- *Corresponding Author:
- Xuerun Wu
Tianjin University of Traditional Chinese Medicine, Hospital of Integrated Chinese and Western Medicine, Tianjin 301617, China
E-mail: Wywyyjk@126.com
| Date of Received | 25 December 2022 |
| Date of Revision | 15 July 2023 |
| Date of Acceptance | 22 December 2023 |
| Indian J Pharm Sci 2023;85(6):1789-1795 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The paper aimed to explore the mechanism by which high mobility group box-1 and nicotinamide adenine dinucleotide phosphate oxidase-derived reactive oxygen species enhance each other’s influence on the amplitude of electroretinogram and the permeability of the vitreous. According to the purpose of the experiment, the experimental rats were separated into a control group, a diabetes group and a Shenling Baizhu powder group. The messenger ribonucleic acid expression of microRNA-23 and microRNA-27a in rat muscle tissue was quantitatively analyzed by real-time quantitative polymerase chain reaction. The contractility of rat soleus skeletal muscle was analyzed by electrical stimulation program. Western blot analysis of rat muscle growth protein synthesis and the expression of myostatin signaling pathway was carried out. The expression of renal fibrosis-related albumin was tested by Western blot. The messenger ribonucleic acid expression of microRNA-23, 27a in the diabetic group was lower than control group (p<0.05), and the expression in the Shenling Baizhu powder group was higher than diabetic group (p<0.05). Compared with the control group, the diabetic group had lower body weight (p<0.05), and raised blood glucose concentration and diabetes volume (p<0.05). The ratio of phosphorylated protein kinase B/protein kinase B and phospho forkhead box protein O1/forkhead box protein O1 protein expression in the Shenling Baizhu powder group was higher than diabetic group (p<0.05), while the phosphatase and tensin homolog protein expression was lower (p<0.05). The protein expression of myostatin, FBXO32 and TRIM63 in the diabetes group was higher than control group (p<0.05), and the expression in the Shenling Baizhu powder group was lower than diabetes group (p<0.05). Compared with the control group, the protein expression of transforming growth factor-beta, suppressor of mothers against decapentaplegic 2/3, alpha-smooth muscle actin, collagen-1a and fibronectin was higher in the diabetes group (p<0.05), and the expression in the Shenling Baizhu powder group was lower than diabetes group (p<0.05). Shenling Baizhu powder can improve diabetes-induced sarcopenia by up-regulating microRNA expression, activating the insulin-like growth factor 1/phosphoinositide 3-kinases/ protein kinase B signaling pathway and inhibiting the myostatin cascade.
Keywords
Shenling Baizhu powder, microRNA-23a, microRNA-27a, diabetes, sarcopenia
Diabetes Mellitus (DM) is a complex illness, and its global prevalence rate is increasing rapidly. DM shows a wide range of pathophysiological changes, which are caused by the decrease of insulin secretion or hormone action[1]. According to the definition of European working group on myopenia in the elderly, DM may lead to sarcopenia, and cause different degrees of muscle damage on the basis of the DM severity and muscle fiber pattern[2]. The research record found that if diabetes is not intervened to worsen, it is easy to bring about more serious cardiovascular illness, which will not only cause adverse effects on the invalid organs, but even endanger their lives in serious cases. In recent years, due to the increasing aging problem, people’s lifestyle, diet structure, work and rest habits and other factors, not only the incidence of diabetes has continued to rise, but also the mortality of patients has continued to rise, which has seriously damaged the health of patients. Muscle atrophy is a breakaway indicator of mortality in all patients with Chronic Kidney Disease (CKD)[3]. DM is the main cause of CKD. In America and other western societies, DM accounts for 30 % of all documentation of end-stage renal illness[4]. Although the treatment of controlling blood sugar and renal protective medicant are effective for diabetic nephropathy, this disease is the main reason for the increase of mortality of diabetic patients[5].
MicroRNAs (miRNAs) have become the key regulator of metabolic homeostasis. These small non-coding RNAs target insulin signal transduction and Transforming Growth Factor Beta (TGFβ)/myostatin cascade reaction[6]. Recent studies show that miRNA is involved in the gene expression changes relevant to DM pathophysiology, including miR-23a and miR- 27a[7]. Shenling Baizhu powder is a traditional Chinese medicine prescription containing 10 herbs. Shenling Baizhu powder has the ability of invigorating spleen and is generality used for spleen and stomach weakness[8]. Shenling Baizhu powder has been proved that it can strengthen the body, advance the immune system, measure gastrointestinal peristalsis[9]. Myopenia belongs to the category of “disease syndrome” in traditional Chinese medicine, which is characterized by flaccidity, weakness and inability to voluntary movement due to deficiency of essence, and qi and blood. This study is to explore whether Shenling Baizhu powder can regulate miR expression, so as to improve diabetes-induced sarcopenia.
Materials and Methods
Experimental materials:
Experimental rats: Thirty 60 d old male rats, weighing 250 g, were purchased from University of Sao Paulo, kept with constant 23°±2° and 12 h of light 12 h of darkness, and received standard rat feed and tap water. The experimental procedure was approved by the institute of biomedical sciences and the ethics committee of animal research, and followed the ethical guidelines for the animal use.
Diabetes model and medication of Shenling Baizhu powder: Rats were fasted for 12 h after adapting to the environment for 2 w. Streptozotocin (Sigma Chemical Company, St. Louis, Missouri) was dissolved in sodium citrate buffer and injected intraperitoneally into rats at a dose of 50 mg/kg body weight. During this process, rats were sedated with halothane. After Shenling Baizhu powder was dissolved in water, rats were given orally at a dose of 15 ml/kg for 2 w .
Diabetes model and medication of Shenling Baizhu powder: Rats were fasted for 12 h after adapting to the environment for 2 w. Streptozotocin (Sigma Chemical Company, St. Louis, Missouri) was dissolved in sodium citrate buffer and injected intraperitoneally into rats at a dose of 50 mg/kg body weight. During this process, rats were sedated with halothane. After Shenling Baizhu powder was dissolved in water, rats were given orally at a dose of 15 ml/kg for 2 w.
Experimental methods:
Quantitative Polymerase Chain Reaction (PCR): Ribonuclic Acid (RNA) was extracted by RNeasy Mini Kit. QScript complementary Deoxyribonucleic Acid (cDNA) SuperMix (Quanta) software was used to reverse transcription. Reverse Transcription (RT)-PCR was carried out by using the Delta Delta Cycle Threshold (δδ CT) method with StepOnePlus system (Applied Biosystems) and perfect Sybr green Fast Mix (Quanta). The expressions of single miR-23a-3p and miR-27a-3p were normalized to U6.
Blood, urine, and tissue sampling: Rats were put in a metabolic cage with urine gathered for glucose determination for 24 h. Animals were anesthetized, blood was collected, and the soleus muscle was anatomy, so as to carry out the electrical stimulation program. After euthanasia with excessive thiopental sodium, the length of the tibia was carefully tested to correct the muscle weight. As mentioned above, urine glucose and plasma glucose were tested by glucose oxidase.
In vitro muscle electrical stimulation: A horizontal metal bracket fixed one tendon and connected the other to an isometric sensor through a pulley. Muscle length was adjusted to produce maximum twitching tension. Muscle was immersed in 100 ml Krebs-Heinseleit buffer, pH 7.4. The left muscle was not stimulated as a control sample. The right soleus muscle was stimulated with a low frequency pulse (0.5 Hz) with duration of 0.2 ms for 60 s. Therefore, the muscles were pre-incubated for 30 min. After pre-incubation, the right muscle was stimulated by ultra-large square wave pulse with duration of 0.2 ms. By stimulating at a frequency of 100 Hz for 10 s, it produced 10 tonic contractions in 10 min. After tonic stimulation, replace the buffer with fresh buffer and incubate the muscles for another 20 min.
Analysis of sarcopenia: The muscle was analyzed according to the diagnostic criteria of sarcopenia established by EWGSOP, which suggested that low muscle mass and function should be considered. The weight of flounder was analyzed relative to the length of tibia (S/T index) for adult rat’s sustained growth, testing contractile function using electrical stimulation of muscles. The following characteristics were analyzed; Thrombotic Thrombocytopenic Purpura (TTP) and Hormone Replacement Therapy (HRT), Maximal Contraction Rates (MCR) and Material Removal Rate (MRR), FI, etc.
Immunohistochemistry: Muscle was embedded in tissue freezing medium of isopentane cooled. Tissue was infiltrated in 0.05 % TritonX-100 for 10 min, and quenched and fixed in 50 mM Ammonium chloride (NH4Cl) for 10 min. 5 % bovine serum albumin sealed the samples incubated with primary antibody. The sections were then labeled with Fluorescein Isothiocyanate (FITC) anti-rabbit Immunoglobulin G (IgG) (111-095- 144; dilution 1:100; Jackson immunology research laboratory, West Grove, Pennsylvania) for 60 min. The nucleus was stained by 4′,6-Diamidino-2- Phenylindole (DAPI). The image was visualized by Olympus1X51 inverted fluorescence microscope. By using anti-laminin antibody, muscle fibers cross-sectional area in TA muscle was measured, with at least 500 individual muscle fibers in each muscle.
Western blot evaluation: Frozen muscle samples were homogenized for 10 min. Transfer the homogenate to a propylene tube. The same amount of protein (30 μg) was electrophoresed in polyacrylamide gel, and immunoblotting with related antibodies (AP2041, #MP3401). According to the manufacturing specification (1:5000), the enhanced chemiluminescence procedure was carried out using the appropriate secondary conjugated antibody. Imprint strength was analyzed using ImageJ software and standardized by optical density measurement of each lane of PonceauSstained membrane. The results were expressed in arbitrary units, ND average value was considered to be 1.0.
Statistical analysis:
All data were expressed as mean±Standard Error of the Mean (SEM). Muscle contraction effect was analyzed by data comparison in each experimental group with paired t-test. Results were then compared under each condition by one-way analysis of variance using posthoc Student-Newman-Keuls. Pearson correlation test was used to analyze the correlation between protein and miRNA in stimulated muscles. p<0.05 suggested statistical significance.
Results and Discussion
The mRNA expressions of miR-23 and 27a in muscle tissue of rats were quantitatively analyzed by realtime qPCR. Those in diabetic group decreased, and the mRNA expression of miR-23 and miR-27a in Shenling Baizhu powder group increased (p<0.05). The administration of Shenling Baizhu powder can improve their expression in diabetic rats as shown in Table 1.
| Group | miR-23a | miR-27a |
|---|---|---|
| Control | 1.86±0.16 | 1.93±0.18 |
| Diabetes | 1.15±0.06 | 1.04±0.03 |
| Shenling Baizhu powder | 1.79±0.13 | 1.85±0.16 |
| Variance ratio | 11.625 | 9.187 |
| p | 0.014 | 0.021 |
Table 1: Qpcr Analysis of Mir-23 and Mir-27a Expression (X̄±S)
Blood, urine and tissues of rats were collected for biochemical analysis. The weight, blood sugar and 24 h urine sugar of rats in the diabetic group decreased than control group (p<0.05), the blood sugar concentration and urine sugar raised (p<0.05), and the weight, blood sugar concentration and urine sugar decreased (p<0.05) in the Shenling Baizhu powder group. The results showed that streptozotocin successfully induced diabetic rat model, and Shenling Baizhu powder could alleviate the related symptoms of diabetes as shown in Table 2.
| Group | Weight (g) | Blood sugar (mg/dl) | Diabetes (mg/h) |
|---|---|---|---|
| Control | 375.36±25.44 | 116.37±4.23 | 15.11±3.42 |
| Diabetes | 229.57±12.88 | 482.60±13.44 | 285.42±38.57 |
| Shenling Baizhu Powder | 326.39±18.51 | 275.46±10.64 | 47.23±5.33 |
| Variance ratio | 12.396 | 10.243 | 9.115 |
| p | 0.013 | 0.008 | 0.026 |
Table 2: Analysis and Expression of Biochemical Signs in Rats (X̄±S)
Muscle was analyzed according to the diagnostic criteria of sarcopenia established by EWGSOP, and S/T index, MCR, MRR and FI of rats were compared. The S/T index, MCR and MRR of diabetic rats decreased, while the FI increased than control group (p<0.05). The S/T index, MCR and MRR of Shenling Baizhu powder increased, while the FI decreased than diabetic rats (p<0.05). Shenling Baizhu powder significantly reduced the symptoms of diabeticinduced muscular dystrophy by up-regulating the expression of miR-23a/27a as shown in Table 3.
| Group | S/T index (mg/mm) | MCR (N/s/g) | MRR (N/s/g) | FI (% twitch-1) |
|---|---|---|---|---|
| Control | 4.12±0.14 | 4.81±0.22 | 3.01±0.86 | 6.07±1.25 |
| Diabetes | 2.19±0.08 | 3.41±0.11 | 1.67±0.35 | 7.35±1.44 |
| Shenling Baizhu powder | 3.64±0.16 | 4.46±0.17 | 2.98±0.67 | 6.28±1.28 |
| Variance ratio | 9.628 | 11.337 | 13.594 | 10.085 |
| p | 0.003 | 0.015 | 0.007 | 0.022 |
Table 3: Analysis of Myopenia in Rats (X̄±S)
The contractility of rat soleus muscle was analyzed by electrical stimulation program. The results showed that the absolute isotonic force and absolute rigidity of skeletal muscle in diabetic group were reduced than control group (p<0.05), while the absolute isotonic force and absolute rigidity in Shenling Baizhu powder group were raised than diabetic group (p<0.05), and there was no distinguish between the two groups (p>0.05) as shown in Table 4.
| Group | Absolute constant tension (mN) | Isometric tension (mN/g) | Absolute rigidity (mN) | Specific rigidity (mN/g) |
|---|---|---|---|---|
| Control | 108.54±16.37 | 85.32±8.54 | 88.30±11.32 | 58.44±6.37 |
| Diabetes | 75.29±9.41 | 79.36±6.28 | 64.27±5.47 | 65.23±6.88 |
| Shenling Baizhu powder | 97.55±13.29 | 81.23±6.88 | 93.82±13.46 | 69.25±7.49 |
| Variance ratio | 8.339 | 11.416 | 13.735 | 10.823 |
| p | 0.005 | 0.016 | 0.024 | 0.011 |
Table 4: Analysis of Skeletal Muscle Contraction of Rat Soleus Muscle (X̄±S)
The expression of my growth protein synthesis signal in rats was analyzed by Western blot. The expression ratios of phosphorylated Protein Kinase B (p-Akt)/Akt and phosphor-Forkhead box protein O1 (p-FOXO1)/FOXO1 in diabetic group decreased (p<0.05), while the expression ratio of Phosphatase and Tensin Homolog (PTEN) protein increased than control group (p<0.05). The expression ratios of p-Akt/Akt and p-FOXO1/FOXO1 in Shenling Baizhu powder group were higher than diabetic group as shown in fig. 1 and Table 5.
| Group | p-Akt/Akt | PTEN | p-FOXO1/FOXO1 |
|---|---|---|---|
| Control | 1.96±0.18 | 1.04±0.03 | 1.94±0.18 |
| Diabetes | 1.15±0.04 | 1.83±0.16 | 1.13±0.05 |
| Shenling Baizhu powder | 1.89±0.17 | 1.15±0.14 | 1.88±0.15 |
| Variance ratio | 9.625 | 13.249 | 8.168 |
| p | 0.004 | 0.012 | 0.006 |
Table 5: The Expression Of Myogrowth Protein Synthesis Signal In Rats (X̄±S)
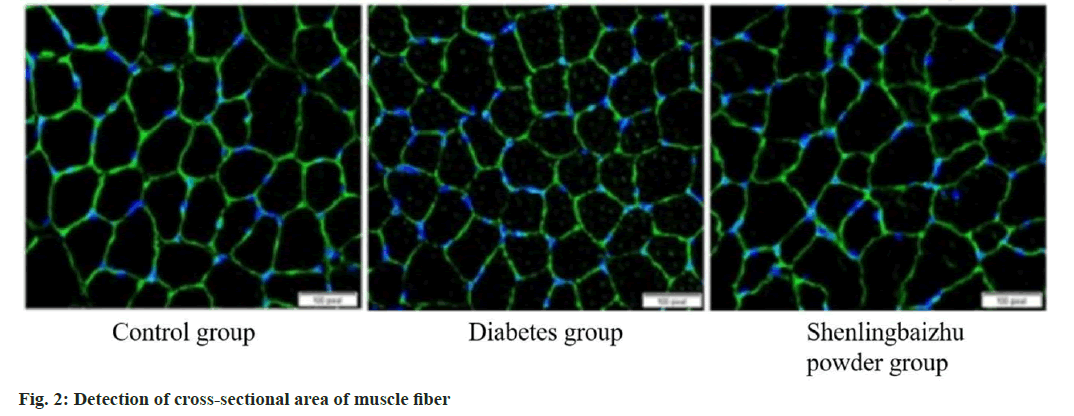
To study whether raising miR in muscle can improve the muscle fiber size reduction caused by diabetes, fiber’s cross-sectional region was tested in the tibialis anterior muscle frozen section. The diabetic group decreased the cross-sectional region of muscle fibers, while Shenling Baizhu powder weakened this decrease (p<0.05) as shown in fig. 2.
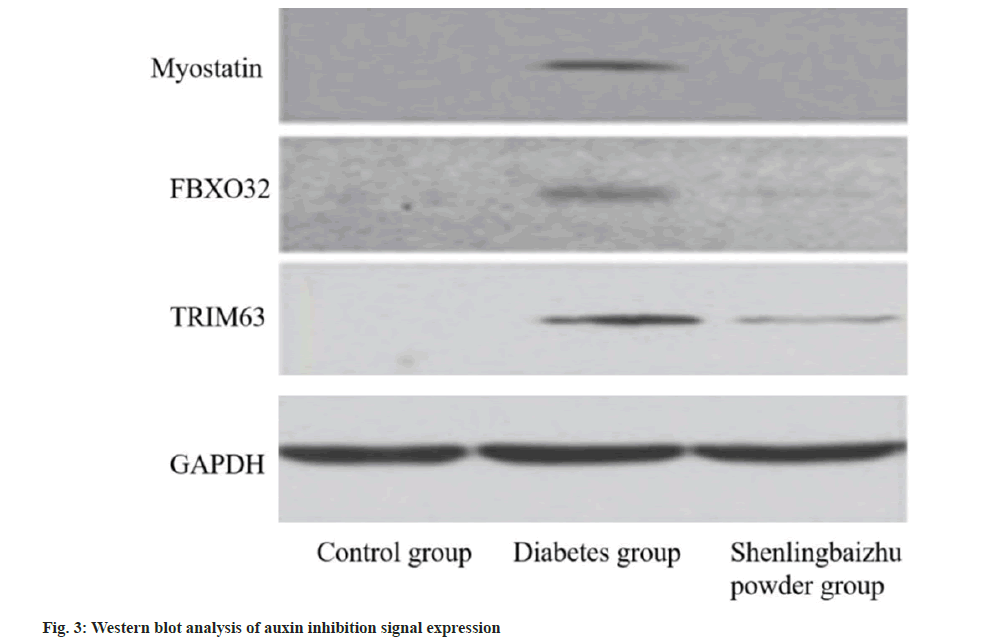
The expression of myostatin signal in rats was tested by Western blot. The protein expressions of myostatin, FBXO32 and TRIM63 in diabetic group were higher than control group (p<0.05), while those in Shenling Baizhu powder group were lower than diabetic group (p<0.05). miR decreased myostatinrelated protein expression and promoted muscle growth as shown in fig. 3 and Table 6.
| Group | Myostatin | FBXO32 | TRIM63 |
|---|---|---|---|
| Control | 1.13±0.05 | 1.05±0.03 | 1.02±0.02 |
| Diabetes | 1.95±0.18 | 2.01±0.21 | 1.97±0.18 |
| Shenling Baizhu powder | 1.08±0.03 | 1.05±0.05 | 1.11±0.07 |
| Variance ratio | 11.636 | 9.417 | 12.055 |
| p | 0.012 | 0.003 | 0.026 |
Table 6: Expression of Auxin Inhibitory Signal by Western Blot Analysis (X̄±S)
The expression of proteins related to renal fibrosis was tested by Western blot. The protein expressions of TGFβ, Suppressor of Mothers Against Decapentaplegic 2 (SMAD2)/3, Alpha-Smooth Muscle Actin (αSMA), Collagen-1a (COL1A1) and fibronectin in the diabetic group were higher, while Shenling Baizhu powder expression was lower (p<0.05), which indicated that Shenling Baizhu powder could significantly weaken the renal fibrosis caused by diabetes as shown in Table 7.
| Group | TGF-β | SMAD2/3 | α-SMA | COL1A1 | Fibronectin |
|---|---|---|---|---|---|
| Control | 1.01±0.03 | 1.07±0.05 | 1.14±0.10 | 1.04±0.07 | 1.05±0.03 |
| Diabetes | 1.95±0.18 | 2.01±0.19 | 1.85±0.16 | 1.94±0.17 | 1.99±0.20 |
| Shenling Baizhu powder | 1.13±0.11 | 1.08±0.10 | 1.12±0.08 | 1.02±0.04 | 1.22±0.12 |
| Variance ratio | 9.553 | 11.202 | 13.417 | 8.328 | 10.581 |
| p | 0.005 | 0.018 | 0.026 | 0.004 | 0.013 |
Table 7: Western Blot Analysis of Renal Fibrosis Related Protein Expression (X̄±S)
The evidence provided by this study showed that Shenling Baizhu powder can reduce muscle loss caused by diabetes and advance muscle ability by restraining the expression of miR-23a/27a, raising insulin signaling pathway and restraining myostatin cascade reaction. The study also found that the upregulation of miR-23a/27a by Ji Shenling Baizhu powder can reduce renal fibrosis, which supported the basic principle of new way to treat various diabetes complications in muscles, kidneys at the same time. Thus, the horizontal of miR-23a and miR 27a in vivo was up-regulated after taking Shenling Baizhu powder, both of which are mRNA encoding protein related to atrophy and fibrosis.
It is reported that insulin deficiency and resistance bring about the insulin and Insulin-Like Growth Factor 1 (IGF-1) down-regulation, which is very important for inducing muscle atrophy in catabolic diseases[10,11]. Akt is an albumin kinase, which effects muscle quality regulation[12]. Generally speaking, their up-regulation will increase protein synthesis and reduce albumin degradation, thus fighting against muscle atrophy[13]. Myostatin is a momentous member of TGF-β superfamily that regulates cell multiplication[14]. SMAD2/3 are transcription elements that are phosphorylated in response to TGF-β and myostatin[15]. In CKD, kidney TGF-β increase brings about SMAD2/3 activation, which leads to the multiplication of myofibroblasts, and finally leads to renal fibrosis[16]. SMAD2/3 activated by myostatin can inhibit myoblast from differentiating into mature muscle fibers[17]. On the contrary, the pharmacological inhibition of myostatin can significantly enhance the function of satellite cells, decrease the degradation rate of protein and increase the synthesis of protein in skeletal muscle[18].
The results showed that miR-23a and miR-27a in skeletal muscle of diabetic rats were lower than control group. This decrease promoted myostatin and PTEN expression, and reduced the phosphorylation of Akt, which directly led to the disorder of synthesis and decomposition of protein, and then developed sarcopenia. In addition, SMAD3 expression increased in diabetic rats, and miR-23a targeted SMAD3 in chondrocytes[19]. It is found that raised SMAD2/3 signal bring about Akt phosphorylation inhibition, caspase-3 and ubiquitinproteasome system activation, which are related to the process of muscle atrophy[20]. In many types of muscular atrophy, a group of genes have a hand in protein degradation are regulated, up-regulation of TRIM63 and FBXO32 are considered as proteolysis markers[21]. In this study, we detected the increased protein expression of TRIM63 and FBXO32 in diabetic rat’s soleus muscle, which was accordance with the description that these genes were upregulated in gastrocnemius muscle and heart induced by diabetes. However, after treatment, the coding of these proteins was reduced. It can be reasonably assumed that in the case of long-term atrophy, muscle proteolysis should be inhibited to retain residual muscle mass. Especially, its maintenance, accompanied by high protein hydrolysis, means high protein synthesis conditions. In addition, our results showed that insulin therapy raised muscle weight and TRIM63 and FBXO32 expression. This shows once again that protein synthesis plays a leading role in the muscle protein turnover induced by diabetes. In view of the above situation, it is an important issue to maintain reduced muscle mass, in which protein production of damaged muscles must play a fundamental effect. Muscle contraction adjusts cell’s metabolic homeostasis, including increasing muscle glucose treatment and positive protein balance.
As expected, streptozotocin induced hyperglycemia and diabetes in rats, and the treatment of Shenling Baizhu powder can partially reverse these conditions. In fact, it has been described in flatfish to slow down contraction and relax convulsion time and reduce MRR[22]. In this study, although the convulsion time remained unchanged, MCR and MRR decreased. In addition, absolute convulsion and tonic tension are also reduced due to diabetes. However, the specific force is still similar, which indicates that the observed muscle force decrease is highly correlated with the smaller muscle size[23]. In the DM’s longterm evolution, motor dysfunction is related to neuropathy existence[24]. In type 1 and type 2 DM, it is reported that ankle plantar and dorsiflexion myasthenia is progressive and relevant to the severity of neuropathy. This shows that muscle strength injury involves muscle change itself and nerve control[25]. Therefore, in this study, the skeletal muscle mass loss caused by diabetes was widely reported. In this study, it was observed that the S/T index of the model group was lower than control group. The treatment of Shenling Baizhu powder can reverse the muscle mass and strength damage of soleus caused by diabetes, indirectly highlighting the effect of miR-23a/27a in the development of sarcopenia.
To sum up, our research shows that Shenling Baizhu powder can improve diabetes-induced sarcopenia by up-regulating miR-23a/27a expression, activating the IGF1/Phosphoinositide 3-Kinases (PI3Ks)/Akt signaling pathway and inhibiting the myostatin cascade reaction.
Conflict of interests:
The authors declared no conflict of interests.
References
- Zhu Y, Qian X, Li J, Lin X, Luo J, Huang J, et al. Astragaloside-IV protects H9C2 (2-1) cardiomyocytes from high glucose-induced injury via miR-34a-mediated autophagy pathway. Artif Cells Nanomed Biotechnol 2019;47(1):4172-81.
[Crossref] [Google Scholar] [PubMed]
- Su T, Xiao Y, Xiao YE, Guo QI, Li C, Huang Y, et al. Bone marrow mesenchymal stem cells-derived exosomal miR-29b-3p regulates aging-associated insulin resistance. ACS Nano 2019;13(2):2450-62.
[Crossref] [Google Scholar] [PubMed]
- Li CW, Yu K, Shyh-Chang NG, Li GX, Jiang LJ, Yu SL, et al. Circulating factors associated with sarcopenia during ageing and after intensive lifestyle intervention. J Cachexia Sarcopenia Muscle 2019;10(3):586-600.
[Crossref] [Google Scholar] [PubMed]
- He Z, Yang JJ, Zhang R, Li HT, Wu L, Jiang F, et al. Circulating miR-29b positively correlates with non-alcoholic fatty liver disease in a Chinese population. J Dig Dis 2019;20(4):189-95.
[Crossref] [Google Scholar] [PubMed]
- Li X, Tang Y, Jia Z, Zhao X, Chen M. Decreased expression of miR-24 in peripheral plasma of type 2 diabetes mellitus patients associated with diabetic foot ulcer. Wound Repair Regen 2020;28(6):728-38.
[Crossref] [Google Scholar] [PubMed]
- Su SS, Li BP, Li CL, Xiu FR, Wang DY, Zhang FR. Downregulation of miR-218 can alleviate high-glucose-induced renal proximal tubule injury by targeting GPRC5A. Biosci Biotechnol Biochem 2020;84(6):1123-30.
[Crossref] [Google Scholar] [PubMed]
- Jia Y, Reddy MA, Das S, Oh HJ, Abdollahi M, Yuan H, et al. Dysregulation of histone H3 lysine 27 trimethylation in transforming growth factor-β1-induced gene expression in mesangial cells and diabetic kidney. J Biol Chem 2019;294(34):12695-707.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Wang Q, Yang K, Zheng H, Hu Y. Effects of miR-22-3p targeted regulation of Socs3 on the hepatic insulin resistance in mice with gestational diabetes mellitus. Am J Transl Res 2020;12(11):7287.
- Cheng Y, Wang D, Wang F, Liu J, Huang B, Baker MA, et al. Endogenous miR-204 protects the kidney against chronic injury in hypertension and diabetes. J Am Soc Nephrol 2020;31(7):1539-54.
[Crossref] [Google Scholar] [PubMed]
- Cawthon PM, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, et al. Establishing the link between lean mass and grip strength cut points with mobility disability and other health outcomes: Proceedings of the sarcopenia definition and outcomes consortium conference. J Gerontol Series A 2020;75(7):1317-23.
[Crossref] [Google Scholar] [PubMed]
- Lv LL, Feng Y, Wu M, Wang B, Li ZL, Zhong X, et al. Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ 2020;27(1):210-26.
[Crossref] [Google Scholar] [PubMed]
- Choon Lim Wong G, Narang V, Lu Y, Camous X, Nyunt MS, Carre C, et al. Hallmarks of improved immunological responses in the vaccination of more physically active elderly females. Exercise Immunol Rev 2019;25:20-33.
[Google Scholar] [PubMed]
- Sugimoto K, Tabara Y, Ikegami H, Takata Y, Kamide K, Ikezoe T, et al. Hyperglycemia in non-obese patients with type 2 diabetes is associated with low muscle mass: The multicenter study for clarifying evidence for sarcopenia in patients with diabetes mellitus. J Diabetes Investig 2019;10(6):1471-9.
[Crossref] [Google Scholar] [PubMed]
- Fukuoka Y, Narita T, Fujita H, Morii T, Sato T, Sassa MH, et al. Importance of physical evaluation using skeletal muscle mass index and body fat percentage to prevent sarcopenia in elderly Japanese diabetes patients. J Diabetes Investig 2019;10(2):322-30.
[Crossref] [Google Scholar] [PubMed]
- Zhu P, Liu J, Lu M, Wu G, Lin X, Cai L, et al. Influence and mechanism of miR-99a suppressing development of colorectal cancer (CRC) with diabetes mellitus (DM). Onco Targets Ther 2019;12:10311.
[Crossref] [Google Scholar] [PubMed]
- Gonçalves DA, Silveira WA, Manfredi LH, Graça FA, Armani A, Bertaggia E, et al. Insulin/IGF1 signalling mediates the effects of β2-adrenergic agonist on muscle proteostasis and growth. J Cachexia Sarcopenia Muscle 2019;10(2):455-75.
[Crossref] [Google Scholar] [PubMed]
- Maliszewska K, Adamska-Patruno E, Krętowski A. The interplay between muscle mass decline, obesity, and type 2 diabetes. Pol Arch Intern Med 2019;129(11):809-16.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Song L, Ma Y, Yin Y, Liu X, Luo X, et al. lncRNA MEG8 upregulates miR-770-5p through methylation and promotes cell apoptosis in diabetic nephropathy. Diabetes Metab Syndr Obes 2020:2477-83.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Yao Y, Wang K, Li J, Chu T, Shen H. microRNA-148a-3p alleviates high glucose-induced diabetic retinopathy by targeting TGFB2 and FGF2. Acta Diabetol 2020;57(12):1435-43.
[Crossref] [Google Scholar] [PubMed]
- Wang S, Wei D, Sun X, Li Y, Li D, Chen B. miR-190b impedes pancreatic β cell proliferation and insulin secretion by targeting NKX6-1 and may associate to gestational diabetes mellitus. J Recept Signal Transduct Res 2021;41(4):349-56.
[Crossref] [Google Scholar] [PubMed]
- Xiao Y, Ding J, Shi Y, Lin L, Huang W, Shen D, et al. miR-330-3p contributes to INS-1 cell dysfunction by targeting glucokinase in gestational diabetes mellitus. J Obstetr Gynaecol Res 2020;46(6):864-75.
[Crossref] [Google Scholar] [PubMed]
- Zhang A, Li M, Wang B, Klein JD, Price SR, Wang XH. miRNA-23a/27a attenuates muscle atrophy and renal fibrosis through muscle-kidney crosstalk. J Cachexia Sarcopenia Muscle 2018;9(4):755-70.
[Crossref] [Google Scholar] [PubMed]
- Li H, Fan J, Zhao Y, Zhang X, Dai B, Zhan J, et al. Nuclear miR-320 mediates diabetes-induced cardiac dysfunction by activating transcription of fatty acid metabolic genes to cause lipotoxicity in the heart. Circ Res 2019;125(12):1106-20.
[Crossref] [Google Scholar] [PubMed]
- Cao Y, Zhong M, Zhang Y, Zheng Z, Liu Y, Ni X, et al. Presarcopenia is an independent risk factor for carotid atherosclerosis in Chinese population with metabolic syndrome. Diabetes Metab Syndr Obes 2020;13:81-8.
[Crossref] [Google Scholar] [PubMed]
- Billot M, Calvani R, Urtamo A, Sánchez-Sánchez JL, Ciccolari-Micaldi C, Chang M, et al. Preserving mobility in older adults with physical frailty and sarcopenia: Opportunities, challenges, and recommendations for physical activity interventions. Clin Interv Aging 2020;15:1675-90.
[Crossref] [Google Scholar] [PubMed]