipid/Lipid:Drug molar ratio | Targets/Indications | Company |
|---|---|---|---|---|---|
| AmBisome | 1990 | AmB | HSPC, cholesterol, NF, distearoylphosphatidylglycerol; alpha tocopherol | Fungal infections, leishmaniasis | Vestar |
| Abelcet® | 1995 | AmB | DMPC:DMPG (7:3 molar ratio) | Invasive severe fungal infections | Sigma-Tau Pharmaceuticals |
| Doxil® | 1995 | DOX | HSPC:cholesterol:PEG 2000-DSPE (56:39:5 molar ratio) | Ovarian, breast cancer, Kaposi’s sarcoma | Sequus Pharmaceuticals |
| DaunoXome® | 1996 | Daunorubicin | DSPC and cholesterol (2:1 molar ratio) | AIDS-related Kaposi’s sarcoma | NeXstar Pharmaceuticals |
| Amphotec® | 1996 | AmB | Cholesteryl sulphate:AmB (1:1 molar ratio) | Severe fungal infections | Ben Venue Laboratories Inc. |
| Depocyt® | 1999 | Cytarabine/Ara-C | DOPC, DPPG, cholesterol and triolein | Neoplastic meningitis | Sky Pharma Inc. |
| Myocet® | 2000 | DOX | EPC:Cholesterol (55:45 molar ratio) | Combination therapy with cyclophosphamide in metastatic breast cancer | Elan Pharmaceuticals |
| Mepact® | 2004 | Mifamurtide | DOPS:POPC (3:7 molar ratio) | High-grade, resectable, non-metastatic osteosarcoma | Takeda Pharmaceutical Limited |
| Marqibo® | 2012 | Vincristine | SM:Cholesterol (60:40 molar ratio) | Acute lymphoblastic leukaemia | Talon Therapeutics, Inc. |
| Onivyde™ | 2015 | Irinotecan | DSPC:MPEG-2000:DSPE (3:2:0.015 molar ratio) | Combination therapy with fluorouracil and leucovorin in metastatic adenocarcinoma of the pancreas | Merrimack Pharmaceuticals Inc. |
| Visudyne® | 2000 | Verteporphin | Verteporphin:DMPC and EPG (1:8 molar ratio) | Choroidal neovascularisation | Novartis |
| DepoDur™ | 2004 | Morphine sulfate | DOPC, DPPG, cholesterol and triolein | Pain management | Sky Pharma Inc. |
| Exparel® | 2011 | Bupivacaine | DEPC, DPPG, cholesterol and tricaprylin | Pain management | Pacira Pharmaceuticals, Inc. |
| Epaxal® | 1993 | Inactivated hepatitis A virus (strain RGSB) | DOPC:DOPE (75:25 molar ratio) | Hepatitis A | Crucell, Berna Biotech Ltd. |
| COVID vaccine | 2020 | mRNA 1227 in LNP | Nucleoside-modified mRNA vaccine encoding the stabilized prefusion spike glycoprotein of SARS-CoV-2 | SARS-CoV-19 | Moderna |
| COVID vaccine | 2020 | mRNA 1227 in LNP | Nucleoside-modified mRNA vaccine encoding the prefusion spike glycoprotein of SARS-CoV-2 | SARS-CoV-19 | Pfizer |
Table 1: Liposomes in Clinical Use
Conclusion
Liposome based drugs have the honor of becoming the first nanoscale drugs approved for clinical use. This is borne as a result of extensive work since their inception more than five decades ago and today they hold a recognized position in the mainstream of drug delivery systems, influencing many biomedical areas. With distinct properties like biocompatibility, biodegradability, accompanied by their nanosize, liposomes have potential applications in diverse areas such as the delivery of antibiotics, anti-fungal, anticancer drugs, genetic medicines and imaging. Presently, a number of liposomal drugs are clinically approved and commercially available, while many more formulations are either being investigated in different stages of clinical trials or awaiting approval. Understanding the current state, advances and challenges of liposomal technology is important to pave the way for future research that will build upon existing platforms. Despite tremendous development in the field, there remain many challenges like pharmaceutical manufacturing, quality assurance, cost, government regulation and intellectual property etc., which need to be overcome.
Advancements in liposomal drug delivery require consistent effort to improve the frontiers of knowledge in design and manufacturing, assessment of toxicology, cellular interactions and clinical evaluation. Further success in the field will be associated with cooperation and communication with the experts in all stages of pharmaceutical development of liposome technologies, to overcome present shortcomings.
Acknowledgements:
The author wishes to thank the Principal and the management B. M. S. College of Engineering for continuous support and encouragement provided.
Conflict of interests:
The authors declared no conflict of interest.
References
- Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol 1965;13(1):238-52.
[Crossref] [Google Scholar] [PubMed]
- Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, et al. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit Rev Ther Drug Carrier Syst 2009;26(6):523-80.
[Crossref] [Google Scholar] [PubMed]
- Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Deliv Rev 2013;65(1):36-48.
[Crossref] [Google Scholar] [PubMed]
- Ventola CL. Progress in nanomedicine: Approved and investigational nanodrugs. PT 2017;42(12):742-55.
[Google Scholar] [PubMed]
- Beltrán-Gracia E, López-Camacho A, Higuera-Ciapara I, Velázquez-Fernández JB, Vallejo-Cardona AA. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol 2019;10(1):1-40.
- Olusanya TO, Haj Ahmad RR, Ibegbu DM, Smith JR, Elkordy AA. Liposomal drug delivery systems and anticancer drugs. Molecules 2018;23(4):907.
[Crossref] [Google Scholar] [PubMed]
- Mohammed AR, Weston N, Coombes AG, Fitzgerald M, Perrie Y. Liposome formulation of poorly water soluble drugs: Optimisation of drug loading and ESEM analysis of stability. Int J Pharm 2004;285(1-2):23-34.
[Crossref] [Google Scholar] [PubMed]
- Allen TM, Martin FJ. Advantages of liposomal delivery systems for anthracyclines. In Semin Oncol 2004;31(13):5-15.
[Crossref] [Google Scholar] [PubMed]
- Ding BS, Dziubla T, Shuvaev VV, Muro S, Muzykantov VR. Advanced drug delivery systems that target the vascular endothelium. Mol Interv 2006;6(2):98-112.
[Crossref] [Google Scholar] [PubMed]
- Jain A, Tiwari A, Verma A, Saraf S, Jain SK. Combination cancer therapy using multifunctional liposomes. Crit Rev Ther Drug Carrier Syst 2020;37(2):105-34.
[Crossref] [Google Scholar] [PubMed]
- Patel G, Thakur NS, Kushwah V, Patil MD, Nile SH, Jain S, et al. Liposomal delivery of mycophenolic acid with quercetin for improved breast cancer therapy in SD rats. Front Bioeng Biotechnol 2020;8:631.
- Fritze A, Hens F, Kimpfler A, Schubert R, Peschka-Süss R. Remote loading of doxorubicin into liposomes driven by a transmembrane phosphate gradient. Biochim Biophys Acta Biomembr 2006;1758(10):1633-40.
[Crossref] [Google Scholar] [PubMed]
- Niculescu AG, Chircov C, Bîrc? AC, Grumezescu AM. Nanomaterials synthesis through microfluidic methods: An updated overview. Nanomaterials 2021;11(4):864.
[Crossref] [Google Scholar] [PubMed]
- Chang DK, Lin CT, Wu CH, Wu HC. A novel peptide enhances therapeutic efficacy of liposomal anti-cancer drugs in mice models of human lung cancer. PLoS One 2009;4(1):e4171.
[Crossref] [Google Scholar] [PubMed]
- Andresen TL, Thompson DH, Kaasgaard T. Enzyme-triggered nanomedicine: Drug release strategies in cancer therapy (Invited Review). Mol Membr Biol 2010;27(7):353-63.
[Crossref] [Google Scholar] [PubMed]
- Zhang XX, McIntosh TJ, Grinstaff MW. Functional lipids and lipoplexes for improved gene delivery. Biochimie 2012;94(1):42-58.
[Crossref] [Google Scholar] [PubMed]
- Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine 2015;10:975-99.
[Crossref] [Google Scholar] [PubMed]
- Lee MK. Liposomes for enhanced bioavailability of water-insoluble drugs: In vivo evidence and recent approaches. Pharmaceutics 2020;12(3):264.
[Crossref] [Google Scholar] [PubMed]
- Inglut CT, Sorrin AJ, Kuruppu T, Vig S, Cicalo J, Ahmad H, et al. Immunological and toxicological considerations for the design of liposomes. Nanomaterials 2020;10(2):190.
[Crossref] [Google Scholar] [PubMed]
- Hansen AH, Lomholt MA, Hansen PL, Mouritsen OG, Arouri A. Optimization and modeling of the remote loading of luciferin into liposomes. Int J Pharm 2016;508(1):128-34.
[Crossref] [Google Scholar] [PubMed]
- Fenske DB, Cullis PR. Entrapment of small molecules and nucleic acid-based drugs in liposomes. Methods Enzymol 2005;391:7-40.
[Crossref] [Google Scholar] [PubMed]
- Agarwal K, Bali A, Gupta CM. Influence of the phospholipid structure on the stability of liposomes in serum. Biochim Biophys Acta Biomembr 1986;856(1):36-40.
[Crossref] [Google Scholar] [PubMed]
- Kaddah S, Khreich N, Kaddah F, Charcosset C, Greige-Gerges H. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem Toxicol 2018;113:40-8.
[Crossref] [Google Scholar] [PubMed]
- Cullis PR, Hope MJ. The bilayer stabilizing role of sphingomyelin in the presence of cholesterol. A 31P NMR study. Biochim Biophys Acta Biomembr 1980;597(3):533-42.
[Crossref] [Google Scholar] [PubMed]
- Agarwal K, Bali A, Gupta CM. Synthesis of carbamyl and ether analogs of phosphatidylcholines. Chem Phys Lipids 1984;36(2):169-77.
- Gupta CM, Bali A, Dhawan S. Modification of phospholipid structure results in greater stability of liposomes in serum. Biochim Biophys Acta Biomembr 1981;648(2):192-8.
- Torchilin V. Antibody-modified liposomes for cancer chemotherapy. Expert Opin Drug Deliv 2008;5(9):1003-25.
[Crossref] [Google Scholar] [PubMed]
- Agarwal K, Bali A, Gupta CM. Effect of phospholipid structure on stability and survival times of liposomes in circulation. Biochim Biophys Acta Gen Subj 1986;883(3):468-75.
[Crossref] [Google Scholar] [PubMed]
- Juszkiewicz K, Sikorski AF, Czogalla A. Building blocks to design liposomal delivery systems. Int J Mol Sci 2020;21(24):9559.
[Crossref] [Google Scholar] [PubMed]
- Barenholz YC. Doxil®-The first FDA-approved nano-drug: Lessons learned. J Control Release 2012;160(2):117-34.
[Crossref] [Google Scholar] [PubMed]
- Joguparthi V, Anderson BD. Liposomal delivery of hydrophobic weak acids: Enhancement of drug retention using a high intraliposomal pH. J Pharm Sci 2008;97(1):433-54.
[Crossref] [Google Scholar] [PubMed]
- Pauli G, Tang WL, Li SD. Development and characterization of the solvent-assisted active loading technology (SALT) for liposomal loading of poorly water-soluble compounds. Pharmaceutics 2019;11(9):465.
[Crossref] [Google Scholar] [PubMed]
- Sur S, Fries AC, Kinzler KW, Zhou S, Vogelstein B. Remote loading of preencapsulated drugs into stealth liposomes. Proc Natl Acad Sci U S A 2014;111(6):2283-8.
[Crossref] [Google Scholar] [PubMed]
- Johnston MJ, Semple SC, Klimuk SK, Edwards K, Eisenhardt ML, Leng EC, et al. Therapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulations. Biochim Biophys Acta Biomembr 2006;1758(1):55-64.
[Crossref] [Google Scholar] [PubMed]
- Yanagida Y, Namba M, Fukuta T, Yamamoto H, Yanagida M, Honda M, et al. Release rate is a key variable affecting the therapeutic effectiveness of liposomal fasudil for the treatment of cerebral ischemia/reperfusion injury. Biochem Biophys Res Commun 2020;531(4):622-7.
[Crossref] [Google Scholar] [PubMed]
- Torchilin VP. Passive and active drug targeting: Drug delivery to tumors as an example. Handb Exp Pharmacol 2010;(197):3-53.
[Crossref] [Google Scholar] [PubMed]
- Xu S, Olenyuk BZ, Okamoto CT, Hamm-Alvarez SF. Targeting receptor-mediated endocytotic pathways with nanoparticles: Rationale and advances. Adv Drug Deliv Rev 2013;65(1):121-38.
[Crossref] [Google Scholar] [PubMed]
- ElBayoumi TA, Torchilin VP. Tumor-targeted nanomedicines: Enhanced antitumor efficacy in vivo of doxorubicin-loaded, long-circulating liposomes modified with cancer-specific monoclonal antibody. Clin Cancer Res 2009;15(6):1973-80.
[Crossref] [Google Scholar] [PubMed]
- Gullotti E, Yeo Y. Extracellularly activated nanocarriers: A new paradigm of tumor targeted drug delivery. Mol Pharm 2009;6(4):1041-51.
[Crossref] [Google Scholar] [PubMed]
- Mamot C, Ritschard R, Wicki A, Küng W, Schuller J, Herrmann R, et al. Immunoliposomal delivery of doxorubicin can overcome multidrug resistance mechanisms in EGFR-overexpressing tumor cells. J Drug Target 2012;20(5):422-32.
[Crossref] [Google Scholar] [PubMed]
- Leserman LD, Weinstein JN, Blumenthal R, Sharrow SO, Terry WD. Binding of antigen-bearing fluorescent liposomes to the murine myeloma tumor MOPC 315. J Immunol 1979;122(2):585-91.
[Google Scholar] [PubMed]
- Arias-Alpizar G, Kong L, Vlieg RC, Rabe A, Papadopoulou P, Meijer MS, et al. Light-triggered switching of liposome surface charge directs delivery of membrane impermeable payloads in vivo. Nat Commun 2020;11(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res 2008;41(1):120-9.
[Crossref] [Google Scholar] [PubMed]
- Garde SV, Forté AJ, Ge M, Lepekhin EA, Panchal CJ, Rabbani SA, et al. Binding and internalization of NGR-peptide-targeted liposomal doxorubicin (TVT-DOX) in CD13-expressing cells and its antitumor effects. Anticancer Drugs 2007;18(10):1189-200.
[Crossref] [Google Scholar] [PubMed]
- Pangburn TO, Petersen MA, Waybrant B, Adil MM, Kokkoli E. Peptide-and aptamer-functionalized nanovectors for targeted delivery of therapeutics. J Biomech Eng 2009;131(7):074005.
[Crossref] [Google Scholar] [PubMed]
- Puri A, Kramer-Marek G, Campbell-Massa R, Yavlovich A, Tele SC, Lee SB, et al. HER2-specific affibody-conjugated thermosensitive liposomes (Affisomes) for improved delivery of anticancer agents. J Liposome Res 2008;18(4):293-307.
[Crossref] [Google Scholar] [PubMed]
- Eloy JO, Petrilli R, Trevizan LN, Chorilli M. Immunoliposomes: A review on functionalization strategies and targets for drug delivery. Colloids Surf B Biointerfaces 2017;159:454-67.
[Crossref] [Google Scholar] [PubMed]
- Lu L, Ding Y, Zhang Y, Ho RJ, Zhao Y, Zhang T, et al. Antibody-modified liposomes for tumor-targeting delivery of timosaponin AIII. Int J Nanomed 2018;13:1927-44.
[Crossref] [Google Scholar] [PubMed]
- Geretti E, Leonard SC, Dumont N, Lee H, Zheng J, de Souza R, et al. Cyclophosphamide-mediated tumor priming for enhanced delivery and antitumor activity of HER2-targeted liposomal doxorubicin (MM-302) predose with cyclophosphamide enhances MM-302 tumor delivery. Mol Cancer Ther 2015;14(9):2060-71.
[Crossref] [Google Scholar] [PubMed]
- Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res 2006;66(13):6732-40.
[Crossref] [Google Scholar] [PubMed]
- Sawant RR, Torchilin VP. Challenges in development of targeted liposomal therapeutics. AAPS J 2012;14(2):303-15.
[Crossref] [Google Scholar] [PubMed]
- Merino M, Zalba S, Garrido MJ. Immunoliposomes in clinical oncology: State of the art and future perspectives. J Control Release 2018;275:162-76.
[Crossref] [Google Scholar] [PubMed]
- Immordino ML, Dosio F, Cattel L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomed 2006;1(3):297-315.
[Google Scholar] [PubMed]
- Hussain Z, Khan S, Imran M, Sohail M, Shah SW, de Matas M. PEGylation: A promising strategy to overcome challenges to cancer-targeted nanomedicines: A review of challenges to clinical transition and promising resolution. Drug Deliv Transl Res 2019;9(3):721-34.
[Crossref] [Google Scholar] [PubMed]
- Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev 2016;99:28-51.
[Crossref] [Google Scholar] [PubMed]
- Jung SH, Jung SH, Seong H, Cho SH, Jeong KS, Shin BC. Polyethylene glycol-complexed cationic liposome for enhanced cellular uptake and anticancer activity. Int J Pharm 2009;382(1-2):254-61.
[Crossref] [Google Scholar] [PubMed]
- Kontogiannopoulos KN, Tsermentseli SK, Assimopoulou AN, Papageorgiou VP. Sterically stabilized liposomes as a potent carrier for shikonin. J Liposome Res 2014;24(3):230-40.
[Crossref] [Google Scholar] [PubMed]
- Yatuv R, Robinson M, Dayan-Tarshish I, Baru M. The use of PEGylated liposomes in the development of drug delivery applications for the treatment of hemophilia. Int J Nanomedicine 2010;5:581-91.
[Crossref] [Google Scholar] [PubMed]
- Nag OK, Awasthi V. Surface engineering of liposomes for stealth behavior. Pharmaceutics 2013;5(4):542-69.
[Crossref] [Google Scholar] [PubMed]
- Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep 2020;69(50):1922-4.
[Crossref] [Google Scholar] [PubMed]
- He H, Yuan D, Wu Y, Cao Y. Pharmacokinetics and pharmacodynamics modeling and simulation systems to support the development and regulation of liposomal drugs. Pharmaceutics 2019;11(3):110.
[Crossref] [Google Scholar] [PubMed]
- Lee EH, Kim A, Oh YK, Kim CK. Effect of edge activators on the formation and transfection efficiency of ultradeformable liposomes. Biomaterials 2005;26(2):205-10.
[Crossref] [Google Scholar] [PubMed]
- Lee JS, Ankone M, Pieters E, Schiffelers RM, Hennink WE, Feijen J. Circulation kinetics and biodistribution of dual-labeled polymersomes with modulated surface charge in tumor-bearing mice: Comparison with stealth liposomes. J Control Release 2011;155(2):282-8.
[Crossref] [Google Scholar] [PubMed]
- Zalipsky S, Qazen M, Walker JA, Mullah N, Quinn YP, Huang SK. New detachable poly (ethylene glycol) conjugates: Cysteine-cleavable lipopolymers regenerating natural phospholipid, diacyl phosphatidylethanolamine. Bioconjug Chem 1999;10(5):703-7.
[Crossref] [Google Scholar] [PubMed]
- Pasut G, Paolino D, Celia C, Mero A, Joseph AS, Wolfram J, et al. Polyethylene glycol (PEG)-dendron phospholipids as innovative constructs for the preparation of super stealth liposomes for anticancer therapy. J Control Release 2015;199:106-13.
[Crossref] [Google Scholar] [PubMed]
- Sakurai Y, Kajimoto K, Harashima H. Anti-angiogenic nanotherapy via active targeting systems to tumors and adipose tissue vasculature. Biomater Sci 2015;3(9):1253-65.
[Crossref] [Google Scholar] [PubMed]
- Merrimack Pharmaceuticals Inc. Annual reports for 9187 international companies. AnnualReports.com, NASDAW_MACK; 2015.
- Mohamed M, Abu Lila AS, Shimizu T, Alaaeldin E, Hussein A, Sarhan HA, et al. PEGylated liposomes: Immunological responses. Sci Technol Adv Mater 2019;20(1):710-24.
[Crossref] [Google Scholar] [PubMed]
- Zhang F, Liu MR, Wan HT. Discussion about several potential drawbacks of PEGylated therapeutic proteins. Biol Pharm Bull 2014;37(3):335-9.
[Crossref] [Google Scholar] [PubMed]
- Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M. Ethosomes-novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J Control Release 2000;65(3):403-18.
[Crossref] [Google Scholar] [PubMed]
- Touitou E, Godin B, Dayan N, Weiss C, Piliponsky A, Levi-Schaffer F. Intracellular delivery mediated by an ethosomal carrier. Biomaterials 2001;22(22):3053-9.
[Crossref] [Google Scholar] [PubMed]
- Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM. Lipid vesicles for skin delivery of drugs: Reviewing three decades of research. Int J Pharm 2007;332(1-2):1-6.
[Crossref] [Google Scholar] [PubMed]
- Ghasemiyeh P, Mohammadi-Samani S. Potential of nanoparticles as permeation enhancers and targeted delivery options for skin: Advantages and disadvantages. Drug Des Devel Ther 2020;14:3271-89.
- Verma P, Pathak K. Therapeutic and cosmeceutical potential of ethosomes: An overview. J Adv Pharm Technol Res 2010;1(3):274-82.
[Crossref] [Google Scholar] [PubMed]
- Yang L, Wu L, Wu D, Shi D, Wang T, Zhu X. Mechanism of transdermal permeation promotion of lipophilic drugs by ethosomes. Int J Nanomed 2017;12:3357-64.
- Bendas ER, Tadros MI. Enhanced transdermal delivery of salbutamol sulfate via ethosomes. AAPS PharmSciTech 2007;8(4):E107.
[Crossref] [Google Scholar] [PubMed]
- Jain S, Tiwary AK, Sapra B, Jain NK. Formulation and evaluation of ethosomes for transdermal delivery of lamivudine. AAPS PharmSciTech 2007;8(4):E111.
[Crossref] [Google Scholar] [PubMed]
- Duangjit S, Pamornpathomkul B, Opanasopit P, Rojanarata T, Obata Y, Takayama K, et al. Role of the charge, carbon chain length and content of surfactant on the skin penetration of meloxicam-loaded liposomes. Int J Nanomed 2014;9:2005-17.
[Crossref] [Google Scholar] [PubMed]
- Zhang Z, Wo Y, Zhang Y, Wang D, He R, Chen H, et al. In vitro study of ethosome penetration in human skin and hypertrophic scar tissue. Nanomedicine 2012;8(6):1026-33.
[Crossref] [Google Scholar] [PubMed]
- Bhalaria MK, Naik S, Misra AN. Ethosomes: A novel delivery system for antifungal drugs in the treatment of topical fungal diseases. Indian J Exp Biol 2009;47(5):368-75.
[Google Scholar] [PubMed]
- Dubey V, Mishra D, Dutta T, Nahar M, Saraf DK, Jain NK. Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J Control Release 2007;123(2):148-54.
[Crossref] [Google Scholar] [PubMed]
- Sayed OM, Abo El-Ela FI, Kharshoum RM, Salem HF. Treatment of basal cell carcinoma via binary ethosomes of vismodegib: In vitro and in vivo studies. AAPS PharmSciTech 2020;21(2):1-11.
[Crossref] [Google Scholar] [PubMed]
- Bodade SS, Shaikh KS, Kamble MS, Chaudhari PD. A study on ethosomes as mode for transdermal delivery of an antidiabetic drug. Drug Deliv 2013;20(1):40-6.
[Crossref] [Google Scholar] [PubMed]
- US8652512B2. Nebulized liposomes for the pulmonary application of drug compound. Google patents; 2020.
- Elhissi A. Liposomes for pulmonary drug delivery: The role of formulation and inhalation device design. Curr Pharm Des 2017;23(3):362-72.
[Crossref] [Google Scholar] [PubMed]
- Rudokas M, Najlah M, Alhnan MA, Elhissi A. Liposome delivery systems for inhalation: A critical review highlighting formulation issues and anticancer applications. Med Princ Pract 2016;25(2):60-72.
[Crossref] [Google Scholar] [PubMed]
- Monforte V, Ussetti P, López R, Gavaldà J, Bravo C, de Pablo A, et al. Nebulized liposomal amphotericin B prophylaxis for Aspergillus infection in lung transplantation: Pharmacokinetics and safety. J Heart Lung Transplant 2009;28(2):170-5.
[Crossref] [Google Scholar] [PubMed]
- Clancy JP, Dupont L, Konstan MW, Billings J, Fustik S, Goss CH, et al. Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection. Thorax 2013;68(9):818-25.
[Crossref] [Google Scholar] [PubMed]
- Bassetti M, Vena A, Russo A, Peghin M. Inhaled liposomal antimicrobial delivery in lung infections. Drugs 2020;80(13):1309-18.
[Crossref] [Google Scholar] [PubMed]
- Cipolla D, Blanchard J, Gonda I. Development of liposomal ciprofloxacin to treat lung infections. Pharmaceutics 2016;8(1):6.
[Crossref] [Google Scholar] [PubMed]
- Meers P, Neville M, Malinin V, Scotto AW, Sardaryan G, Kurumunda R, et al. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J Antimicrob Chemother 2008;61(4):859-68.
[Crossref] [Google Scholar] [PubMed]
- Tai TT, Wu TJ, Wu HD, Tsai YC, Wang HT, Wang AM, et al. A strategy to treat COVID?19 disease with targeted delivery of inhalable liposomal hydroxychloroquine: A preclinical pharmacokinetic study. Clin Transl Sci 2021;14(1):132-6.
[Crossref] [Google Scholar] [PubMed]
- Puri A. Phototriggerable liposomes: Current research and future perspectives. Pharmaceutics 2013;6(1):1-25.
[Crossref] [Google Scholar] [PubMed]
- Nardecchia S, Sánchez-Moreno P, de Vicente J, Marchal JA, Boulaiz H. Clinical trials of thermosensitive nanomaterials: An overview. Nanomaterials 2019;9(2):191.
[Crossref] [Google Scholar] [PubMed]
- Bi H, Xue J, Jiang H, Gao S, Yang D, Fang Y, et al. Current developments in drug delivery with thermosensitive liposomes. Asian J Pharm Sci 2019;14(4):365-79.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Xu P, He D, Xu B, Tu J, Shen Y. Long-circulating thermosensitive liposomes for the targeted drug delivery of oxaliplatin. Int J Nanomed 2020;15:6721-34.
[Crossref] [Google Scholar] [PubMed]
- Yaghini E, Dondi R, Edler KJ, Loizidou M, MacRobert AJ, Eggleston IM. Codelivery of a cytotoxin and photosensitiser via a liposomal nanocarrier: A novel strategy for light-triggered cytosolic release. Nanoscale 2018;10(43):20366-76.
- NIH, U.S. National Library of Medicine. Phase 3 study of thermodox with radiofrequency ablation (RFA) in treatment of hepatocellular carcinoma (HCC). Clinical trials.gov (NIH); 2017.
- Peng Z, Wang C, Fang E, Lu X, Wang G, Tong Q. Co-delivery of doxorubicin and SATB1 shRNA by thermosensitive magnetic cationic liposomes for gastric cancer therapy. PLoS One 2014;9(3):e92924.
[Crossref] [Google Scholar] [PubMed]
- Hemati M, Haghiralsadat F, Jafary F, Moosavizadeh S, Moradi A. Targeting cell cycle protein in gastric cancer with CDC20siRNA and anticancer drugs (doxorubicin and quercetin) co-loaded cationic PEGylated nanoniosomes. Int J Nanomedicine 2019;14:6575-85.
[Crossref] [Google Scholar] [PubMed]
- Kikuchi A, Okano T. Pulsatile drug release control using hydrogels. Adv Drug Deliv Rev 2002;54(1):53-77.
[Crossref] [Google Scholar] [PubMed]
- Movahedi F, Hu RG, Becker DL, Xu C. Stimuli-responsive liposomes for the delivery of nucleic acid therapeutics. Nanomedicine 2015;11(6):1575-84.
- Kulkarni JA, Cullis PR, van Der Meel R. Lipid nanoparticles enabling gene therapies: From concepts to clinical utility. Nucleic Acid Ther 2018;28(3):146-57.
[Crossref] [Google Scholar] [PubMed]
- Ashizawa AT, Cortes J. Liposomal delivery of nucleic acid-based anticancer therapeutics: BP-100-1.01. Expert Opin Drug Deliv 2015;12(7):1107-20.
[Crossref] [Google Scholar] [PubMed]
- Audouy SA, de Leij LF, Hoekstra D, Molema G. In vivo characteristics of cationic liposomes as delivery vectors for gene therapy. Pharm Res 2002;19(11):1599-605.
[Crossref] [Google Scholar] [PubMed]
- Zylberberg C, Gaskill K, Pasley S, Matosevic S. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther 2017;24(8):441-52.
[Crossref] [Google Scholar] [PubMed]
- Smyth Templeton N. Liposomal delivery of nucleic acids in vivo. DNA Cell Biol 2002;21(12):857-67.
[Crossref] [Google Scholar] [PubMed]
- Basha G, Novobrantseva TI, Rosin N, Tam YY, Hafez IM, Wong MK, et al. Influence of cationic lipid composition on gene silencing properties of lipid nanoparticle formulations of siRNA in antigen-presenting cells. Mol Ther 2011;19(12):2186-200.
[Crossref] [Google Scholar] [PubMed]
- Filion MC, Phillips NC. Toxicity and immunomodulatory activity of liposomal vectors formulated with cationic lipids toward immune effector cells. Biochim Biophys Acta Biomembr 1997;1329(2):345-56.
[Crossref] [Google Scholar] [PubMed]
- Zhu Y, Meng Y, Zhao Y, Zhu J, Xu H, Zhang E, et al. Toxicological exploration of peptide-based cationic liposomes in siRNA delivery. Colloids Surf B Biointerfaces 2019;179:66-76.
[Crossref] [Google Scholar] [PubMed]
- Mok KW, Cullis PR. Structural and fusogenic properties of cationic liposomes in the presence of plasmid DNA. Biophys J 1997;73(5):2534-45.
[Crossref] [Google Scholar] [PubMed]
- Hoffmann M, Hersch N, Gerlach S, Dreissen G, Springer R, Merkel R, et al. Complex size and surface charge determine nucleic acid transfer by fusogenic liposomes. Int J Mol Sci 2020;21(6):2244.
[Crossref] [Google Scholar] [PubMed]
- Langereis S, Geelen T, Grüll H, Strijkers GJ, Nicolay K. Paramagnetic liposomes for molecular MRI and MRI?guided drug delivery. NMR Biomed 2013;26(7):728-44.
[Crossref] [Google Scholar] [PubMed]
- Huang WC, Zhou S, He X, Chiem K, Mabrouk MT, Nissly RH, et al. SARS?CoV?2 RBD neutralizing antibody induction is enhanced by particulate vaccination. Adv Mater 2020;32(50):2005637.
[Crossref] [Google Scholar] [PubMed]
- Liu L, Liu Z, Chen H, Liu H, Gao Q, Cong F, et al. Subunit nanovaccine with potent cellular and mucosal immunity for COVID-19. ACS Appl Bio Mater 2020;3(9):5633-8.
- Ohno S, Kohyama S, Taneichi M, Moriya O, Hayashi H, Oda H, et al. Synthetic peptides coupled to the surface of liposomes effectively induce SARS coronavirus-specific cytotoxic T lymphocytes and viral clearance in HLA-A* 0201 transgenic mice. Vaccine 2009;27(29):3912-20.
[Crossref] [Google Scholar] [PubMed]
- Anderluzzi G, Lou G, Gallorini S, Brazzoli M, Johnson R, O’Hagan DT, et al. Investigating the impact of delivery system design on the efficacy of self-amplifying RNA vaccines. Vaccines 2020;8(2):212.
[Crossref] [Google Scholar] [PubMed]
- Jackson NA, Kester KE, Casimiro D, Gurunathan S, deRosa F. The promise of mRNA vaccines: A biotech and industrial perspective. NPJ Vaccines 2020;5(1):1-6.
- Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. The advisory committee on immunization practices’ interim recommendation for use of Moderna COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep 2021;69(5152):1653-6.
[Crossref] [Google Scholar] [PubMed]
- Davidson RN, Martino LD, Gradoni L, Giacchino R, Russo R, Gaeta GB, et al. Liposomal amphotericin B (AmBisome) in Mediterranean visceral leishmaniasis: A multi-centre trial. Q J Med 1994;87(2):75-81.
[Crossref] [Google Scholar] [PubMed]
- Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017;9(2):12.
[Crossref] [Google Scholar] [PubMed]
- Gulati M, Bajad S, Singh S, Ferdous AJ, Singh M. Development of liposomal amphotericin B formulation. J Microencapsul. 1998;15(2):137-151.