- *Corresponding Author:
- T. Huang
Department of Hepatobiliary and Pancreatic Surgery, China
E-mail: huangtcdr@163.com
| This article was originally published in a special issue, “Innovations in Biomedical Research and Drug Development” |
| Indian J Pharm Sci 2023:85(3) Spl Issue “134-140” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the mechanism of receptor-interacting protein 3 on the proliferation, invasion and reactive oxygen species levels of gallbladder cancer cells induced by tumor necrosis factor alpha. Gallbladder cancer SGC-996 cells were divided into a control group, a plasmid cloning deoxyribonucleic acid-negative control group, a plasmid cloning deoxyribonucleic acid-receptor-interacting protein 3 group, a plasmid cloning deoxyribonucleic acid-receptor-interacting protein 3+tumor necrosis factor alpha group and a plasmid cloning deoxyribonucleic acid-receptor-interacting protein 3+si-receptor-interacting protein 3 group. Reverse transcription polymerase chain reaction for messenger ribonucleic acid expression in cells; detection of cell proliferation, invasion and reactive oxygen species in cells by cell counting kit-8, Transwell and flow cytometry, respectively; detection of receptor-interacting protein 3, matrix metallopeptidase-9, matrix metallopeptidase-2 proteins in cells (Western blotting method). The expression of receptor-interacting protein 3 messenger ribonucleic acid was lower in gallbladder cancer cells than in gallbladder epithelial cells (p<0.05) and lowest in SGC-996 cells (p<0.05). receptor-interacting protein 3 messenger ribonucleic acid, cell survival rate, invasion ability, reactive oxygen species level, receptorinteracting protein 3, matrix metallopeptidase-2 and matrix metallopeptidase-9 protein were similar in the control and plasmid cloning deoxyribonucleic acid-negative control groups (p>0.05), while receptorinteracting protein 3 messenger ribonucleic acid, receptor-interacting protein 3 protein, reactive oxygen species content were elevated. And cell survival, invasion ability, matrix metallopeptidase-2 and matrix metallopeptidase-9 protein were decreased in the plasmid cloning deoxyribonucleic acid-receptorinteracting protein 3 group (p<0.05). Compared with the plasmid cloning deoxyribonucleic acid-receptorinteracting protein 3 group, receptor-interacting protein 3 protein, reactive oxygen species content were increased and cell survival, invasion ability, matrix metallopeptidase-2 and matrix metallopeptidase-9 protein were decreased in the plasmid cloning deoxyribonucleic acid-receptor-interacting protein 3+tumor necrosis factor alpha group and receptor-interacting protein 3 protein, reactive oxygen species content were decreased and cell survival, invasion ability, matrix metallopeptidase-2 and matrix metallopeptidase-9 protein were increased in the plasmid cloning deoxyribonucleic acid-receptor-interacting protein 3+sireceptor interacting protein 3 group (p<0.05). Overexpression of receptor-interacting protein 3 inhibited tumor necrosis factor alpha induced proliferation and invasion of gallbladder cancer cells and promoted reactive oxygen species levels in tumor necrosis factor alpha induced gallbladder cancer cells and its mechanism was related to matrix metallopeptidase-2 and matrix metallopeptidase-9 protein expression.
Keywords
Receptor-interacting protein 3, gallbladder cancer, proliferation, invasion, reactive oxygen species level
Gallbladder Cancer (GBC) ranks 6th in incidence among all gastrointestinal tract tumors. There are more than 52 800 new patients with GBC in China every year[1,2]. GBC is extremely malignant and prone to recurrence and metastasis, resulting in a 5 y survival rate of only 5 % to 15 %[3]. Currently, radical surgical resection is often used to treat GBC in clinical practice; but the early symptoms are not significant leading to the best time for surgery is often missed. Although 10 % of patients with GBC can be treated by surgery, the treatment effect is still unsatisfactory due to the high recurrence rate and high chemotherapy resistance[4]. Therefore, exploring the mechanism of metastasis and treating GBC is currently a hot spot of clinical research. Cellular necrosis, a common mode of death, can mediate multiple modes of death including organelle swelling, cell membrane rupture and local inflammatory response due to release of cellular contents[5]. It has been demonstrated that programmed necrosis is a modifiable mode of death, among which Tumor Necrosis Factor Alpha (TNF-α) induced programmed necrosis has been widely studied by scholars in recent years[6]. Receptor- Interacting Protein 3 (RIP3) is a programmed necrosis protein with specific serine/threonine kinase activity. Although its C-segment structure does not possess a death-related protein structural domain, RIP3 can exert a regulatory effect on cell death by sensing changes in the intracellular environment[7]. It was found that when the cytokine TNF-α binds to TNFR[8], it can induce the binding of RIP1 and RIP3, which contributes to the activation of RIP3 and thus mediates the development of programmed necrosis. RIP3-dependent programmed necrosis is engaged in lots of disease processes, including ischemic injury, inflammatory diseases and tumors, but little has been reported about its effect on GBC cell activity[9]. Reactive Oxygen Species (ROS) are oxygen-derived reactive small molecules and numerous studies have confirmed that ROS are associated with tumors. Reduced concentrations of ROS in cells can play a role in promoting invasion and proliferation, while high concentrations of ROS can promote cell death by triggering oxidative damage to proteins, lipids, Deoxyribonucleic Acid (DNA) and Ribonucleic Acid (RNA)[10]. Therefore, in this study, we induced GBC with TNF-α and investigated the effect of RIP3 on its activity and ROS level.
Materials and Methods
Reagents and instruments:
GBC, NOZ cells (HSRRB, Japan); gallbladder epithelial GBC-SD cells and GBC SGC-996 cells (Shanghai Cellular Institute, China); RIP3- overexpressed plasmid cloning (pcDNA-RIP3) and its pcDNA-Negative Control (pcDNA-NC) (Gikai Gene Synthesis, China); TRIzol, Reverse Transcription Polymerase Chain Reaction (RT- PCR) kit (Chengdu Weike Biotechnology Co., Ltd.); RIP3 interference plasmid (si-RIP3) (Beijing Genesis Biotechnology Co., Ltd.); TNF-α (Shanghai Biobot Biomedical Ltd.); Cell Counting Kit-8 (CCK-8) reagent (Tong Ren Chemical Research Institute, Japan); transfection reagent LipofectamineTM 2000 (Invitrogen, United States of America (USA)); rabbit anti-human RIP2, Beta (β)-actin polyclonal antibody (Sigma, USA); RNA extraction kit (Takara, Japan) and Thermo FC Enzyme Labeler (Thermo, USA).
Cell culture:
The GBC cell NOZ and SGC-996 and gallbladder epithelial cell GBC-SD were obtained and cultured in Dulbecco's Modified Eagle Medium (DMEM) medium (10 % Fetal Bovine Serum (FBS)). Then, penicillin-streptomycin double antibodies were added, with a constant temperature incubator at 37° and the Carbon dioxide (CO2) content was set at 5 %. The culture medium was changed once in 48 h.
Transfection and grouping:
Logarithmically grown SGC-996 cells were taken and inoculated in 6-well plates and the cells were cultured until the fusion reached 80 %-90 % for transfection. The control, pcDNA-NC, pcDNA- RIP3, pcDNA-RIP+TNF-α and pcDNA-RIP+si-RIP3 groups were established. The pcDNA-RIP group was transfected with RIP3 plasmid (pcDNA- RIP3); the pcDNA-NC group was transfected with pcDNA-NC; the pcDNA-RIP3+TNF-α group was first induced with TNF-α (15 μg/l) and then transfected with the pcDNA-RIP3 plasmid. The pcDNA-RIP+si-RIP3 group was transfected with the pcDNA-RIP3 plasmid and RIP3 interference plasmid (si-RIP3) into SGC-996 cells; the control group was not transfected with any plasmid into SGC-996 cells and no TNF-α was added. LipofectamineTM 2000 liposome transfection reagent instructions were used.
RIP3 messenger RNA (mRNA) expression by RT-PCR:
Total RNA was extracted after cell lysis. RIP3 upstream primers: 5 '- GGT GT GAAC C AT GAGAAGTAT GA-3 '; downstream primers: 5'-GAGTCCTTCCACG ATACCAAAG-3'; β-actin upstream primers: 5 ' - A T A C A A C T G C T C T G G G G T G C - 3 ' and downstream primers: 5'-GGCCTTCTTGCGAACCTACT-3'. The primers were transiently centrifuged and deionized water was added, thoroughly mixed and made into a 100 μmol/l storage solution. The concentration of the primers was diluted to 10 μmol/l. PCR was repeated 40 times 2-ΔΔCT method to analyze RIP3, mRNA expression.
CCK-8 for proliferation:
Adjust the density of SGC-996 cells to 1.5×104 cells/ml and inoculate the cells in 96-well plates. The cells were cultured with complete DMEM medium and the medium was incubated at 37° for 48 h, setting the CO2 concentration at 5 % and the cells were incubated with 100 μl of CCK-8 solution for a total of 1.5 h. The OD values were measured by an enzyme marker (450 nm).
Transwell assay for cell invasiveness:
Cell suspensions were made from each group of cells, centrifuged in a centrifuge, washed after discarding the medium, added to DMEM with 0. 1 % FBS, resuspended, and adjusted to a cell concentration of 1.5×104 cells/ml. Matrigel was laid flat in the Transwell upper chamber and sterilized by Ultra-Violet (UV) light for 6 h; cell suspension (200 μl) were added to upper chamber and DMEM (10 % FBS) to lower chamber; incubated at 37° for 24 h, setting the CO2 concentration at 5 %. Then, the cells and Matrigel remaining in the upper chamber were scraped off and placed in formaldehyde solution for fixation; 10 min later, the upper chamber was stained with crystal violet (0. 1 %). After 10 min, the chambers were washed and placed with the bottom facing upward. Then, the chambers were placed under a microscope and cell invasion ability was analyzed.
ROS level measurement:
1.5×104/ml of SGC-996 cells were inoculated in 6-well plates, each group of cells was digested with trypsin for 24 h. Dichloro-Dihydro-Fluorescein Diacetate (DCFH-DA) (10 μmol/l) was added, 30 min later, flow cytometry to detect ROS levels.
Western blotting:
Trypsin digestion of cells and 3,3′-Diaminobenzidine (DAB) assay for total protein concentration in cells. An equal concentration of protein solution was prepared at 2 μg/ml and the corresponding volume of boiling solution was added at a ratio of 6:1. Boil for 10 min and store in the refrigerator (-20°). Take 30 μg of the protein sample, perform electrophoresis on the sample and transfer it to the polyvinylidene Difluoride (PVDF) membrane, then 5 % skimmed milk powder was added, 1 h later, add the primary antibody (1:1000), incubate the primary antibody at 4° overnight, PVDF membrane was then washed, add the corresponding secondary antibody (1:1000) and incubation at 25° for 2 h.
Statistical analysis:
Data analysis using GraphPad Priam 8.0 software. Measurement data were expressed by mean±standard deviation (x͞ ±s). One-way Analysis of Variance (ANOVA) was used for comparison among multiple groups and Least Significant Difference test (LSD-t) was used for pairwise comparison between groups. p<0.05 represents significance.
Results and Discussion
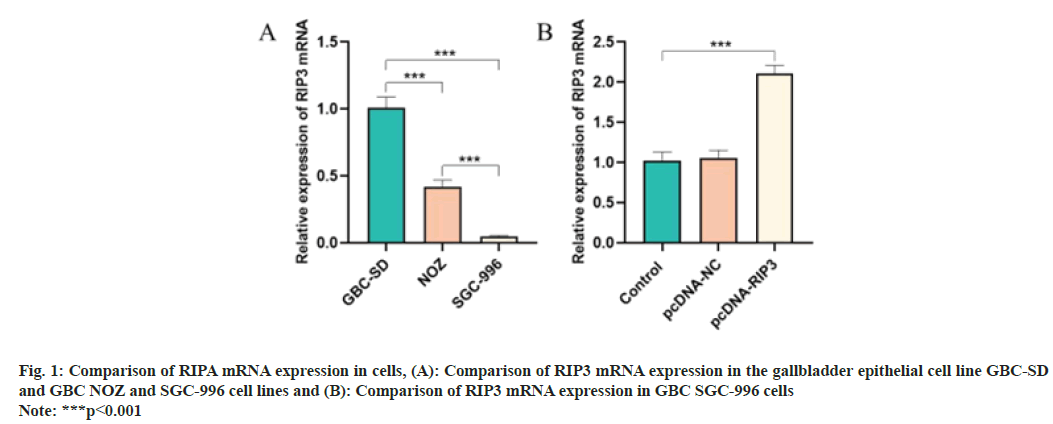
Compared with the GBC-SD cells, RIP3 mRNA expression in NOZ and SGC-996 cells was reduced (p<0.05) and the reduction was most pronounced in SGC-996 cells (p<0.05) (fig. 1A). Compared with the control group, RIP3 mRNA expression of the pcDNA-RIP3 group was higher (p<0.05) as shown in fig. 1B.
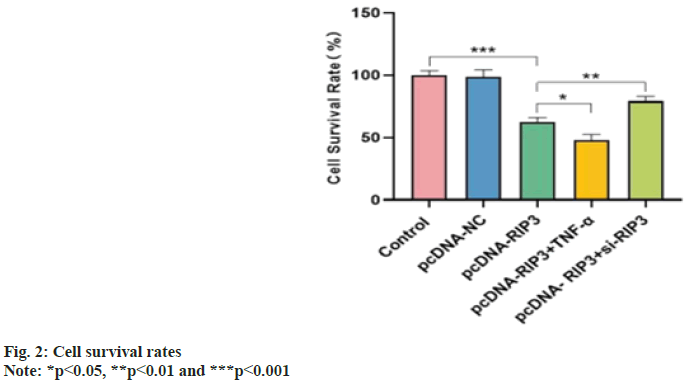
Cell survival rates were similar in the control and pcDNA-NC groups (p>0.05) and decreased in the pcDNA-RIP3 group (p<0.05). Compared with the pcDNA-RIP3 group, the cell survival rate was reduced in the pcDNA-RIP3+TNF-α group and increased in the pcDNA-RIP3+si-RIP3 group (p<0.05) as shown in fig. 2.
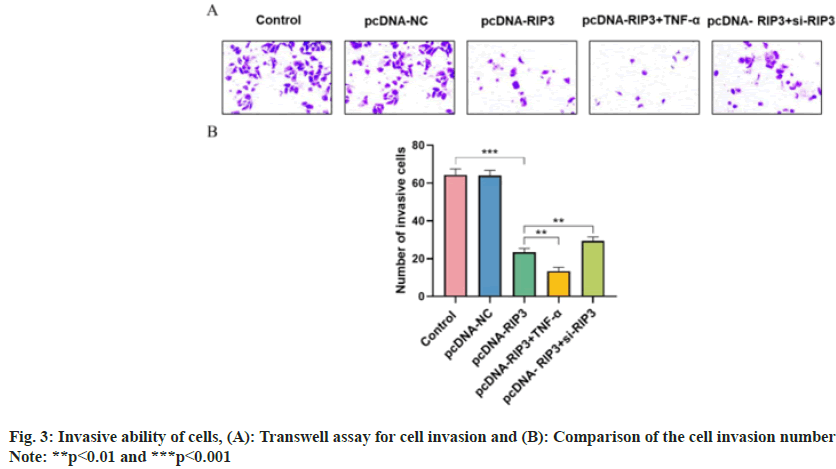
Number of cell invasion were similar in the control and pcDNA-NC groups (p>0.05) and decreased in the pcDNA-RIP3 group (p<0.05). Compared with the pcDNA-RIP3 group, the number of cell invasion was reduced in the pcDNA-RIP3+TNF-α group and increased in the pcDNA-RIP3+si-RIP3 group (p<0.05) as shown in fig. 3.
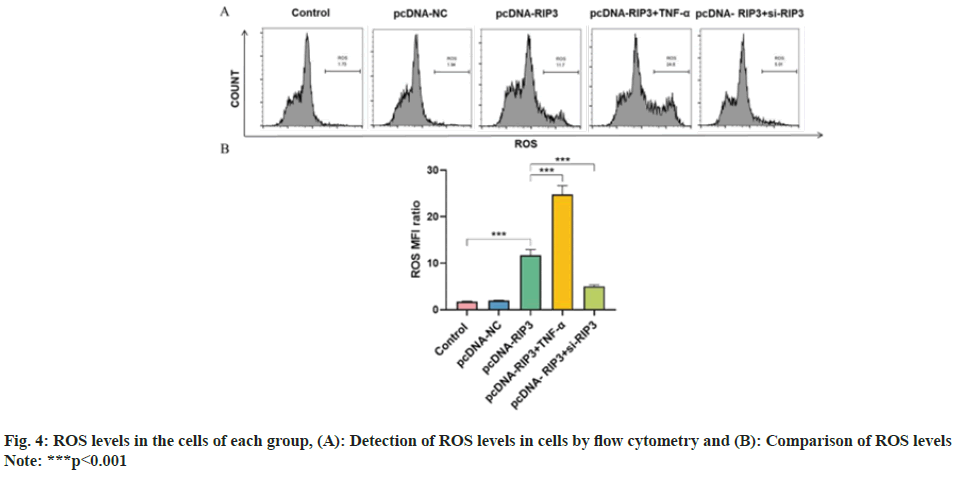
ROS content were similar in the control and pcDNA-NC groups (p>0.05) and increased in the pcDNA-RIP3 group (p<0.05). Compared with the pcDNA-RIP3 group, ROS content was increased in the pcDNA-RIP3+TNF-α group and reduced in the pcDNA-RIP3+si-RIP3 group (p<0.05) as shown in fig. 4.
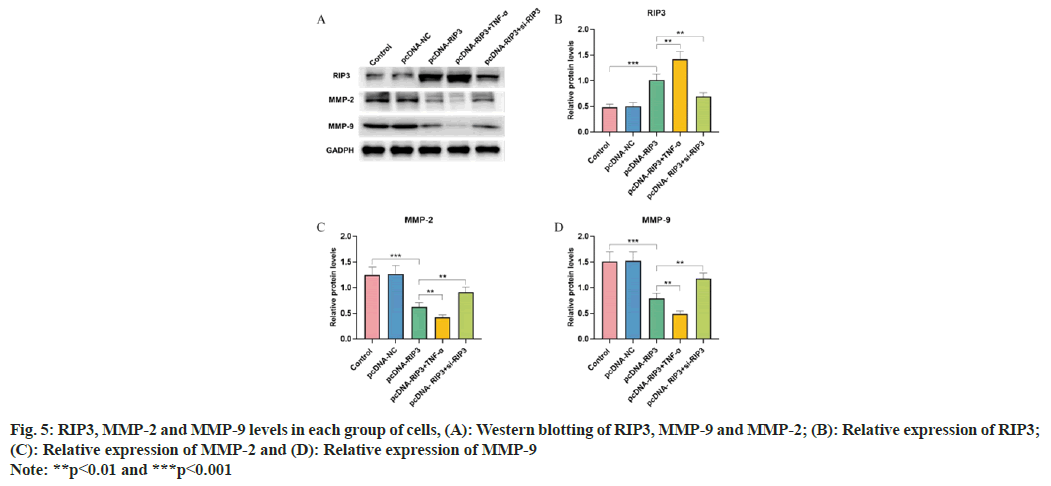
RIP3, Matrix Metallopeptidase (MMP)-2 and MMP-9 protein expression were similar in the control and pcDNA-NC groups (p>0.05), while RIP3 was elevated and MMP-2 and MMP-9 were decreased in the pcDNA-RIP3 group (p<0.05). Compared with the pcDNA-RIP3 group, RIP3 protein expression was increased and, MMP-9 and MMP-2 were decreased in the pcDNA- RIP3+TNF-α group and RIP3 was decreased and, MMP-9 and MMP-2 were increased in the pcDNA- RIP3+si-RIP3 group (p<0.05) as shown in fig. 5.
Studies show that GBC accounts for more than 90 % of all biliary tract malignancies[11]. This observation shows that finding effective treatments for GBC is particularly important for clinical treatment. Numerous studies have confirmed[12] that programmed necrosis can mediate the development of various diseases including ischemic injury, inflammatory diseases and tumors. RIP3 is one of the key regulatory proteins of programmed necrosis and it has been found that the absence of RIP3 expression in malignant tumors can help tumor cells escape programmed necrosis[13]. In various tumors, deletion of RIP3 expression can be caused by elevating the methylation pattern of the RIP3 promoter region of necrosis factor, which can exert a promoting effect on tumor cell growth through defective programmed necrosis. RIP3 induces differentiation of leukemia-initiating cells by promoting cell death, which in turn exerts an inhibitory effect on the development of myeloid leukemia[14]. Our study confirmed that RIP3 levels in GBC cells were lower than those in gallbladder epithelial cells. Overexpression of RIP3 could increase RIP3 levels in GBC cells and inhibit proliferation, invasion and inhibition of RIP3 expression produces an opposite effect, suggesting that overexpression of RIP3 is important for the treatment of GBC. It has been demonstrated that ectopic expression of RIP3 in RIP3-deficient tumor cells contributes to the inhibition of tumor growth[15].
Metastasis of tumor cells depends on the ability of cells to break through tissue barriers because tumor cells can secrete many proteases that provide favorable conditions for the metastasis of tumor cells through the extracellular matrix degradation. MMPs are proteases that degrade the extracellular matrix and play an important role in tumor metastasis. MMP-2 and MMP-9 have strong degradation effects on the extracellular matrix of tumors and can affect tumor recurrence and metastasis by promoting the migration of tumor cells[16]. We found that overexpression of RIP3 inhibited GBC cell invasion, which may be involved in inhibiting the activation of MMP-2 and MMP-9 proteins.
ROS homeostasis can play an important role in cellular life activities. ROS can regulate the biological activity of tumor cells through many different signaling pathways, including apoptosis, invasion and proliferation[10]. Under normal conditions, the metabolism of intracellular ROS is in dynamic equilibrium and when the redox balance of cells is disrupted, large amounts of ROS can be generated, causing apoptosis[17]. Our study confirmed that overexpression of RIP3 increased ROS levels in GBC cells, suggesting that overexpression of RIP3 may promote apoptosis in GBC cells by generating large amounts of ROS.
TNF-α is the most common cytokine in the body, which is produced in large quantities mainly by activated monocytes and macrophages, and has a direct tumor-killing effect. In vitro experimental studies have shown that wild-type TNF-α can shrink or eliminate tumors by killing various tumor cells[18]. RIP3 expressed by cells after treatment with TNF-α shifts the cells toward programmed necrosis. It has been shown that RIP3 enhances TNF-α-induced programmed necrosis in NIH3T3 cells and that knockdown of RIP3 in cells avoids programmed necrosis of cells[19]. Our study confirmed that compared to the pcDNA- RIP3 group, the proliferation, invasion ability and MMP-2 and MMP-9 levels in the pcDNA- RIP3+TNF-α group were lower and the ROS level was higher, suggesting that overexpression of RIP3 could inhibit proliferation and invasion of GBC cells induced by TNF-α and promote ROS levels in cells, thus increasing TNF-α mediated programmed necrosis, which is consistent with the abovementioned findings.
In conclusion, overexpression of RIP3 inhibits proliferation and invasion of GBC cells induced by TNF-α, and promotes the content of ROS in GBC cells. The mechanism was related to MMP-2 and MMP-9 protein expression.
Funding:
This study was supported by the Quanzhou Science and Technology Planning Project (No: 2019N106S) and Fujian Health Research Talent Training Project (No: 2019-QZNB- 10).
Conflict of interests:
The authors declared no conflict of interests.
References
- Ozer M, Goksu SY, Sanford NN, Porembka M, Khurshid H, Ahn C, et al. A propensity score analysis of chemotherapy use in patients with resectable gallbladder cancer. JAMA Netw Open 2022;5(2):e2146912.
[Crossref] [Google Scholar] [PubMed]
- Jang J, Yoo DS, Chun BC. Spatial epidemiologic analysis of the liver cancer and gallbladder cancer incidence and its determinants in South Korea. BMC Public Health 2021;21(1):2090.
[Crossref] [Google Scholar] [PubMed]
- Low SK, Giannis D, Thuong ND, Nam NH, Alshareef A, Koulas I, et al. Trends in primary gallbladder cancer incidence and incidence-based mortality in the United States, 1973 to 2015. Am J Clin Oncol 2022;45(7):306-15.
[Crossref] [Google Scholar] [PubMed]
- Okumura K, Gogna S, Gachabayov M, Felsenreich DM, McGuirk M, Rojas A, et al. Gallbladder cancer: Historical treatment and new management options. World J Gastrointest Oncol 2021;13(10):1317-5.
[Crossref] [Google Scholar] [PubMed]
- Amaral EP, Foreman TW, Namasivayam S, Hilligan KL, Kauffman KD, Barbosa Bomfim CC, et al. GPX4 regulates cellular necrosis and host resistance in Mycobacterium tuberculosis infection. J Exp Med 2022;219(11):e20220504.
[Crossref] [Google Scholar] [PubMed]
- Shi X, Zhu W, Chen T, Cui W, Li X, Xu S. Paraquat induces apoptosis, programmed necrosis and immune dysfunction in CIK cells via the PTEN/PI3K/AKT axis. Fish Shellfish Immunol 2022;130:309-16.
[Crossref] [Google Scholar] [PubMed]
- Mei P, Xie F, Pan J, Wang S, Gao W, Ge R, et al. E3 ligase TRIM25 ubiquitinates RIP3 to inhibit TNF induced cell necrosis. Cell Death Differ 2021;28(10):2888-99.
[Crossref] [Google Scholar] [PubMed]
- Zhou J, Li G, Han G, Feng S, Liu Y, Chen J, et al. Emodin induced necroptosis in the glioma cell line U251 via the TNF-α/RIP1/RIP3 pathway. Invest New Drugs 2020;38(1):50-9.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Liu S, Liu H, Yang C, Jiang A, Wei H, et al. Versatile cationic liposomes for RIP3 overexpression in colon cancer therapy and RIP3 down regulation in acute pancreatitis therapy. J Drug Target 2020;28(6):627-42.
[Crossref] [Google Scholar] [PubMed]
- Cheung EC, Vousden KH. The role of ROS in tumour development and progression. Nat Rev Cancer 2022;22(5):280-97.
[Crossref] [Google Scholar] [PubMed]
- Chen T, Liu H, Liu Z, Li K, Qin R, Wang Y, et al. FGF19 and FGFR4 promotes the progression of gallbladder carcinoma in an autocrine pathway dependent on GPBAR1-cAMP-EGR1 axis. Oncogene 2021;40(30):4941-53.
[Crossref] [Google Scholar] [PubMed]
- Yu YQ, Gamez-Belmonte R, Patankar JV, Liebing E, Becker C. The role of programmed necrosis in colorectal cancer. Cancers 2022;14(17):4295.
[Crossref] [Google Scholar] [PubMed]
- Zhao X, Lu J, Chen X, Gao Z, Zhang C, Chen C, et al. Methamphetamine exposure induces neuronal programmed necrosis by activating the receptor-interacting protein kinase 3-related signalling pathway. FASEB J 2021;35(5):e21561.
[Crossref] [Google Scholar] [PubMed]
- Dan W, Zhong L, Zhang Z, Wan P, Lu Y, Wang X, et al. RIP1-dependent apoptosis and differentiation regulated by Skp2 and Akt/GSK3β in acute myeloid leukemia. Int J Med Sci 2022;19(3):525.
[Crossref] [Google Scholar] [PubMed]
- Yang CK. Study on the regulatory mechanism of the expression of the necrotic gene RIP3 in tumor cells and its role in tumor genesis and development. Suzhou Univ J 2002;3(9):6-8.
- Tian K, Du G, Wang X, Wu X, Li L, Liu W, et al. MMP-9 secreted by M2-type macrophages promotes Wilms’ tumour metastasis through the PI3K/AKT pathway. Mol Biol Rep 2022;49(5):3469-80.
[Crossref] [Google Scholar] [PubMed]
- Chen Y, Zhang J, Lin Y, Lei Q, Guan KL, Zhao S, et al. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep 2011;12(6):534-41.
[Crossref] [Google Scholar] [PubMed]
- Adjuto-Saccone M, Soubeyran P, Garcia J, Audebert S, Camoin L, Rubis M, et al. TNF-α induces endothelial–mesenchymal transition promoting stromal development of pancreatic adenocarcinoma. Cell Death Dis 2021;12(7):649.
[Crossref] [Google Scholar] [PubMed]
- Zhou Y, Feng Q, Li Y, Liu Q, Zhao X, Duan C, et al. Aluminum induced necroptosis of PC12 cells via TNFR1-RIP1/RIP3 signaling pathway. Neurochem Res 2022;47(10):3037-50.
[Crossref] [Google Scholar] [PubMed]