- *Corresponding Author:
- Z. Černíčková
Department of Social Pharmacy, University of Veterinary and Pharmaceutical Sciences Brno, Palackého tř. 1946/1, 612 42 Brno, Czech Republic
E-mail: cernickova.z@seznam.cz
| Date of Submission | 05 December 2016 |
| Date of Revision | 25 April 2017 |
| Date of Acceptance | 07 March 2018 |
| Indian J Pharm Sci 2018;80(3): 412-419 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The marketing authorisation process is an integral part of the life cycle of a veterinary medicinal product, which the applicant wishes to market. The costs of the marketing authorisation of veterinary medicinal products are different from state to state. The differences in marketing authorisation fees are also between particular types of the marketing authorisation procedure such as the national procedure, mutual recognition procedure, decentralised procedure and centralised procedure and whether certain state is the reference member state or concerned member state particularly applicable in either a mutual recognition procedure or a decentralised procedure. The fees is also depended on the type of the marketing authorisation application, such as full, generic, hybrid, similar biological, well established use, fixed combination or informed consent and eventually whether a reference veterinary medicinal product is marketed in the state or not specifically for generic, hybrid and similar biological applications. If it is focused on the states in northern Europe, where Finland, Sweden, Norway, Iceland, Denmark and Baltic States Estonia, Latvia and Lithuania are included, it is a very varied group of states, where each competent authority has its own approach to the determination of marketing authorisation fees. It is important to mention that the marketing authorisation fees for veterinary medicinal products mostly are not the same as the fees for human medicinal products and also even this is paid attention.
Keywords
Veterinary medicinal product, member state, national procedure, MRP, DCP
The marketing authorisation of a medicinal product is a verification process of its efficacy, quality and safety whether it is a veterinary or a human medicinal product [1,2]. The marketing authorisation process is ensured by the competent authorities of individual member states where marketing authorisation applicant wants to have granted marketing authorisation of the veterinary medicinal product. But if centralised procedure is used then evaluation process is provided by the European Medicines Agency (EMA) [3]. If it is focused on the member states of northern Europe (Finland, Sweden, Norway, Iceland, Denmark, Estonia, Lithuania and Latvia) it is important to mention that all these states belong to the European Economic Area (EEA) but not all these states belong to the European Union. Norway and Iceland are not members of the European Union. However these two countries have accepted the complete community acquires regarding veterinary medicinal products via the EEA agreement and it follows that Norway and Iceland are considered the member states of the community and are parties of the community procedures. Also it is important to mention that legally binding acts from the community are not direct rights and obligations for these member states but have to be transferred into legally binding acts in Norway and Iceland [3].
In the member states of the community the marketing authorisation applicant can progress via four marketing authorisation procedures for the veterinary medicinal products i.e. national, mutual recognition, decentralised and centralised procedures. If the marketing authorisation for the veterinary medicinal product has not been granted yet in any country of EEA and the marketing authorisation applicant wants to market this veterinary medicinal product only in the one member state in northern Europe then national procedure is chosen [3]. But if the applicant wants to include several member states into the marketing authorisation procedure then decentralised procedure (DCP) is necessary. In this case, one state has to be chosen as a reference member state (RMS), which prepares an assessment report and other member states included to the DCP are concerned member states (CMS). On the other hand, if marketing authorisation for a veterinary medicinal product has been already granted in some member state in northern Europe then mutual recognition procedure (MRP) is the right choice. The member state, where the veterinary medicinal product is already marketed, would be the RMS. A competent authority of this RMS prepares or eventually updates the assessment report regarding this veterinary medicinal product. Other member states included to MRP, where the marketing authorisation applicant wants to expand the marketing authorisation of veterinary medicinal product are CMS [4]. The last possibility of marketing authorisation procedure is centralised procedure. In this case, a marketing authorisation application form is submitted only to EMA but not to individual competent authorities of the member states. An assessment is carried out by the committee for medicinal products for veterinary use falling under EMA. Thus issued marketing authorisation is valid in the all member states of the community. Using the centralised procedure is mandatory for some veterinary medicinal products and in some cases the marketing authorisation applicant can choose between centralised or the other procedure. But it is not possible that every veterinary medicinal product could be authorised by the centralised procedure. For using centralised procedure, the clear rules stated in the regulation (EC) no: 726/2004 are applicable [5].
In all the marketing authorisation procedures, a marketing authorisation applicant has to pay appropriate marketing authorisation fees. The fees are paid to the competent authority of the member state in the case of national procedure or to the competent authorities of all CMSs and to the competent authority of RMS in the case of MRP and DCP. But in the centralised procedure the marketing authorisation fee is paid only to the EMA instead of to all competent authorities of the member states. The amount of the marketing authorisation fees is depended on the type of procedure and whether the member state is in the position of RMS or CMS (MRP and DCP). In most states in Northern Europe the application type (full application according to Article 12 (3) of Directive 2001/82/EC; generic application according to Article 13 (1); hybrid application according to Article 13 (3); similar biological application according to Article 13 (4); well-established use application according to Article 13a; fixed combination application according to Article 13b; informed consent application according to Article 13c is also an important parameter for determining the amount of the marketing authorisation fee. Typical states where on the application type is not taken into account are Estonia, Lithuania and Latvia. Particular competent authorities of Northern European states have their own approach to determination of marketing authorisation fees and the amount of these fees is also independent on other member states of the community.
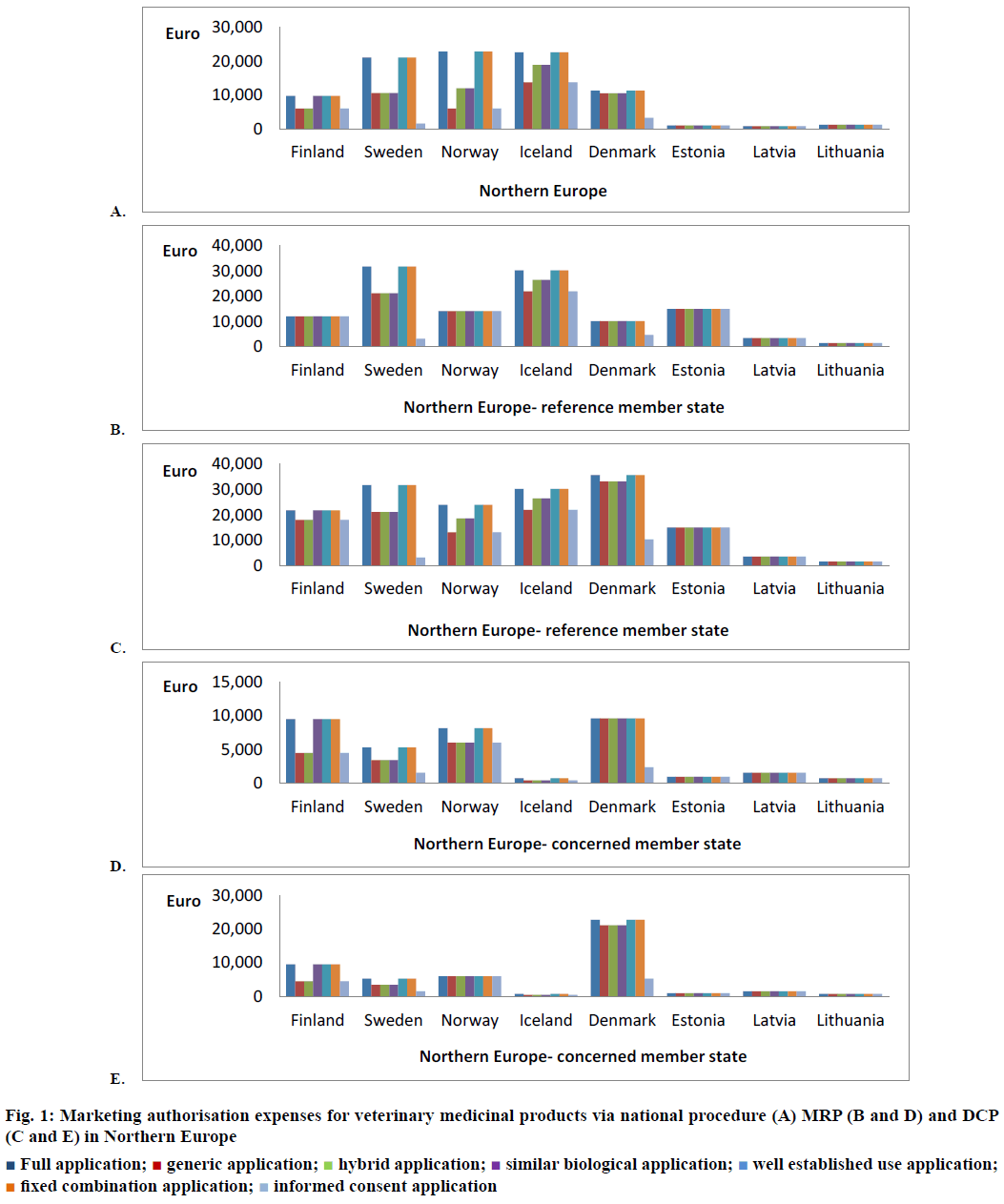
If a marketing authorisation applicant wants to authorise a veterinary medicinal product only in Finland and marketing authorisation of this medicinal product is not granted in any other state of the EEA, then a national procedure is requested. The national marketing authorisation fee, which applicant has to pay is depended on the application type. In the case of full, similar biological, well-established use and fixed combination application, the national fee is 9750 €. But for the generic, hybrid or informed consent application, this fee is 6000 € (Figure 1A). If an applicant wants to apply for marketing authorisation of several strengths or pharmaceutical forms of this veterinary medicinal product simultaneously then the marketing authorisation fee is 6000 € for each additional strength/pharmaceutical form independently on the application type. When one compares these marketing authorisation fees for veterinary and human medicinal products, one finds that marketing authorisation costs for human medicinal product are higher than costs for the veterinary medicinal product. For the medicinal product for human use the national fees are 13 000 €, 8000 € for generic applications and 8000 € for each additional strength or pharmaceutical form submitted at one moment [6]. In MRP, fees is crucial whether Finland is RMS (veterinary medicinal product is marketed in Finland and applicant wants to expand the marketing authorisation to other states of the EEA) or CMS (veterinary medicinal product is marketed in the other states of the EEA and applicant wants to have granted marketing authorisation for this product also in Finland). The only case when Finnish competent authority does not distinguish the fees between application types is when Finland is RMS in the MRP. In this case the marketing authorisation fee is 12 000 € (Figure 1B) irrespective of the application type and type of the medicinal product (veterinary or human). All strengths and pharmaceutical forms of the medicinal product are included in this fee unlike national procedure. In the case that Finland is only CMS in the MRP the marketing authorisation fee is lower than when Finland is RMS. Moreover, the division by application type is here applied. The full, wellestablished use, fixed combination or similar biological application are valued at 9500 € and generic, hybrid and informed consent application at 4500 € (Figure 1D) [6].
Figure 1: Marketing authorisation expenses for veterinary medicinal products via national procedure (A) MRP (B and D) and DCP (C and E) in Northern Europe
 Full application;
Full application;  generic application;
generic application;  hybrid application;
hybrid application;  similar biological application;
similar biological application;  well established use application;
well established use application;  fixed combination application;
fixed combination application;  informed consent application
informed consent application
Finland can be included to the DCP when the marketing authorisation applicant decides that a veterinary medicinal product, which is not marketed in any state of the EEA will be introduced in the market in Finland and some other member states. If Finland is chosen as RMS, which will then prepare the whole assessment report regarding this veterinary medicinal product, then fee will be 21 750 € (18 000 € for generic, hybrid and informed consent application; Figure 1C). This is the highest marketing authorisation fee of the Finnish competent authority and into this fee the marketing authorisations of the all strengths and pharmaceutical forms of this veterinary medicinal product, which were submitted at the same moment are included (same as Finland is the RMS in MRP). If Finland is the only CMS in the DCP then marketing authorisation fees are the same as fees paid in the case that Finland is CMS in the MRP (9500 € and 4500 €; Figure 1E) [6]. In comparison with marketing authorisation fees for human medicinal products the veterinary fees are lower except in the case when Finland is RMS in the MRP. In this single case the fee is same for veterinary and human medicinal product (12 000 €). In both types of medicinal product same principles are applied and only amounts of the marketing authorisation fees are different [6].
In Sweden marketing authorisation fees for the veterinary medicinal products are substantially higher than in Finland but within this fee, in Sweden the marketing authorisations of all strengths, pharmaceutical forms and routes of administration of this veterinary medicinal product, which were submitted simultaneously are included (it also applies to human medicinal products). In the case of full, wellestablished use and fixed combination application in the national procedure, the marketing authorisation fee is 21 142 €. Fifty percent of this fee (10 571 €) is requested for generic, hybrid and similar biological applications (Figure 1A). In these application types, the competent authority of Sweden distinguishes whether veterinary reference medicinal product is authorised in Sweden or it is a European veterinary reference medicinal product. If marketing authorisation of the veterinary reference medicinal product has not been granted in Sweden (European veterinary reference medicinal product) then national marketing authorisation fee is the same as for full application (21 142 €). Between states of Northern Europe this aspect in the marketing authorisation fees is taken into account only in Sweden. The costs of the informed consent application are substantially lower than other application types, it is only 1586 € (Figure 1A) [7]. The competent authority of Sweden does not distinguish the marketing authorisation fees whether it is the RMS in MRP or DCP, the fee is 31 712 € for full applications and 21 142 € for generic applications (Figure 1B and C). Likewise, the authority does not distinguish whether Sweden is the CMS in MRP or DCP. The marketing authorisation fees when Sweden is CMS are substantially lower than it is RMS. In the case of full, well-established use and fixed combination application this fee is 5285 € (RMS 31 712 €) and for generic, hybrid and similar biological application 3436 € (RMS 21 142 €; Figure 1D and E). The very low fees are for the informed consent application i.e. 3172 € in the position of RMS and 50 % of this fee (1586 €) for CMS. In the MRP and DCP, the generic, hybrid and similar biological application is also different whether the veterinary reference medicinal product is marketed in Sweden or it is European veterinary reference medicinal product. If it is the European veterinary reference medicinal product then marketing authorisation fees is the same as for appropriate full application (31 712 € in the position of RMS and 5285 € in the position of CMS). In all these fees regarding MRP and DCP the marketing authorisations of other strengths, pharmaceutical forms and routes of administration of this veterinary medicinal product, which were submitted at one moment are included [7].
Any marketing authorisation fee valid for the veterinary medicinal product corresponds 50 % of appropriate fee for the human medicinal product. In Sweden the marketing authorisation of human medicinal products follows the same rules as for veterinary medicinal products (the marketing authorisation fee for the generic application is 50 % of the fee for full application, the distinction whether veterinary reference medicinal product is or is not marketed in Sweden, the marketing authorisation fee for the informed consent application is substantially lower than for other application types, the fees include marketing authorisations of all strengths, pharmaceutical forms and routes of administration of the medicinal product, which were submitted at the same moment [7].
As mentioned in the introduction Norway is not the member of European Union but belongs to the EEA and there are the same possibilities of marketing authorisation procedures. Norwegian competent authority divides the national fees into three groups. Into the first group the full, well-established use and fixed combination applications are placed and this marketing authorisation fee is 22 888 €. The other fee is 11 989 €, which is valid for hybrid and similar biological applications. The lowest national marketing authorisation fee is in the case of generic and informed consent applications and amounts to 5994 € (Figure 1A) [8]. The only marketing authorisation fee in Norway, which is not distinguished according to application type is when Norway is the RMS in MRP (the same in Finland). The marketing authorisation fee 14 169 € has to be paid irrespective of application type (Figure 1B). This fee is related to the case when Norway is RMS in MRP the first time for this veterinary medicinal product. If it is a repeat use procedure and the competent authority updates the prepared assessment report, then this fee is only 8719 €. For generic veterinary medicinal products this fee (RMS in MRP) is higher than Norway is RMS in the DCP (14 169 € MRP versus 13 079 € DCP). For this reason Norway is a unique case because other states in Northern Europe have this DCP fee higher than in the MRP or alternatively the same in as much as the marketing authorisation of veterinary medicinal product has not been granted in any country of the EEA yet. This exception relates only to the generic veterinary medicinal product and veterinary medicinal product submitted on the basis of informed consent application. Other application types confirm this rule and DCP fees are higher than in the MRP. The marketing authorisation applicant has to pay for full, well-established use or fixed combination application 23 978 € and for hybrid or similar biological application 18 528 € (Figure 1C) [8]. If Norway is only the CMS in DCP then the fee 5994 € is requested independently on the application type (Figure 1E). When Norway is CMS in the MRP then the fee is the same as in the DCP (5994 €) except of the full, well-established use and fixed combination application when this fee is 8174 € (Figure 1D). It is not common that marketing authorisation fee paid to CMS in the MRP is higher than in the case of the DCP. So even in this Norway has the unique approach [8].
The marketing authorisation fees for veterinary medicinal products are substantially lower than fees for the human medicinal products. The national marketing authorisation fee for the first group of applications (full, well-established use and fixed combination application) is for human medicinal products 45 213 €, for the second group of applications (hybrid and similar biological application) 19 618 € and for the third group of applications (generic and informed consent application) 17 438 €. As well as in the veterinary medicinal products the human fee in the MRP with Norway in the position of RMS is the same for the all application types but it is higher than in veterinary medicinal products (16 348 €). In the DCP with Norway like RMS the marketing authorisation fees are 45 776 €, 23 978 € and 20 708 € so always higher than in the case of RMS in the MRP which is different from veterinary medicinal products. The marketing authorisation fee with Norway in the position of CMS is in the all application types in DCP higher than in the MRP (22 888 €, 17 438 € for the DCP versus 16 893 €, 13 624 € and 11 988 € for the MRP) [8].
In Norway the marketing authorisations of other strengths, pharmaceutical forms and routes of administration of the medicinal product (veterinary and also human) are not included into these basic fees like it is in Sweden. The marketing authorisation applicant has to pay 5450 € for each additional strength/pharmaceutical form of the veterinary medicinal product and 10 900 € or 13 079 € (RMS in DCP) for each additional strength/pharmaceutical form of the human medicinal product independently on the application type [8].
Iceland as well as Norway do not belong to the European Union but has taken over the marketing authorisation legislation of the European Union. From the all states in northern Europe the Iceland has the highest national marketing authorisation fees for the veterinary medicinal products. There are three levels of the fees the same as in Norway. The highest marketing authorisation fee is 22 675 € (very similar in Norway 22 888 €) for the full, well-established use and fixed combination application. Followed fee for the hybrid, similar biological application (18 896 €) and the lowest national fee is for the generic and informed consent application (13 756 €; Figure 1A). If the marketing authorisation applicant would like to include to the marketing authorisation process the other pharmaceutical form/strength of the veterinary medicinal product then has to pay 1512 € for each variant [9]. The marketing authorisation fees in the DCP and MRP are identical. In comparison with other States in northern Europe the fees when Iceland is RMS in the MRP/DCP are among the highest and for the full, wellestablished use and fixed combination application this fee is 30 233 €, for the hybrid and similar biological application 26 454 € and for the generic and informed consent application 21 919 € (Figure 1B and C). But difference between MRP and DCP fee is that in the case of MRP the national marketing authorisation fee, which had to be paid during the marketing authorisation process in Iceland is deducted from this MRP fee. Inclusion the other strength or pharmaceutical form of the veterinary medicinal product into this marketing authorisation process is determined on 2645 € [9]. If Iceland is only the CMS the fees are also identical in the MRP and DCP. In contrast with other marketing authorisation fees which are the highest in Northern Europe the Icelandic CMS fees are the lowest in northern Europe. Specifically, it is 454 € (generic, hybrid, similar biological and informed consent application) and 756 € (full, well-established use and fixed combination application; Figure 1D and E). Even an extra fee for the additional strength/pharmaceutical form of the veterinary medicinal product is very low (189 €) [9].
The competent authority of Iceland has the marketing authorisation fees for the veterinary medicinal products the same as for the human medicinal products. The only exception is when Iceland is the CMS and in this case the fees for the human medicinal product are higher than for the veterinary medicinal product (2268 € or 1965 € for human versus 756 € or 454 € for veterinary) [9].
Denmark is the State in northern Europe, which has the smallest difference between national marketing authorisation fee for the full application (11 269 €) and generic application (10 506 €; Figure 1A). This difference is only 763 €. The marketing authorisation fee 10 506 € corresponds also to hybrid and similar biological application. A far smaller national marketing authorisation fee is paid for the informed consent application (3219 €). If the marketing authorisation applicant would like to include to the national marketing authorisation process the other pharmaceutical form/ strength of the veterinary medicinal product then has to paid 2396 € for each other pharmaceutical form and 2219 € for each other strength [10]. The marketing authorisation fee in the position of RMS in the MRP is slightly lower than in the case of national procedure and it is the same for all application types (10 146 €) excluding informed consent application (4669 €; Figure 1B). Differences between MRP costs when Denmark is RMS and CMS are minimal. Denmark as CMS requires 9609 € (2383 € informed consent application; Figure 1D) it follows that only 537 € less than Denmark is the RMS in MRP. And in addition into the CMS fees are not included the marketing authorisations of other strengths or pharmaceutical forms of the veterinary medicinal product, which were submitted at one moment. Each additional strength or pharmaceutical form of the veterinary medicinal product means an extra fee 2202 € [10].
If the marketing authorisation applicant has chosen Denmark as RMS in the DCP then has to pay the highest fee from the all states in northern Europe. The marketing authorisation fee for the veterinary medicinal products with full marketing authorisation dossier is 35 732 €, for generic, hybrid and similar biological medicinal products it is 33 165 €. Only the informed consent application does not belong among the most expensive of this type in northern Europe (only 10 281 €; Figure 1C). It is necessary to mention that into these fees all strengths and pharmaceutical forms of this veterinary medicinal product, which have been submitted all at once are included. CMS fees in the DCP also belong among the highest in northern Europe (even several folds) and these fees are even higher than national fees or fees when Denmark is RMS in the MRP. In the case of full, well established use and fixed combination application this fee is 22 726 €, for generic, hybrid and similar biological application 21 110 € and for informed consent application only 5277 € (Figure 1E). These high fees also include the all pharmaceutical forms and strengths of this veterinary medicinal product [10]. Denmark as the only State in northern Europe has all marketing authorisation fees the same for veterinary and human medicinal products. Iceland has the identical fees for veterinary and human medicinal products only in the national procedure and when Iceland is the RMS in MRP or DCP [9,10].
Baltic states are also assigned to northern Europe. Valid marketing authorisation fees for the veterinary medicinal products in these Baltic States are mostly substantially lower than in other States in northern Europe. For Baltic States it is very important to mention that fees are not divided according to application type. The marketing authorisation fee is the same for original, generic or hybrid veterinary medicinal product. If marketing authorisation applicant has chosen the national procedure then has to pay 990 € in Estonia, 750 € in Latvia or 1199 € in Lithuania (Figure 1A). These fees are the lowest national fees for the veterinary medicinal products in Northern Europe. Regarding Baltic States it is also important to mention that the marketing authorisation fees are the same if this state is RMS in the MRP or DCP. Differences between MRP and DCP are also not in fees when the state is CMS. As the only Estonia in the position RMS has several times higher fee than in the national procedure (14 990 € versus 990 €). But competent authority of Estonia in MRP takes into account whether assessment report which was prepared during previous MRP for this veterinary medicinal product is updated and for the repeat use procedure in MRP only 3990 € is required. When Latvia or Lithuania is the RMS this difference is not so rapid (Latvia RMS in MRP/DCP 3520 € versus national procedure 750 €; Lithuania RMS in MRP/DCP 1500 € versus national procedure 1199 €; Figure 1B and C). If Baltic States are only the CMSs in the MRP or DCP then they do not represent a large financial burden for marketing authorisation applicants. After Iceland the Baltic States have these fees the lowest from Northern Europe. The Estonian competent authority requests 990 €, Latvian authority 1565 € and Lithuanian agency 750 € (Figure 1D and E) [11-13].
Into all these fees the marketing authorisation for one strength and one pharmaceutical form of the veterinary medicinal product is included. In Estonia 511 € is paid for each additional strength or pharmaceutical form submitted at one moment irrespective of used type of the marketing authorisation procedure. In Latvia this fee is divided according to type of the procedure and whether additional strength or pharmaceutical form is added. The other pharmaceutical form represents the expense 320 € and the other strength 235 € in the national procedure. If Latvia is the RMS in MRP or DCP these fees are 2740 € (pharmaceutical form) a 2480 € (strength). In the case of CMS in MRP or DCP it is 785 € (pharmaceutical form) and 525 € (strength). The attitude of Lithuania is somewhere between Estonia and Latvia because the competent authority distinguishes only the type of the procedure but no whether the additional strength or pharmaceutical form is added. In the national procedure appropriate fee is 600 €, in MRP or DCP in the position of RMS 750 € and CMS 375 € [11-13].
In all these Baltic States marketing authorisation fees for the veterinary medicinal products are mostly lower than for the human medicinal products. In Estonia the veterinary product’s fees correspond with human fees only for the generic, hybrid and similar biological medicinal products. In Latvia each marketing authorisation fee for the veterinary medicinal product is lower than for the human medicinal product without any exception. In Lithuania the fees for medicinal products for veterinary use are also lower than for medicinal products for human use but there is the one exception when Lithuania is the CMS in MRP or DCP and it is generic, hybrid or similar biological medicinal product or it is informed consent application. Only in these cases the marketing authorisation fee for the veterinary medicinal product is higher than for the human medicinal product [11-14].
The Northern Europe represents a very diverse group of the states. Over Scandinavian countries belonging to the European Union (Finland, Sweden), economically advanced countries of the EEA, which not belonging to European Union but have accepted the European legislation (Norway, Iceland), Denmark, to the countries of the former Union of Soviet Socialist Republics (Baltic States-Estonia, Latvia, Lithuania). This diversity and economic level are clearly seen in the amount of required marketing authorisation fees for the veterinary medicinal products.
Mostly national fee is between the fee when the state is RMS and when it is in the position of CMS in MRP or DCP. If you wanted to compare the fees when the state is RMS in the MRP and DCP then the Northern European States could be divided into two groups. The first group are the states which do not distinguish if they are the RMS in MRP or DCP and the fees are still same (Sweden, Iceland, Estonia, Latvia, Lithuania). The second group is covered the states in Northern Europe where the fees in the case of DCP are higher than in the MRP (Finland, Norway, Denmark). In Norway only one exception exists when the marketing authorisation of the veterinary medicinal product is requested based on generic application or informed consent application then this fee is higher in the case of MRP. If attention is given to States in Northern Europe in the position of CMS in MRP and DCP then the most of these states (Finland, Sweden, Iceland, Estonia, Latvia, Lithuania) do not have any difference between these two fees. The other approach is in Denmark (CMS) where the marketing authorisation fee in DCP is higher than in MRP and even substantially. Absolute exception of these fees is Norway which for full, well-established use and fixed combination applications the MRP fee has higher than DCP fee. However, in other application types is conformity between MRP and DCP. In general CMS marketing authorisation fees are lower than when the same member state is RMS and this applies to MRP and also DCP. The difference between fee when the member state is RMS and CMS can be minimal but also can be huge. The biggest difference between these fees is seen in Iceland which as the RMS has even some of the highest fees in Northern Europe and on the other hand as CMS has the lowest fees in Northern Europe.
If the marketing authorisation fees are compared for veterinary and human medicinal products in Northern Europe then it will find out that the one state has the same fees for veterinary and human medicinal products (Denmark), some states have the fees for human medicinal products higher than for veterinary medicinal products (Sweden, Norway and Latvia) and the third group are created by states, which have some fees for human medicinal products higher than for veterinary medicinal products and some fees are the same for both. The combination of these two principles is in Finland, Iceland, Estonia and Lithuania.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- European Commission. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. Official J Eur Union 2001;311:67-128.
- European Commission. Directive 2001/82/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to veterinary medicinal products. Official J Eur Union 2001;311:1-66.
- European Commission. Notice to Applicants Veterinary Medicinal Products. Volume 6A. Procedures for marketing authorisation, Chapter 1 Marketing Authorisations; 2007. p. 1-41.
- European Commission. Notice to Applicants Veterinary Medicinal Products, Volume 6A Procedures for marketing authorisation, Chapter 2 Mutual Recognition Procedure and Decentralised Procedure; 2005. p. 1-35.
- European Commission. Regulation (EC) No 726/2004 of the European Parliament and of the Council of 31 March 2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency. Official J Eur Union 2004;136:1-70.
- http://www.fimea.fi/documents/542809/841791/Maksuasetus+2016_EN.pdf/3cf7403a-5578-4918-82ad-e9f45c0f2bd3. Accessed on Apr 17, 2016.
- https://lakemedelsverket.se/english/product/Medicinal-products/Fees--/. Accessed on Apr 17, 2016.
- https://legemiddelverket.no/english/regulatory-affairs/regulatory-fees. Accessed on Apr 17, 2016.
- http://www.ima.is/media/Leyfisveitingar_lyfja/Tariff_No__635_2011_translation.pdf. Accessed on Apr 17, 2016.
- http://laegemiddelstyrelsen.dk/en/licensing/fees. Accessed on May 17, 2016.
- http://www.sam.ee/en/state-fees. Accessed on May 17, 2016.
- http://www.zm.gov.lv/en/partikas-un-veterinarais-dienests/statiskas-lapas/authorisation-of-veterinary-medicines/price-list-and-payment-application?nid=2344#jump.
- http://www.vvkt.lt/Fees. Accessed on May 17, 2016.
- https://www.zva.gov.lv/doc_upl/SAM-pricelist-20160108.pdf. Accessed on Jun 04, 2016.