- *Corresponding Author:
- H. Dai
Department of Orthopedics, The Fifth Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi 530022, China
E-mail: daihai2014@163.com
| Date of Received | 24 August 2021 |
| Date of Revision | 30 October 2022 |
| Date of Acceptance | 02 February 2023 |
| Indian J Pharm Sci 2023;85(1):145-151 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate whether calprotectin is involved in the protective effect of resveratrol on rheumatoid arthritis injury and its potential mechanism. 12 of the 72 rats were randomly selected as blank group; rheumatoid arthritis model was constructed by bovine collagen II complete Freund's adjuvant method. The rats with successful modeling were divided into model group, high, medium and low-dose resveratrol groups (60, 30, and 15 mg/kg), and toll-like receptor 4 agonist (neoseptin 3) group (resveratrol 60 mg/kg+neoseptin 3 1 mg/kg), with 12 rats in each group. Each group was intervened by gavage of corresponding drugs once a day for 42 d. The general state of the rats was observed; the rat ankle joint volume was detected and the swelling degree was calculated; rats were scored for arthritis index; serum levels of interleukin 6, tumor necrosis factor-alpha and interleukin 1 beta contents were measured by enzyme-linked immunosorbent assay; the histological changes of ankle joints were observed by hematoxylin and eosin staining; calprotectin, toll-like receptor 4 and nuclear factors-kappa B p65 protein expression in ankle tissues were determined by Western blot analysis. The rats in resveratrol group had good mental state, significantly reduced joint swelling, increased body weight, decreased inflammatory cell infiltration and new pannus in synovial tissue, the joint swelling rate, arthritis index, serum tumor necrosis factor-alpha, interleukin 6 and interleukin 1 beta contents, calprotectin, toll-like receptor 4, nuclear factors-kappa B p65 protein expression in ankle joint tissue were significantly lower (p<0.05); in a dose-dependent manner. Neoseptin 3 can reverse the protective effect of resveratrol on rheumatoid arthritis rats. Resveratrol may inhibit calprotectin expression, which in turn may inhibit the activation of toll-like receptor 4/nuclear factors-kappa B p65 signaling pathway, thereby exerting a protective effect on rheumatoid arthritis rats.
Keywords
Rheumatoid arthritis, resveratrol, calprotectin, synovial inflammation, toll-like receptor 4/ nuclear factor-kappa B
Rheumatoid Arthritis (RA) is a systemic autoimmune disease characterized pathologically by synovial hyperplasia and pannus production; the infiltration of inflammatory mediators and hyperplasia of synoviocytes play an important role in the pathogenesis of RA[1]. Calprotectin (S100A8/A9), a proinflammatory cytokine, is closely related to the occurrence and development of several autoimmune diseases[2]. Recently, studies have found that S100A8/A9 protein is highly expressed in the synovium of RA patients and in RA fibroblast like synoviocytes[3,4], and that its expression level can directly reflect the inflammatory activity of the synovium and synovial fluid in RA patients and is an ideal indicator for assessing the disease activity of RA patients[5]. Resveratrol, a bioactive compound mainly found in fruits such as grapes and peanuts, has been found to possess antioxidant, anti-inflammatory, anticancer and cardiovascular protective activities[6]; in vitro and in vivo experimental evidence has shown that resveratrol attenuates inflammation in RA synoviocytes[7,8] and has great clinical potential as a novel drug for RA treatment. However, whether the effect of resveratrol in treating RA is related to S100A8/A9 has not been reported, and therefore, this study aims to further investigate whether S100A8/ A9 is involved in the protective effect of resveratrol on RA injury and its underlying mechanism.
Materials and Methods
Experimental animals:
72 Sprague-Dawley (SD) rats of Specific Pathogen Free (SPF) grade were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. [License No.: SCXK (J) 2016-0011], male, body mass 200-240 g, housed under conditions of temperature 22°-26°, humidity 50 %-60 %, 12 light/dark cycle and had free access to food and water.
Main instruments and reagents:
Resveratrol (Shanghai Macklin Biochemical Co., Ltd., China); complete Freund's adjuvant, bovine collagen II (Sigma-Aldrich, China); Toll-Like Receptor 4 (TLR4) agonist-neoseptin 3 (Med Chem Express Inc., United States of America (USA)); Tumor Necrosis Factor-Alpha (TNF-α), Interleukin (IL)-6 and IL-1 beta (β) Enzyme-Linked Immunosorbent Assay (ELISA) kit (Wuhan Bio swamp Biotechnology Co., Ltd., China); Mouse anti-S100A8/A9 complex antibodies (Novus Corporation, USA), TLR4, Nuclear Factors-Kappa B p65 (NF-κB p65), beta-Actin, goat anti-mouse Immunoglobulin G (IgG) (Heavy and Light chains (H+L))/Horseradish Peroxidase (HRP), goat anti-rabbit IgG (H+L)/HRP (Beijing Biosynthesis Biotechnology Co., Ltd., China); iMark680 multifunctional micro plate reader (Bio-Rad Inc., USA), BX61 light microscope (Olympus, Japan).
Methods:
Constructing RA models: 12 rats were selected as blank group by random number table method and the remaining rats were used to induce RA rat model by bovine collagen II complete Freund's adjuvant method[9]. Bovine collagen II was formulated as a 2 mg/ml solution (solvent was 0.1 M acetic acid) with an equal volume of complete Freund's adjuvant, mixed and placed in a refrigerator at 4° until fully emulsified to make a 1 mg/ml solvent emulsion. Primary immunization; three points were selected on the back of the rats, one point each on the caudal root and right hind paw, and emulsifier was injected subcutaneously into the rats at a volume of 0.2 ml per rat; after 7 d, an equal volume of emulsifier was injected subcutaneously again for booster immunization. The blank group was injected with an equal volume of normal saline. On the 20th d, the foot and ankle joints developed acute inflammatory swelling and were observed by rat plantar camera photographs and radiographs, with severe redness and continuous low-density shadow and osteolysis, and the Arthritis Index (AI) score ≥2 points were classified as the replication success of the model[10,11]; 0 point for normal, no redness; 1 point for redness and swelling of toe joints; 2 points for redness and swelling of toe joints and plantar joints; 3 points for redness and swelling of the entire foot below the ankle joint; 4 points for total redness and deformity of foot and ankle joints. All rats in this study were successfully modeled.
Grouping and administration: 60 rats with successful modeling were randomly divided into model group, high-medium and low-dose resveratrol groups (60, 30, and 15 mg/kg)[12], and neoseptin 3 group (resveratrol 60 mg/kg+neoseptin 3 1 mg/kg)[13], with 12 rats in each group. Resveratrol high, medium and low-dose groups were administered the corresponding doses of the drug by intragastric gavage at a volume of 1 ml/100 g, and neoseptin 3 group received 1 mg/kg neoseptin 3 by tail vein injection after 60 mg/kg gavage; the blank group and the model group were given the same volume of normal saline, 1 time/d, continuous administration for 42 d.
Observation of rat status: During the experiment, the rats were observed for mobility, diet, body weight, coat color and joint swelling.
Joint swelling detection: The rat ankle volumes were measured by an articulator before, immediately after (before administration) and at the end of drug administration to calculate swelling. The ratio of the volume of joint replacement water measured at the first time before modeling (used as the base value) to that measured at each subsequent time was the rate of joint swelling.
AI: The rats were scored for AI before and after drug administration, respectively. The cumulative score for individual joints was AI for each rat.
Serum inflammatory factor content by ELISA: 1 h after the last administration, rats were anesthetized with an intraperitoneal injection of 40 mg/kg sodium pentobarbital, blood was collected from the abdominal aorta, serum was isolated, and TNF-α, IL-6 and IL-1β contents were measured in accordance with the instructions of the ELISA kit.
Histological changes in the ankle joint were observed by Hematoxylin and Eosin (HE) staining:
After the completion of blood collection, the rats were sacrificed, and the right ankle joints of 6 rats were fixed in 4 % paraformaldehyde, embedded in paraffin, sectioned and deparaffinized and hydrated for HE staining. Histological changes in the ankle joints were observed under a light microscope.
Western blot was used to detect S100A8/A9, TLR4 and NF-κB p65 protein expression in ankle tissues:
Total protein was extracted from the synovial tissues of 6 rat ankles and equal protein samples were separated by Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and transferred and after the membranes were blocked in 5 % non-fat milk for 1 h and incubated at 4° with primary antibodies (S100A8/A9, TLR4, NF-κB p65, 1:1000; β-actin, 1:2000) for 24 h and then, membranes were incubated with HRP labeled secondary antibodies (1:5000) for 1 h at room temperature, developed, protein bands were observed and band gray values were quantified, relative expression of each protein of interest was calculated, where β-actin was used as internal reference.
Statistical methods:
Statistical Package for the Social Sciences (SPSS) 22.0 software was used for statistical analysis and the metrology data conformed to normal distribution expressed in (x?±s), and one-way Analysis of Variance (ANOVA) and Least Significant Difference (LSD)-t test were used for multiple comparisons among groups. p<0.05 indicated statistical significance.
Results and Discussion
The rats in the blank group before and after modeling and after administration exhibited normal activity, smooth and shiny hair, large appetite and normal body weight gain; after modeling, the model rats were apathetic with minimal activity, sparse and dull hair, decreased appetite, significantly swollen joints and reduced body weight (p<0.05; after the intervention, compared with the blank group, the rats in the model group were apathetic and less active, with dull hair, decreased appetite, swollen joints and decreased body weight (p<0.05); compared with the model group, the rats in the different doses of resveratrol group showed increased activity, higher diet, shiny hair, reduced joint swelling and higher body weight (p<0.05); compared with the high-dose resveratrol group, the rats in the neoseptin 3 group had poorer general status and significantly lower body weight (p<0.05) as shown in Table 1.
| Group | Dose mg·kg-1 | Before modeling | Before administration | After administration | |
|---|---|---|---|---|---|
| Blank group | - | 200.11±5.43 | 289.33±19.83 | 376.74±18.31 | |
| Model group | - | 202.64±7.55 | 226.91±18.38a | 251.84±17.54a | |
| Resveratrol | High-dose group | 60 | 201.35±5.19 | 227.88±19.91a | 322.58±17.62ab |
| Medium-dose group | 30 | 203.88±6.15 | 231.78±11.71a | 298.31±16.99ab | |
| Low-dose group | 15 | 202.45±7.08 | 229.82±17.85a | 279.87±18.35ab | |
| Neoseptin 3 group | 1 | 205.73±5.66 | 225.01±19.07a | 260.87±16.78ac | |
Note: (a) vs. blank group; (b) vs. model group and (c) vs. resveratrol high-dose group, p<0.05
Table 1: Effect of Resveratrol on Body Weight of RA RATS before and after administration (x?±s, g, n=12)
Before administration, the swelling of joints in the model rats increased compared with the blank group (p<0.05); after administration, the joint swelling rate of the model rats increased compared with that of the blank group (p<0.05), and decreased compared with that of the model rats in the high, medium and low-dose resveratrol groups (p<0.05), and resveratrol exhibited a certain dose-dependent manner, with the joint swelling rate significantly higher in the neoseptin 3 group compared with the high-dose resveratrol group (p<0.05) as shown in Table 2.
| Group | Dose mg·kg-1 | Before administration | After administration | |
|---|---|---|---|---|
| Blank group | - | 1.06±0.10 | 1.09±0.11 | |
| Model group | - | 2.23±0.12a | 1.83±0.14a | |
| Resveratrol | High-dose group | 60 | 2.21±0.14a | 1.28±0.12ab |
| Medium-dose group | 30 | 2.25±0.11a | 1.49±0.11ab | |
| Low-dose group | 15 | 2.22±0.15a | 1.57±0.15ab | |
| Neoseptin 3 group | 1 | 2.23±0.13a | 1.75±0.13ac | |
Note: (a) vs. blank group; (b) vs. model group and (c) vs. resveratrol high-dose group, p<0.05
Table 2: Effect of Resveratrol on the Rate of Joint Swelling in RA RATS (x?±s, g, n=12)
Before administration, the AI of the model rats increased compared with the blank group (p<0.05); after administration, the AI of the model rats increased (p<0.05) compared with that of the blank group, and decreased (p<0.05) compared with that of the model rats in the high, medium and low-dose resveratrol groups; the AI was significantly higher (p<0.05) in the neoseptin 3 group compared with the high-dose resveratrol group as shown in Table 3.
| Group | Dose mg·kg-1 | Before administration | After administration | |
|---|---|---|---|---|
| Blank group | - | 0.00±0.00 | 0.00±0.00 | |
| Model group | - | 4.17±0.54a | 6.78±1.02a | |
| Resveratrol | High-dose group | 60 | 4.21±0.49a | 4.82±0.87ab |
| Medium-dose group | 30 | 4.23±0.51a | 5.37±0.96ab | |
| Low-dose group | 15 | 4.19±0.46a | 6.04±0.95ab | |
| Neoseptin 3 group | 1 | 4.22±0.51a | 6.45±1.03ac | |
Table 3: Effects of Resveratrol on AI in RA RATS (x?±s, n=12)
After administration, compared with the blank group, TNF-α, IL-6 and IL-1 β levels in the model group were increased (p<0.05) and the levels of the above indicators were decreased (p<0.05) in the high, medium and low-dose resveratrol groups compared with the model group. In contrast to the high-dose resveratrol group, TNF-α, IL-6 and IL-1 β levels in the neoseptin 3 were significantly higher (p<0.05) as shown in Table 4.
| Group | Dose mg·kg-1 | TNF-α | IL-6 | IL-1β | |
|---|---|---|---|---|---|
| Blank group | - | 189.7±18.11 | 154.38±27.07 | 121.06±16.89 | |
| Model group | - | 252.7±27.64a | 397.97±24.58a | 172.14±23.23a | |
| Resveratrol | High-dose group | 60 | 215.99±22.55ab | 213.56±26.78ab | 126.56±15.91ab |
| Medium-dose group | 30 | 224.32±23.16ab | 281.56±27.39ab | 142.08±17.05ab | |
| Low-dose group | 15 | 237.31±19.94ab | 310.56±27.23ab | 152.6±18.47ab | |
| Neoseptin 3 group | 1 | 245.22±20.87ac | 390.75±29.12ac | 167.34±21.03ac | |
Note: (a) vs. blank group; (b) vs. model group and (c) vs. resveratrol high-dose group, p<0.05
Table 4: Effect of Resveratrol on Serum TNF-α, IL-6 and IL-1 Β Contents in RA RATS (x?±s, pg/ml, n=12)
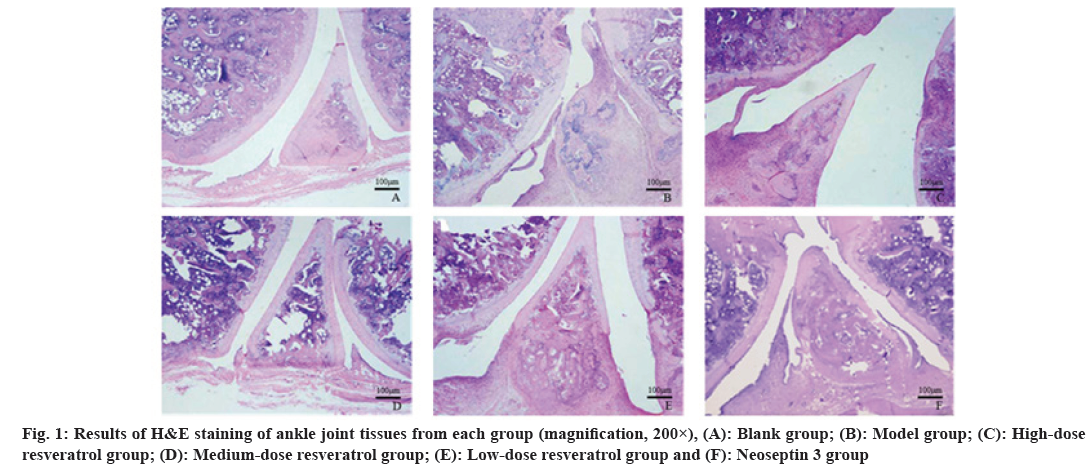
Rats in the blank group had normal joints and no synovial lesions; in the model group, the articular chondrocytes of the rats were swollen and necrotic, the joint cavity was narrow, synovial tissue proliferation was seen and inflammatory cell infiltration was obvious; a small number of chondrocyte swelling, necrosis and inflammatory cell infiltration and hyperplasia with increased joint space were seen in the high, medium, and low-dose resveratrol groups; the damage of cartilage and synovium tissues in rats was significantly aggravated in neoseptin 3 group compared with high-dose resveratrol group as shown in fig. 1.
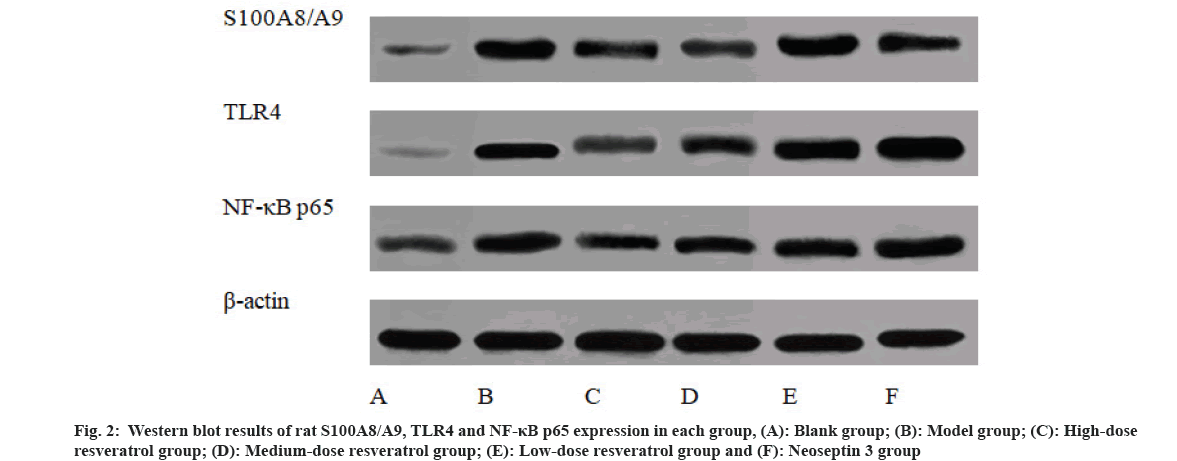
Compared with the blank group, S100A8/A9, TLR4 and NF-κB p65 protein levels in the model group were increased (p<0.05), compared with those in the model group, S100A8/A9, TLR4 and NF-κB p65 protein levels were reduced in the high and medium-dose resveratrol groups (p<0.05), and compared with those in the high-dose resveratrol group, TLR4 and NF-κB p65 protein expression of ankle joint tissue was significantly increased in neoseptin 3 group (p<0.05) as shown in Table 5 and fig. 2.
| Group | Dose mg·kg-1 | S100A8/A9 | TLR4 | NF-κB p65 | |
|---|---|---|---|---|---|
| Blank group | - | 0.32±0.05 | 0.22±0.04 | 0.40±0.06 | |
| Model group | - | 1.02±0.08a | 0.93±0.07a | 0.85±0.08a | |
| Resveratrol | High-dose group | 60 | 0.53±0.07ab | 0.50±0.05ab | 0.59±0.07ab |
| Medium-dose group | 30 | 0.73±0.06ab | 0.65±0.07ab | 0.68±0.09ab | |
| Low-dose group | 15 | 0.94±0.08a | 0.82±0.06ab | 0.77±0.06ab | |
| Neoseptin 3 group | 1 | 0.52±0.06ab | 0.84±0.08abc | 0.76±0.09abc | |
Note: (a) vs. blank group; (b) vs. model group and (c) vs. resveratrol high-dose group, p<0.05
Table 5: Effects of Resveratrol on S100A8/A9, TLR4 and NF-ΚB P65 Protein Expression in Ankle Tissues of RA RATS (x?±s, n=6)
Although the pathogenesis of RA is not fully defined, researchers have identified synovial inflammation as a key feature of the disease after years of effort[14]. During the pathogenesis of RA, a large number of neutrophils and macrophages activates, invades and releases a large number of proinflammatory mediators into the joint cavity, and increases synovial vascularity. The RA rat model induced by bovine collagen II complete Freund's adjuvant method behaves similarly to human RA and is a commonly used animal model to study RA. Swelling degree and AI of the toe was the main index for evaluating the inflammatory reaction in rats, and the symptoms were joint red, swelling, heat, pain, ulceration, damaged chondrocytes, hyperplasia and thickening of synovial tissue, inflammatory cell infiltration, and a large number of pannus in the joint cavity[15]. The above symptoms were also manifested in the results of this experiment, and on the 20th d of modeling, the rat foot and ankle joints developed obvious inflammatory swelling, and the AI was significantly increased, and the results of subsequent HE staining confirmed that the synovial tissues of the model rats were proliferating, accompanied by a large amount of inflammatory cell infiltration, indicating that we successfully prepared a RA rat model.
Calprotectin, as a promising biomarker of RA disease activity, is a heterodimer complex composed of two calcium binding proteins, S100A8 and S100A9, involved in the pathogenesis of multiple chronic inflammatory diseases[16]. Recently, studies have found that S100A8/A9 proteins are highly expressed in the synovium of RA patients and in RA fibroblast like synoviocytes[3,4] and it is involved in the development and progression of RA. Calprotectin acts as an alarmin by binding to the receptor for advanced glycation end products and TLR-4 and amplifies the inflammatory response[17]. In arthritis, activated neutrophils and macrophages in inflamed joints may contribute to S100A8/A9 release[18]. Resveratrol, a polyphenolic compound with diverse biological functions and activities, has been regarded as a novel therapeutic agent for RA in recent years. Resveratrol has been found to prevent NF-κB p65 activation, inhibits RA-Fibroblast Like Synoviocytes (RA-FLSs) proliferation and migration, and can activate B-cell lymphoma protein 2 (Bcl-2)-associated X (BAX) to induce apoptosis in RA-FLSs[19]; and resveratrol in RA rat model induced by bovine type II collagen and in an in vitro RA model of IL-1β stimulating synoviocytes (RSC-364), it can alleviate RA by reducing Reactive Oxygen Species (ROS) and inflammatory responses, inhibiting the Mitogen-Activated Protein Kinase (MAPK) signaling and attenuating Hypoxia-Inducible Factor 1-alpha (HIF-1α)-mediated angiogenesis[20]. The results of this study showed that after administration of resveratrol intervention, the general state of RA rats was significantly alleviated, the body weight was significantly increased, the joint swelling degree and AI were significantly reduced, and the pathological damage was significantly reduced, indicating that resveratrol has an obvious protective effect on RA rats.
S100A8/A9 can bind through TLR4 and activate NF-κB p65, which induces inflammatory responses[17]. Therefore, in this study, we evaluated the contents of inflammatory factors and measured S100A8/A9, TLR4, and NF-κB p65 protein expression in ankle synovial tissues. The results showed that TNF-α, IL-6 and IL-1β contents in the serum of rats in the model group were increased, and the levels of S100A8/A9, TLR4 and NF-κB p65 proteins in synovial tissues were increased, indicating that S100A8/A9 are highly expressed in RA rats and that TLR4/NF-κB p65 signaling pathway is activated; after administration of resveratrol intervention, the serum TNF-α, IL-6 and IL-1β levels of rats were significantly decreased, and S100A8/A9, TLR4 and NF-κB p65 proteins were reduced significantly in the synovial tissues of ankle joints, and there was no significant difference in S100A8/A9 protein expression in rats treated with a TLR4 agonist (neoseptin 3) based on high-dose resveratrol, but the expression of TLR4 and NF-κB p65 increased significantly, indicating that administration of neoseptin 3 reversed the protective effects of resveratrol on RA; it suggested that resveratrol may inhibit S100A8/A9 expression, which in turn inhibits the activation of the TLR4/NF-κB p65 signaling pathway, thereby exerting a protective effect on RA rats.
In conclusion, resveratrol may inhibit S100A8/A9 expression, which in turn may inhibit the activation of TLR4/NF-κB p65 signaling pathway, thereby exerting a protective effect on RA rats. This study further enriches the protective mechanism of resveratrol on RA and provides a theoretical basis for its application. Because of the condition limitation, this study investigated the possible mechanism by which resveratrol inhibited S100A8/A9 expression to exert RA protective effects from the animal level, and the subsequent in-depth study will be conducted from the cellular level; in addition, whether the suppression of S100A8/A9 expression by resveratrol is associated with the receptor for advanced glycation end products needs to be further investigated.
Conflict of interests:
The authors declared no conflict of interests.
References
- Su X, Zhang H, Wang H, Sun P. MiR-130a/Ndrg2 axis inhibits the proliferation of fibroblast-like synoviocytes in rheumatoid arthritis. Inflammation 2020;43(6):2048-60.
[Crossref] [Google Scholar] [PubMed]
- Silvin A, Chapuis N, Dunsmore G, Goubet AG, Dubuisson A, Derosa L, et al. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell 2020;182(6):1401-18.
[Crossref] [Google Scholar] [PubMed]
- de Moel EC, Rech J, Mahler M, Roth J, Vogl T, Schouffoer A, et al. Circulating calprotectin (S100A8/A9) is higher in rheumatoid arthritis patients that relapse within 12 mo of tapering anti-rheumatic drugs. Arthr Res Ther 2019;21(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Di Ceglie I, Kruisbergen NN, van den Bosch MH, van Lent PL. Fc-gamma receptors and S100A8/A9 cause bone erosion during rheumatoid arthritis. Do they act as partners in crime? Rheumatology 2019;58(8):1331-43.
[Crossref] [Google Scholar] [PubMed]
- Sreejit G, Abdel-Latif A, Athmanathan B, Annabathula R, Dhyani A, Noothi SK, et al. Neutrophil-derived S100A8/A9 amplify granulopoiesis after myocardial infarction. Circulation 2020;141(13):1080-94.
[Crossref] [Google Scholar] [PubMed]
- Meng T, Xiao D, Muhammed A, Deng J, Chen L, He J. Anti-inflammatory action and mechanisms of resveratrol. Molecules 2021;26(1):229-44.
[Crossref] [Google Scholar] [PubMed]
- Wang G, Xie X, Yuan L, Qiu J, Duan W, Xu B, et al. Resveratrol ameliorates rheumatoid arthritis via activation of SIRT1-Nrf2 signaling pathway. Biofactors 2020;46(3):441-53.
[Crossref] [Google Scholar] [PubMed]
- Lu J, Zheng Y, Yang J, Zhang J, Cao W, Chen X, et al. Resveratrol alleviates inflammatory injury and enhances the apoptosis of fibroblast-like synoviocytes via mitochondrial dysfunction and ER stress in rats with adjuvant arthritis. Mol Med Rep 2019;20(1):463-72.
[Crossref] [Google Scholar] [PubMed]
- Zhang G, Zhang C, Guo Z, Hou X. Therapeutic effect of methotrexate combined with electroacupuncture in rheumatoid arthritis rats. Chin J Tissue Eng Res 2020;24(29):4667-72.
- Tian X, Zheng Q, Wang J. Therapeutic effect of emodin on rheumatoid arthritis model rats and anti-inflammatory mechanism study. Jiangsu J Tradit Chin Med 2020;52(10):84-7.
- Ruan R, Deng X, Zhu X. Study of effects of carnosic acid on NF-κB signaling pathway in rheumatoid arthritis rats. Mod J Int Tradit Chin Western Med 2020;29(25):2757-61.
- Li L, Gu W. Resveratrol mediates the regulation of inflammation by tenascin-c/TLR4 signaling in a rat model of rheumatoid arthritis. Western J Tradit Chin Med 2020;33(6):20-3.
- Zhong X, Xiao Q, Liu Z. Mechanism study of resatorvid on hepatic ischemia reperfusion injury in a rat model of cardiac death. Chin J Organ Transplant 2019;40(12):753-7.
- Guan Y, Zhao X, Liu W, Wang Y. Galuteolin suppresses proliferation and inflammation in TNF-α-induced RA-FLS cells by activating HMOX1 to regulate IKKβ/NF-κB pathway. J Orthop Surg Res 2020;15(1):484-92.
- Chadha S, Behl T, Kumar A, Khullar G, Arora S. Role of Nrf2 in rheumatoid arthritis. Curr Res Transl Med 2020;68(4):171-81.
[Crossref] [Google Scholar] [PubMed]
- Smiljanovic B, Grützkau A, Sörensen T, Grün JR, Vogl T, Bonin M, et al. Synovial tissue transcriptomes of long-standing rheumatoid arthritis are dominated by activated macrophages that reflect microbial stimulation. Sci Rep 2020;10(1):7907.
[Crossref] [Google Scholar] [PubMed]
- Blom AB, van den Bosch MH, Blaney Davidson EN, Roth J, Vogl T, van de Loo FA, et al. The alarmins S100A8 and S100A9 mediate acute pain in experimental synovitis. Arthr Res Ther 2020;22(1):199-213.
- Vogl T, Stratis A, Wixler V, Völler T, Thurainayagam S, Jorch SK, et al. Autoinhibitory regulation of S100A8/S100A9 alarmin activity locally restricts sterile inflammation. J Clin Invest 2018;128(5):1852-66.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Wang G, Wang T, Cao W, Zhang L, Chen X. Nrf2–Keap1 pathway–mediated effects of resveratrol on oxidative stress and apoptosis in hydrogen peroxide–treated rheumatoid arthritis fibroblast-like synoviocytes. Ann N Y Acad Sci 2019;1457(1):166-78.
[Crossref] [Google Scholar] [PubMed]
- Yang G, Chang CC, Yang Y, Yuan L, Xu L, Ho CT, et al. Resveratrol alleviates rheumatoid arthritis via reducing ROS and inflammation, inhibiting MAPK signaling pathways and suppressing angiogenesis. J Agric Food Chem 2018;66(49):12953-60.
[Crossref] [Google Scholar] [PubMed]