- *Corresponding Author:
- Zhangxia Ren
Department of Breast Surgery,
Huaxi Hospital of Sichuan University,
Chengdu,
Sichuan 610093,
China
E-mail: renzhangxia2022@163.com
| Date of Received | 18 September 2020 |
| Date of Revision | 10 August 2021 |
| Date of Acceptance | 01 April 2022 |
| Indian J Pharm Sci 2022;84(2):477-482 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect of long non coding RNA MAGI2-antisense RNA 3 on the proliferation, invasion and migration of cervical cancer cells and the underlying mechanism. SiHa cells were divided into plasmid cloning DNA group, plasmid cloning DNA-MAGI2-antisense RNA 3 group, anti-microRNA-negative control group, anti-microRNA-31-5p group, plasmid cloning DNA-MAGI2-antisense RNA 3+microRNAnegative control group and plasmid cloning DNA-MAGI2-antisense RNA 3+microRNA-31-5p group; quantitative reverse transcription polymerase chain reaction was used to determine the messenger RNA expression levels of microRNA-31-5p and MAGI2-antisense RNA 3; cell viability was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide assay; the number of cell migration and invasion in each group was detected by Transwell assay; dual luciferase reporter assay was performed to detect the fluorescence activity. The expression levels of microRNA-31-5p were significantly increased and those of MAGI2-antisense RNA 3 messenger RNA were decreased in cervical cancer cell lines (p<0.05); overexpression of MAGI2-antisense RNA 3 or inhibition expression of microRNA-31-5p could suppress SiHa cell proliferation, migration and invasion (p<0.05). MAGI2-antisense RNA 3 targeted regulation of microRNA-31-5p; overexpression of microRNA-31-5p partially reversed the inhibitory effects of overexpressing MAGI2-antisense RNA 3 on the proliferation, migration, invasion of SiHa cells. Long non coding RNA MAGI2-antisense RNA 3 can inhibit cervical cancer cell proliferation, migration and invasion by regulating microRNA-31-5p.
Keywords
Long non coding RNA MAGI2-antisense RNA 3, microRNA-31-5p, cervical cancer, proliferation, migration, invasion
Cervical cancer is one of the common malignancies in women and its incidence is gradually increasing. Although treatment modalities such as surgery, radiotherapy and chemotherapy have improved the survival time and quality of life of patients, it is still more difficult to clinically treat advanced and recurrent forms of cervical cancer; with the development of molecular biology, targeted therapy research has made breakthrough progress, aiming at improving the efficacy and reducing toxic side effects[1]. Long non coding RNA (LncRNA) and microRNA (miRNA) belong to the same class of lncRNAs, which do not encode proteins but play important roles in the normal physiological functions of the body; it may serve as a potential therapeutic target for cervical cancer. Studies have reported that lncRNA MAGI2 Antisense RNA 3 (MAGI2-AS3) inhibits breast cancer cell growth by targeting the Fas Cell Surface Death Receptor/Fas Ligand (Fas/FasL) signaling pathway[2]; in addition, MAGI2-AS3 can also inhibit the migration and invasion of breast cancer cells by sponging miR-374a[3]. Studies have found that aberrant expression of miRNAs is associated with cervical cancer metastasis and differentiation[4]. Studies have reported that inhibition of miR-31-5p targets AXIN1 to activate Wingless-related integration site (Wnt)/ beta (β)-catenin signaling pathway, thereby inhibiting the proliferation, invasion, and tumorigenicity of osteosarcoma cells[5]. The proliferation and migration ability of cervical cancer HeLa cells of overexpressing miR-31 was obviously enhanced[6]. However, the effects of MAGI2-AS3 and miR-31-5p on cervical cancer proliferation, migration and invasion and whether MAGI2-AS3 regulates cervical cancer cell proliferation, migration and invasion by targeting miR- 31-5p are unknown. The present experiment aimed to investigate whether lncRNA MAGI2-AS3 affects cervical cancer progression by regulating miR-31-5p.
Materials and Methods
Materials:
Cervical cancer cells SiHa, CaSki, HeLa and normal cervical Ectocervical Epithelium (Ect1/E6E7) cells were purchased from Shanghai Cell Bank, Chinese Academy of Sciences; Roswell Park Memorial Institute (RPMI)-1640 medium was purchased from Guangzhou Taylor Biological Technology Co., Ltd.; the fluorescence quantitative Polymerase Chain Reaction (PCR) kit was purchased from Shanghai Kemin Biotechnology Co., Ltd.; dual luciferase reporter assay kit was purchased from GeneCopoeia, USA; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) kit was purchased from Shanghai Meixuan Biological Science and Technology Co., Ltd.; Transwell chambers and Matrigel were purchased from Shanghai Yubo Biological Technology Co., Ltd. and Radio Immunoprecipitation Assay (RIPA) protein lysate was purchased from Beyotime Biotech Inc., Shanghai.
Methods:
Cell transfection and grouping: Cervical cancer cells SiHa, CaSki, HeLa and normal cervical cells Ect1/E6E7 were routinely cultured in RPMI-1640 medium; SiHa cells were divided into plasmid cloning DNA (pcDNA) group (transfected with pcDNA), pcDNA-MAGI2- AS3 group (transfected with pcDNA-MAGI2-AS3), anti-miR-Negative Control (NC) group (transfected with anti-miR-NC), anti-miR-31-5p group (transfected with anti-miR-31-5p), pcDNA-MAGI2-AS3+miR-NC group (co-transfected with pcDNA-MAGI2-AS3 and miR-NC) and pcDNA-MAGI2-AS3+miR-31-5p group (co-transfected with pcDNA-MAGI2-AS3 and miR- 31-5p).
Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR) detection of miR-31-5p and MAGI2-AS3 messenger RNA (mRNA) expression levels:
Total cellular RNA was extracted, reversely transcribed into pcDNA and the reaction system was set up according to the instruction of fluorescence quantitation kit, U6 and β-actin was used as an internal reference for PCR amplification and relative expression was calculated by the 2-△△Ct method.
Western blot for protein expression:
Cells in each group were added RIPA lysis solution to extract total protein, subjected to Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDSPAGE), transferred to membrane, blocked with skim milk powder; primary and secondary antibodies were added separately for incubation, exposure in a dark room for development, fixation imaging and analysis of absorbance of protein bands.
MTT assay for cell proliferation:
Cells were incubated for 24 h, 48 h and 72 h, and the absorbance/Optical Density (OD) value at 490 nm was measured according to the instructions of the kit.
Transwell assay for cell migration and invasion:
Migration: The upper chamber of the Transwell was added with cell suspension, the lower chamber was added with culture solution, incubated at 37° for 24 h, the chamber was taken out, the culture solution was removed, 0.1 % crystal violet was stained for 10 min and five fields were randomly selected for counting under the microscope.
Invasion: Matrigel was diluted, added to the upper chamber of the Transwell and dried for the co-migration assay.
Luciferase reporter gene assay:
Luciferase expression vectors Wild-Type (WT)- MAGI2-AS3 and Mutant (MUT)-MAGI2-AS3 were constructed, and log phase SiHa cells were harvested and transfected with miR-NC and miR-31-5p into SiHa and dual luciferase assays were performed according to the instructions.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 20.00 was used for statistical analysis. Measurement data were expressed as x̄±s. t test was performed for comparison between two groups. Single factor analysis of variance was used for comparison among multiple groups. p<0.05 was used for statistical significance.
Results and Discussion
The expression levels of MAGI2-AS3 mRNA were significantly decreased and those of miR-31-5p were increased in cervical cancer cells SiHa, CaSki and HeLa compared with normal cervical cells ECT1/E6E7 (p<0.05) as shown in Table 1.
| Group | MAGI2-AS3 | miR-31-5p |
|---|---|---|
| ECT1/E6E7 | 1.03±0.09 | 1.00±0.09 |
| SiHa | 0.32±0.03* | 2.76±0.27* |
| CaSki | 0.56±0.05* | 2.98±0.29* |
| HeLa | 0.43±0.04* | 2.81±0.28* |
| F | 268.305 | 127.810 |
| p | 0.000 | 0.000 |
Note: *p<0.05, compared with the ECT1/E6E7 group
Table 1: MAGI2-AS3 and MIR-31-5P Expression in Cervical Cancer Cells and Normal Cervical Cells (x̄±s, n=9)
TargetScan predicted the presence of a binding site for MAGI2-AS with miR-31-5p as shown in fig. 1. Luciferase activity of SiHa was significantly reduced in cells co-transfected with WT-MAGI2-AS and miR- 31-5p (p<0.05); however, there was no significant difference in the luciferase activity of SiHa cells cotransfected with MUT-MAGI2-AS and miR-31-5p as shown in Table 2. The miR-31-5p expression level decreased in SiHa after overexpression of MAGI2-AS; the expression level of miR-31-5p in cell SiHa was increased after suppression of MAGI2-AS expression (p<0.05) as shown in Table 3. It was seen that MAGI2-AS could target to regulate the expression of miR-31-5p.
| Group | WT-MAGI2-AS3 | MUT-MAGI2-AS3 |
|---|---|---|
| MiR-NC | 1.04±0.09 | 1.03±0.08 |
| MiR-31-5p | 0.38±0.04* | 1.01±0.09 |
| t | 20.104 | 0.498 |
| p | 0.000 | 0.625 |
Note: Compared with miR-NC group, *p<0.05
Table 2: Dual Luciferase Reporter Assay (x̄±s, n=9)
| Group | miR-31-5p |
|---|---|
| pcDNA | 1.01±0.09 |
| pcDNA-MAGI2-AS3 | 0.48±0.04# |
| si-NC | 1.00±0.08 |
| si-MAGI2-AS3 | 2.87±0.28& |
| F | 419.683 |
| p | 0.000 |
Note: si-NC: small interfering-NC and si-MAGI2-AS3: small interfering-MAGI2-AS3, compared with pcDNA group, #p<0.05 and compared with the si-NC group, &p<0.05
Table 3: MAGI2-AS3 Regulates miR-31-5P (x̄±s, n=9)
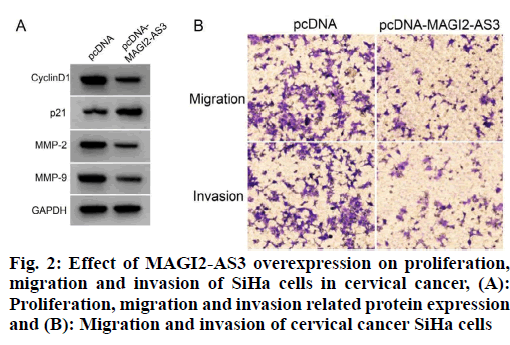
The expression level of MAGI2-AS3 in SiHa cells was significantly increased in the pcDNA-MAGI2-AS3 group compared with the pcDNA group; the activity of SiHa cells was significantly decreased, the expression levels of cyclin D1, Matrix Metalloproteinase-2 (MMP-2) and Matrix Metalloproteinase-9 (MMP-9) proteins were significantly decreased, the expression level of p21 was significantly increased and the migration and invasion numbers of SiHa cells were decreased (p<0.05) as shown in fig. 2 and Table 4. It can be seen that MAGI2-AS3 overexpression inhibited the proliferation, migration and invasion of SiHa in cervical cancer cells.
| Group | MAGI2-AS3 | Cell viability (OD 490 nm) | Migration cell number | Invasive cell number | CyclinD1 protein | p21 protein | MMP-2 protein | MMP-9 protein |
||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||||||||
| pcDNA | 0.99±0.08 | 0.29±0.03 | 0.51±0.05 | 0.68±0.06 | 165.28±13.25 | 141.26±11.73 | 0.78±0.07 | 0.26±0.03 | 0.65±0.06 | 0.72±0.07 |
| pcDNA-MAGI2-AS3 | 2.93±0.28* | 0.26±0.03 | 0.32±0.03* | 0.45±0.04* | 79.29±7.35* | 62.45±6.38* | 0.36±0.03* | 0.61±0.06* | 0.28±0.03* | 0.33±0.03* |
| t | 19.986 | 2.121 | 9.775 | 9.569 | 17.025 | 17.706 | 16.545 | 15.653 | 16.547 | 15.363 |
| p | 0.000 | 0.05 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 when compared with pcDNA group
Table 4: Effects of MAGI2-AS3 Overexpression on Cell Proliferation, Migration and Invasion in Siha Cells (x̄±s, n=9)
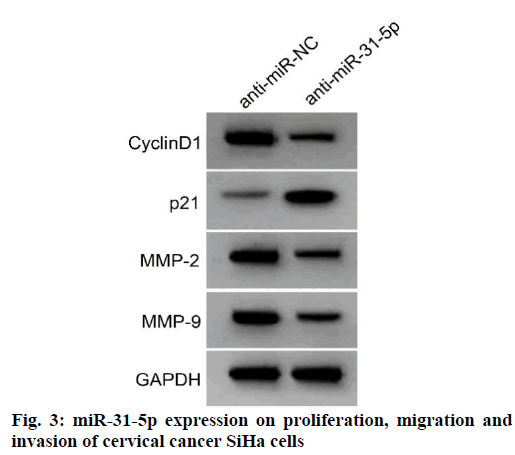
Compared with the anti-miR-NC group, the expression levels of miR-31-5p in SiHa cells were significantly decreased in the anti-miR-31-5p group, the expression levels of cyclin D1, MMP-2 and MMP-9 proteins were significantly decreased, the expression level of p21 was significantly increased, the activity of SiHa cells was significantly decreased and the number of migration and invasion in SiHa cells was significantly decreased (p<0.05) as shown in fig. 3 and Table 5. It can be seen that inhibition of miR-31-5p expression inhibited the proliferation, migration and invasion of SiHa in cervical cancer cells.
| Group | miR-31-5p | Cell viability (OD490 nm) | Migration cell number | Invasive cell number | CyclinD1 protein | p21 protein | MMP-2 protein | MMP-9 protein | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||||||||
| Anti-miR-NC | 1.03±0.09 | 0.28±0.03 | 0.53±0.05 | 0.69±0.06 | 153.28±14.05 | 138.25±10.97 | 0.77±0.07 | 0.25±0.03 | 0.66±0.06 | 0.73±0.07 |
| Anti-miR-31-5p | 0.42±0.04* | 0.27±0.03 | 0.39±0.03* | 0.48±0.04* | 86.38±8.64* | 75.38±7.95* | 0.39±0.03* | 0.57±0.05* | 0.31±0.03* | 0.36±0.03* |
| t | 18.581 | 0.707 | 7.203 | 8.737 | 12.168 | 13.922 | 14.989 | 16.464 | 15.653 | 14.575 |
| p | 0 | 0.49 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Note: Compared with anti-miR-NC group, *p<0.05
Table 5: Effects of Inhibiting miR-31-5P Expression on Proliferation, Migration and Invasion of Cervical Cancer Siha Cells (x̄±s, n=9)
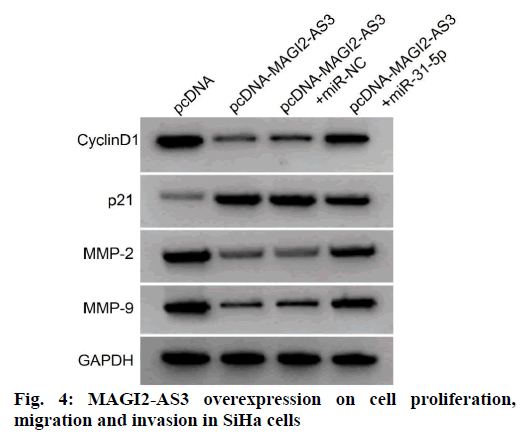
Compared with the pcDNA-MAGI2-AS3+miR-NC, the pcDNA-MAGI2-AS3+miR-31-5p group showed significantly higher levels of miR-31-5p expression in SiHa cells, higher levels of cyclin D1, MMP-2 and MMP-9 protein expression, lower levels of p21, and higher activities in SiHa cells, and higher amounts of SiHa cell migration and invasion (p<0.05) as shown in fig. 4 and Table 6. It can be seen that miR-31-5p overexpression reversed the suppressive effects of MAGI2-AS3 overexpression on the proliferation, migration and invasion of SiHa cells of cervical cancer.
| Group | miR-31-5p | Cell viability (OD490 nm) | Migration cell number | Invasive cell number | CyclinD1 protein | p21 protein | MMP-2 protein | MMP-9 protein | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||||||||
| pcDNA | 0.99±0.09 | 0.30±0.03 | 0.54±0.04 | 0.71±0.06 | 146.28±11.87 | 123.65±9.28 | 0.79±0.07 | 0.24±0.03 | 0.67±0.06 | 0.71±0.06 |
| pcDNA-MAGI2-AS3 | 0.45±0.04* | 0.28±0.03 | 0.33±0.03* | 0.46±0.04* | 76.35±7.06* | 58.32±5.27* | 0.37±0.03* | 0.59±0.05* | 0.29±0.03* | 0.32±0.03* |
| pcDNA-MAGI2-AS3+miR-NC | 0.42±0.04 | 0.27±0.02 | 0.31±0.03 | 0.42±0.04 | 73.08±7.15 | 55.97±5.36 | 0.34±0.03 | 0.61±0.06 | 0.27±0.03 | 0.30±0.03 |
| pcDNA-MAGI2-AS3+miR-31-5p | 0.87±0.08# | 0.29±0.03 | 0.45±0.04# | 0.63±0.05# | 126.38±9.22# | 108.65±8.66# | 0.67±0.06# | 0.36±0.03# | 0.54±0.05# | 0.58±0.05# |
| F | 171.305 | 1.936 | 83.7 | 73.419 | 146.838 | 198.341 | 172.748 | 147.949 | 173.582 | 183.608 |
| p | 0 | 0.144 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Note: Compared with pcDNA group, *p<0.05 and compared with pcDNA-MAGI2-AS3+miR-NC group, #p<0.05
Table 6: MiR-31-5P Overexpression Reverses The Effects of MAGI2-AS3 Overexpression on Cell Proliferation, Migration and Invasion in Siha Cells (x̄±s, n=9)
As a common malignant tumor, cervical cancer seriously threatens women's physical health and quality of life and now targeted therapy has received increasing attention[7]. Recently, studies have found that lncRNA and miRNAs can be used as biomarkers for cervical cancer screening, diagnosis, prognosis, treatment response assessment as well as detecting cancer recurrence[8]. MAGI2-AS3 is an lncRNA MAGI2 AS3 and studies have found that MAGI2-AS3 is downregulated in breast cancer tissues and peripheral blood, and may be involved in breast cancer development, progression, as well as recurrence and metastasis[9].
MAGI2-AS3 is also significantly under-expressed in glioma tissues and represents an important prognostic factor for glioma patients[10]. Overexpression of MAGI2- AS3 can inhibit cell proliferation and migration in liver cancer and bladder cancer[11,12]. In this study, MAGI2- AS3 was under-expressed in cervical cancer cells and overexpression of MAGI2-AS3 also inhibited SiHa cell proliferation, migration and invasion.
The occurrence and development of cervical cancer is a complex process of multiple gene dysregulation and studies have found that miRNA is involved in regulating cervical cancer progression, exploring the mechanism of miRNAs in cervical cancer and is also of great significance in improving the diagnosis and treatment of cervical cancer and improving prognosis[13]. Studies have found that inhibition of miR-31-5p expression can play a pro-atherosclerotic role by targeted inhibition of Insulin Degrading Enzyme (IDE)[14]. MiR-31- 5p expression is down-regulated in acute myeloid leukemia and overexpression of miR-31-5p can inhibit cell proliferation and promote cell differentiation and maturation in the mononuclear direction, exerting a tumor suppressor function by targeting Human antigen R protein (HuR) in acute myeloid leukemogenesis[15]. While miR-31-5p is highly expressed in colorectal tissues, inhibition of miR-31-5p can inhibit the malignant biological behavior of colorectal cancer cells[16]. Our results showed that miR-31-5p was highly expressed in cervical cancer cells, while inhibition of miR-31-5p expression could suppress SiHa cell proliferation, migration and invasion. MAGI2-AS3 was found to target miR-31-5p; overexpression of miR- 31-5p reversed the effects of overexpressing MAGI2- AS3 on proliferation, migration, invasion of SiHa cells. These results suggest that the inhibitory effect of MAGI2-AS3 on the proliferation, migration and invasion of SiHa cells is related to miR-31-5p.
Taken together, lncRNA MAGI2-AS3 may inhibit cervical cancer cell proliferation, migration and invasion by regulating miR-31-5p.
Conflict of interests:
The authors declared no conflicts of interest.
References
- Wang J, Liu XJ. A review of targeted therapy for cervical cancer. Int J Gynaecol Obstet 2017;44(3):292-5.
- Yang Y, Yang H, Xu M, Zhang H, Sun M, Mu P, et al. Long non-coding RNA (lncRNA) MAGI2-AS3 inhibits breast cancer cell growth by targeting the Fas/FasL signalling pathway. Hum Cell 2018;31(3):232-41.
[Crossref] [Google Scholar] [PubMed]
- Du S, Hu W, Zhao YI, Zhou H, Wen W, Xu M, et al. Long non-coding RNA MAGI2-AS3 inhibits breast cancer cell migration and invasion via sponging microRNA-374a. Cancer Biomark 2019;24(3):269-77.
[Crossref] [Google Scholar] [PubMed]
- Peng C, Huang YL. Development of microRNA for diagnosis and treatment of cervical cancer. J Military Surg Southwest Chin 2018;20(2):146-50.
- Chen X, Zhong L, Li X, Liu W, Zhao Y, Li J. Down-regulation of microRNA-31-5p inhibits proliferation and invasion of osteosarcoma cells through Wnt/β-catenin signaling pathway by enhancing AXIN1. Exp Mol Pathol 2019;108:32-41.
[Crossref] [Google Scholar] [PubMed]
- Wang LL, Hu XX, Liu F. MiR-31 lentiviral vector plasmid construction and its effect on the proliferation and migration ability of cervical cancer HeLa cells. Shandong Med J 2017;57(28):7-10.
- Sun Y. Advances in targeted drug therapy for cervical cancer. Mod J Integr Tradit Chin West Med 2016;25(12):1366-8.
- Hu YX, Zhang GN. A review of relevant biomarkers for uterine cervical cancer. J Cancer Control Treat 2019;32(2):148-53.
- Lv MM, Xu N, Wang FL. Expression of long non-coding RNAs in peripheral blood and cancer tissue of breast cancer patients. Chin J Breast Dis 2015;9(4):242-6.
- Chen XD, Zhu MX, Wang SJ. Expression of long non-coding RNA MAGI2-AS3 in human gliomas and its prognostic significance. Eur Rev Med Pharmacol Sci 2019;23(8):3455-60.
[Crossref] [Google Scholar] [PubMed]
- Yin Z, Ma T, Yan J, Shi N, Zhang C, Lu X, et al. LncRNA MAGI2‐AS3 inhibits hepatocellular carcinoma cell proliferation and migration by targeting the miR‐374b‐5p/SMG1 signaling pathway. J Cell Physiol 2019;234(10):18825-36.
[Crossref] [Google Scholar] [PubMed]
- Wang F, Zu Y, Zhu S, Yang Y, Huang W, Xie H, et al. Long noncoding RNA MAGI2-AS3 regulates CCDC19 expression by sponging miR-15b-5p and suppresses bladder cancer progression. Biochem Biophys Res Commun 2018;507(1-4):231-5.
[Crossref] [Google Scholar] [PubMed]
- Dong LX, Li X, Yang S. Research progress of microRNA in cervical cancer. Matern Child Health J Chin 2018;33(11):231-4.
- Wu CY, Wu JL, Wang X. MiR-31-5p exerts pro-atherosclerotic effects by targeting and inhibiting insulin degrading enzyme. Chin J Arterioscler 2015;23(11):1100-6.
- Cao LX, Chen LX. MiR-31-5p expression is down-regulated in acute myeloid leukemia. Basic Clin Med 2017;37(4):537-42.
- Peng H, Wang L, Su Q, Yi K, Du J, Wang Z. MiR-31-5p promotes the cell growth, migration and invasion of colorectal cancer cells by targeting NUMB. Biomed Pharmacother 2019;109:208-16.