- *Corresponding Author:
- Caifeng Gao
Department of Ophthalmology, Guangdong Women and Children Hospital, Guangzhou, Guangdong Province 511400, China
E-mail: 506982896@qq.com
| Date of Received | 22 January 2022 |
| Date of Revision | 04 November 2022 |
| Date of Acceptance | 08 September 2023 |
| Indian J Pharm Sci 2023;85(5):1466-1471 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the mechanism of sodium tanshinone IIA sulfonate inhibiting hypoxia induced retinal neovascularization in rats. Forty 7 d old clean grade C57BL/6J male neonatal rats were randomly divided into control group, model group, low-dose group and high-dose group. The control group was kept in normal air for 10 d. Oxygen-induced retinopathy was studied in the model group, low-dose group, and high-dose group 30 mg/kg sodium tanshinone IIA sulfonate. The body weight of mice in all groups was measured on the 12th and 17th d after birth, and the area without perfusion around the optic disc was observed and calculated. The number of neovascular endothelial cells was calculated using hematoxylin and eosin staining. The expression of vascular endothelial growth factor, vascular endothelial growth factor-2 and hypoxia-inducible factor-1 were detected by Western blot. The no perfusion area around the optic disc in the model group was raised than that in the control group, and the no perfusion area around the optic disc in the low-dose group, and high-dose group was reduced than that in the model group (p<0.01). In the model group, the inner limiting membrane of retina was proliferated and disordered, and the surface was not smooth; in low-dose group, smooth limiting membrane of the retina, and a small number of endothelial cells could be seen breaking through the limiting membrane; in the high-dose group, the limiting membrane of retina was relatively complete and smooth, and the tissue structure of each layer could be seen. The number of neovascular endothelial cells in the model group was raised than that in the control group, and the number of neovascular endothelial cells in the lowdose group, and high-dose group was reduced than that in the model group, as the dose increased, it decreased (p<0.01). Vascular endothelial growth factor, vascular endothelial growth factor-2 and hypoxia-inducible factor-1 in low-dose group, and high-dose group were raised than those in control group, and those proteins expression level of in model group was reduced than control group, as the dose increased, it decreased (p<0.05). Sodium tanshinone IIA sulfonate can inhibit hypoxia induced retinal neovascularization in a dose-dependent manner, and its mechanism may be related to the regulation of vascular endothelial growth factor pathway related proteins by sodium tanshinone IIA sulfonate.
Keywords
Sodium tanshinone IIA sulfonate, hypoxia, retinal neovascularization, hyperplasia
The formation of retinal neovascularization is one of the serious complications of intraocular diseases. The accompanying pathological symptoms such as hemorrhage, hyperplasia and exudation seriously damage the structure of the eye and its function, thus leading to visual impairment and even blindness in patients[1]. The current methods of clinical treatment of retinal neovascularization include surgery and laser treatment. The surgical treatment is difficult, expensive and has a higher incidence of complications and fewer clinical applications[2]. Laser surgery has certain side effects and increases the patient's pain[3]. Therefore, finding a safe and effective drug that effectively inhibits retinal neovascularization has become the focus of attention of ophthalmologists both at home and abroad. Sodium tanshinone IIA sulfonate is an effective monomer extracted from the traditional Chinese medicine Salvia miltiorrhiza (S. miltiorrhiza), it has the effects of scavenging oxygen free radicals, placing myocardial damage, and inhibiting the function of white blood cells, placing myocardial infarction, and ischemic heart disease, pulmonary hypertension, and chronic obstructive pulmonary disease often require its use[4-6]. High glucose-induced retinal neovascularization can be inhibited by tanshinone IIA sodium sulfonate, according to recent studies, and the effect of retinal neovascularization caused by hypoxia is not yet clear[7]. In this experiment, forty 7 d old clean-grade C57BL/6J male neonatal rats were used as observation subjects to analyze the mechanism of tanshinone IIA sulfonate inhibiting hypoxia-induced retinal neovascularization in rats.
Materials and Methods
General information:
Experimental animals: Forty 7 d old clean-grade C57BL/6J male newborn rats were selected and bred together with lactating female rats, provided by Guangdong Medical Experimental Animal Center. This experimental animal was operated in accordance with the "Regulations on the Administration of Laboratory Animals".
Experimental gas: A mixture of nitrogen and oxygen (75 % oxygen+25 % nitrogen) was used, provided by Guangzhou Iron and Steel Co., Ltd.
Experimental drug: With a purity of >98 %, tanshinone IIA sodium sulfonate is provided by the National Institute for the Control of Pharmaceutical and Biological Products.
Methods:
Forty C57BL/6J male newborn rats were randomly divided into the Control Group (CG), the Model Group (MG), the Low-Dose Group (LDG) and the High-Dose Group (HDG). The CG was kept in a normal air environment for 10 d, and the MG, LDG and HDG were constructed. The model of retinopathy induced by oxygen was placed in an autophagy closed container, and the oxygen flow was adjusted to maintain 75 %±2 %. Soda lime was placed at the bottom of the container to absorb the Carbon dioxide (CO2) exhaled by the mice. The mice were kept in the container for 5 d and then placed in a normal air environment for 5 d and raised for 5 d. The LDG and HDG were given intraperitoneal injections of 10 mg/kg and 30 mg/ kg of tanshinone IIA sodium sulfonate from the 1st d of modeling, and the CG and MG were given intraperitoneal injections of the same amount of normal saline.
Observation indicators:
Mice were measured for their body weight at 12th d and 17th d after birth in each group.
Retinal preparation: After weighing on the 17th d, the mice were sacrificed by neck dissection, and the eyeballs were placed in Eppendorf (EP) tubes and fixed with 4 % neutral paraformaldehyde. The sphere with small surgical scissors was cut and the retina was peeled. It was washed with PBS, placed in blocking solution, blocked at 4° overnight, added Fluorescein Isothiocyanate (FITC)-conjugated isolectin B4, and avoided light for 5 h at room temperature; it was washed with Phosphate Buffer Saline (PBS), the retina was placed under a microscope, 5 incisions were made on the retina with small surgical scissors, and placed on a glass slide. The non-perfusion area around the optic disc was measured under a fluorescence microscope.
Hematoxylin and Eosin (HE) staining: After weighing on the 17th d, the mice were sacrificed by neck dissection, and the eyeballs were placed in EP tubes and fixed with 4 % neutral paraformaldehyde. Paraffin sections were taken, dewaxed with xylene, rinsed with Double Distilled Water (DDW), rinsed with gradient alcohol, rinsed with DDW, 2 drops of hematoxylin dye solution was added to each section, stained at room temperature for 10 min, rinsed with tap water for 3 min. 1 drop of 1 % hydrochloric acid ethanol was added to each slice, the color was separated at room temperature for 10 s, rinsed with DDW, 2 drops of 1 % Eosin Y dye solution was added to each slice, stained for 5 min at room temperature, rinsed with tap water and alcohol gradient, 1 drop of resin was dropped to mount the slide. A microscope was used to observe the pathological changes of the retinas of mice in each group, and the number of nuclei of neovascular endothelial cells was calculated.
Western blot: The Vascular Endothelial Growth Factor (VEGF), VEGFR-2 and Hypoxia Inducible Factor Alpha (HIF-α) of each group of mice were monitored by Western blot. After taking out the sample, it was broken with an ultrasonic pulverize, lysed on ice for 30 min, and centrifuged at 14 000 rpm for 15 min at 4°. Take supernatant and determined the protein concentration. Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE), membrane transfer, immune response were performed. The color of the bands obtained by Western blot was developed by the Enhanced Chemiluminescence (ECL) kit. Pictures were taken by the BIO-RAD gel imaging system and the gray value of the protein band data was analyzed. Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) was used as the reference protein to calculate the relative expression of Vascular Endothelial Growth Factor A (VEGFA), VEGFR-2 and HIF-1α proteins.
Statistical methods:
The data in this study were all used Statistical Package for the Social Sciences (SPSS) 20.0 software for statistical data analysis. The comparison of all measurement data conforming to the normal distribution was expressed as (x±s), the comparison between multiple groups was by single- factor analysis of variance, and the pairwise comparison was done by Student–Newman–Keuls (SNK)-q test; enumeration data were expressed as percentages, and the Chi-square (χ2) test was used for comparison between groups. The statistical results were statistically significant at p<0.05, and ap<0.05 compared with the CG; bp<0.05 compared with the MG and cp<0.05 compared with the LDG.
Results and Discussion
At 12 d, mice in each group had similar body weights; at 17 d, the weight of mice in the MG was reduced than that in the CG, and the weight of mice in the LDG and HDG were raised than that in the MG, and it increased with the increase in dose (p<0.01) as shown in Table 1.
| Group | Case | 12 d weight (g) | 17 d weight (g) |
|---|---|---|---|
| Control | 10 | 5.15±0.14 | 8.12±0.56 |
| Model | 10 | 4.95±0.21 | 5.01±0.18a |
| Low dose | 10 | 5.08±0.16 | 6.24±0.24ab |
| High dose | 10 | 5.12±0.18 | 6.85±0.36abc |
| F | 2.551 | 125.59 | |
| p | 0.07 | <0.001 | |
Note: ap<0.001; abp<0.001 and abcp<0.001
Table 1: Changes in Body Weight of Mice in Each Group
The non-perfusion area around the optic disc of mice in the MG was raised than that of the CG. The non-perfusion area around the optic disc of mice in the LDG and HDG was reduced than that in the MG, and with increasing doses, the effect decreased (p<0.01) as shown in Table 2.
| Group | Case | Non-perfusion area (%) |
|---|---|---|
| Control | 10 | 1.28±0.21 |
| Model | 10 | 16.52±2.18a |
| Low dose | 10 | 12.52±2.85ab |
| High dose | 10 | 4.82±1.07abc |
| F | 138.254 | |
| p | <0.001 | |
Note: ap<0.001; abp<0.001 and abcp<0.001
Table 2: The Non-Perfusion Area around the Optic Disc of Mice in Each Group
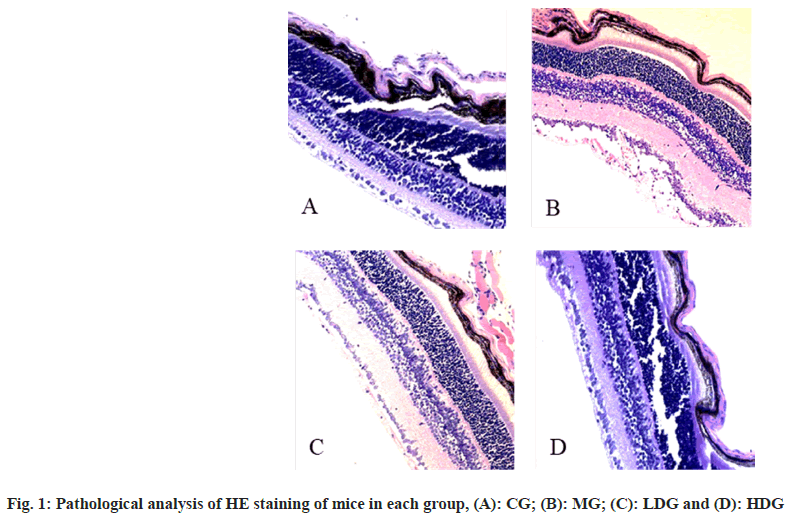
In CG, the Internal Limiting Membrane (ILM) of the retina was intact and smooth, and the tissue structure of each layer was clearly visible. In the MG, the ILM of the retina appeared proliferation and disorder, and the surface was not smooth. The endothelial cell nucleus that broke through the ILM could be seen, either alone or in clusters, or forming a capillary wall containing several red blood cells; in the LDG, the ILM of the retina was smoother, and a small amount of endothelial cell nuclei that broke through the ILM were seen; in the HDG, the ILM of the retina was relatively complete and smooth, with various layers of tissue structure visible as shown in fig. 1.
The number of neovascular endothelial cell nuclei in the MG was raised than that of the CG. The number of neovascular endothelial cell nuclei in the LDG and HDG was reduced than that of the MG, and with increasing doses, the effect decreased (p<0.01) as shown in Table 3.
| Group | Cases | Number of nuclei of neovascular endothelial cells |
|---|---|---|
| Control | 10 | 2.16±0.37 |
| Model | 10 | 52.87±20.19a |
| Low dose | 10 | 26.37±10.59ab |
| High dose | 10 | 14.67±2.89abc |
| F | 35.412 | |
| P | <0.001 |
Note: ap<0.001; abp<0.001 and abcp<0.001
Table 3: Comparison of the Number of Nuclei of Neovascular Endothelial Cells of Mice in Each Group
The levels of VEGFA, VEGFR-2 and HIF-1α in the MG were raised than those in the CG. The levels of VEGFA, VEGFR-2 and HIF-1α in the LDG and HDG were reduced than those in the MG, and increased with the dose increase (p<0.05) as shown in Table 4.
| Group | n | VEGFA | VEGFR-2 | HIF-1α |
|---|---|---|---|---|
| Control | 10 | 0.73±0.09 | 0.62±0.07 | 0.31±0.06 |
| Model | 10 | 0.96±0.06a | 0.93±0.09a | 0.70±0.03a |
| Low dose | 10 | 0.46±0.08ab | 0.39±0.12ab | 0.51±0.09ab |
| High dose | 10 | 0.35±0.03abc | 0.16±0.13abc | 0.41±0.01abc |
| F | 158.367 | 97.67 | 87.22 | |
| P | <0.001 | <0.001 | <0.001 |
Note: ap<0.001; abp<0.001 and abcp<0.001
Table 4: Comparison of VEGFA, VEGFR-2 and HIF-1α Protein Expression of Mice in Each Group
Retinal ischemia hypoxic eye disease is a serious intraocular disease. Presently, most studies believe that factors such as ischemia, hypoxia, inflammation, tumors and trauma can lead to the formation of retinal neovascularization. Among them, ischemia and hypoxia are the most common factors. Retinal ischemia-hypoxic eye disease has become a killer to deprive the light of the people. Retinal neovascularization is a common complication[8]. There is an urgent need to explore the mechanism of retinal neovascularization and find drugs to treat it.
S. miltiorrhiza is a plant of the Salvia family Lamiaceae, and is the main source of a large number of active natural products. Among them, the fat-soluble components and water-soluble components are active and have a wide range of clinical applications[9]. Tanshinone IIA sodium sulfonate is a fat-soluble active ingredient extracted from the rhizome of S. miltiorrhiza, and it is also one of the most active fat-soluble components in S. miltiorrhiza[10]. Recent studies have found that tanshinone IIA sodium sulfonate can slow the proliferation of vascular intima by inhibiting the proliferation of vascular smooth muscle cells[11]. However, there is no research report on the biological effects and related mechanisms of tanshinone IIA sulfonate on hypoxia-induced retinal vascular endothelial cells.
The non-perfusion area of blood vessels is caused by the reactive contraction of retinal blood vessels under hypoxic environment, which can stimulate the formation of abnormal new blood vessels in the corresponding area[12]. In this experiment, we compared the non-perfusion area around the optic disc by retinal spreading technology to analyze the degree of disease and the treatment effects, and found that the non-perfusion area around the optic disc in the MG was raised than that in the CG, and the non-perfusion area around the optic disc in the LDG and HDG were reduced than the CG, it decreases with increasing dose, suggesting that tanshinone IIA sodium sulfonate can slow down the process of hypoxia-induced retinal vasoconstriction, and it is drug-dependent. Under normal circumstances, the structure of each layer of the retina is tightly arranged, and the cell nucleus does not break through the ILM[13]. In this experiment, HE staining of pathological tissue staining analyzed the degree of disease and the treatment effect of tanshinone IIA sodium sulfonate by the cell nuclei number that broke through the ILM and found that the number of neovascular endothelial cell nuclei in the MG was raised than that of the CG. The number of rat neovascular endothelial cell nuclei was reduced than that of the MG, and decreased with increasing dose, suggesting that the sodium tanshinone IIA sulfonate can maintain the structure of each layer of the retina and slow down the damage of hypoxia to the retina structure.
The formation of new blood vessels is a complex biological process dominated by endothelial cells[14]. VEGF is a strong stimulator of endothelial cell-specific mitogen and angiogenesis, it can bind to receptors to participate in the angiogenesis process under pathological or physiological conditions, and is considered to be a key factor involved in the formation of neovascularization[15]. In the process of retinal angiogenesis, up-regulates the level of VEGF, and then activates VEGFR-1 and VEGFR-2 to regulate the angiogenesis process[16]. Batchelor et al.[17] found that activation of VEGFR-2 can stimulate endothelial cell proliferation and survival, and can also induce angiogenesis and increase in microvascular permeability. HIF-1α is a transcription factor that exists as a heterodimer[18]. Studies have shown that hypoxia increases the expression of HIF-1α mRNA and protein, as well as promoting the transcription of VEGF downstream. In this experiment, the levels of VEGFA, VEGFR-2 and HIF-1α proteins in the MG were raised than those in the CG, and those in the LDG and HDG were reduced than MG, suggesting that tanshinone IIA sodium sulfonate can inhibit retinal neovascularization in mice, The VEGF pathway may be involved in the regulation of these proteins.
In conclusion, the sodium tanshinone IIA sulfonate can inhibit hypoxia-induced retinal neovascularization in rats, and the effect is dosedependent. It is possible that sodium tanshinone IIA sulfonate regulates proteins involved in the VEGF pathway.
Acknowledgements:
This work was supported by Administration of Traditional Chinese Medcine of Guangdong Province (No. 20202021).
Author’s contributions:
Ge Mu and Jiao Zheng have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Li Y, Busoy JM, Zaman BA, Tan QS, Tan GS, Barathi VA, et al. A novel model of persistent retinal neovascularization for the development of sustained anti-VEGF therapies. Exp Eye Res 2018;174:98-106.
[Crossref] [Google Scholar] [PubMed]
- Leong BC, Freund KB. Optical coherence tomography angiography in a patient with diabetes and preretinal neovascularization. JAMA Ophthalmol 2019;137(11):e190122.
[Crossref] [Google Scholar] [PubMed]
- Campbell M, Doyle SL. Current perspectives on established and novel therapies for pathological neovascularization in retinal disease. Biochem Pharmacol 2019;164:321-5.
[Crossref] [Google Scholar] [PubMed]
- Gu Y, Deng F, Deng M, Zhang L, Hu Y, Xu G. Effects of tanshinone-IIA sodium injection post-conditioning combined with controlled low central venous pressure on the hepatic ischemia-reperfusion injury. J Clin Anesthesiol 2017;33(7):632-6.
- Zhou J, Zhang BC, Wen CM. Curative effect of sofren injection combined sulfotanshinone sodium injection treatment of patients with cerebral infarction. Liaoning J Tradit Chin Med 2017;44(6):1202-4.
- Chen F, Ma F, Liu YM. Sodium tanshinone IIA sulfonate attenuates aortic valve calcification via regulating ER stress related inflammatory pathways. Chin J Basic Med Tradit Chinese Med 2018;24(7):931-4.
- Cheng J, Chen T, Li P, Wen J, Pang N, Zhang L, et al. Sodium tanshinone IIA sulfonate prevents lipopolysaccharide-induced inflammation via suppressing nuclear factor-κB signaling pathway in human umbilical vein endothelial cells. Can J Physiol Pharmacol 2018;96(1):26-31.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Zhang YQ, Zhang Y. Effect of EphB4/EphrinB2 on neovascularization in rats with diabetic retinopathy. Recent Adv Ophthalmol 2020;10:925-8.
- Liu Z, Luo L, LI F, Chen Y, Qin S, HE M, et al. Experimental study on the effect of sulfotanshinone sodium in lung fibrosis by oleic acid in ALI rats. J Pract Med 2018;34(3):367-70.
[Crossref] [Google Scholar] [PubMed]
- Zhang WJ, Xu S, Weng XT. Systematic evaluation of tanshinone IIA sodium sulfonate on hemorheology in chronic cor pulmonale. Tradit Chin Drug Res Clin Pharmacol 2017;28(6):818-23.
- Yang XJ, Qian JX, Wei Y, Guo Q, Jin J, Sun X, et al. Tanshinone IIA sodium sulfonate attenuates LPS-induced intestinal injury in mice. Gastroenterol Res Pract 2018;2018:9867150.
[Crossref] [Google Scholar] [PubMed]
- Yang XY, Xu GX. Study on the mechanism of action of kallikrein binding protein in retinal neovascular disease. Straits Sci 2019;156(12):35-9.
- Liu WL. microRNA‐9 inhibits retinal neovascularization in rats with diabetic retinopathy by targeting vascular endothelial growth factor A. J Cell Biochem 2019;120(5):8032-43.
[Crossref] [Google Scholar] [PubMed]
- Eresch J, Stumpf M, Koch A, Vutukuri R, Ferreirós N, Schreiber Y, et al. Sphingosine kinase 2 modulates retinal neovascularization in the mouse model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 2018;59(2):653-61.
[Crossref] [Google Scholar] [PubMed]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9(6):669-76.
[Crossref] [Google Scholar] [PubMed]
- Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 1999;18(14):3964-72.
[Crossref] [Google Scholar] [PubMed]
- Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 2007;11(1):83-95.
[Crossref] [Google Scholar] [PubMed]
- Chen P, Duan X, Li X, Li J, Ba Q, Wang H. HIPK2 suppresses tumor growth and progression of hepatocellular carcinoma through promoting the degradation of HIF-1α. Oncogene 2020;39(14):2863-76.
[Crossref] [Google Scholar] [PubMed]