- *Corresponding Author:
- Li Chen
Department of Gynaecology and Obstetrics, Tianjin Medical University, Tianjin 300203, China
E-mail: 18617789629@163.com
| Date of Received | 05 January 2023 |
| Date of Revision | 24 August 2023 |
| Date of Acceptance | 05 December 2023 |
| Indian J Pharm Sci 2023;85(6):1766-1772 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The paper aimed to investigate the mechanism of thymopentin alleviating cisplatin-induced premature ovarian failure by reducing endoplasmic reticulum pressure in granulosa cells. Mice at 2 mo of age were intraperitoneally injected with cisplatin to establish premature ovarian failure model. Ovarian premature failure model was induced by granulosa cells treated with cisplatin (10 μm) for 24 h. The mice were getting into the following groups; control group, premature ovarian failure group, thymopentin+premature ovarian failure group. Isolated and cultured granulosa cells were divided into granulosa cells group, cisplatin induced group and thymopentin group. Cisplatin induces mitochondrial integrity but mitochondrial damage, membrane swelling and rupture. The mitochondrial damage rate in cisplatin induced group was higher than control group (p<0.05), and thymopentin significantly improved the mitochondrial damage induced by cisplatin. Follicle stimulating hormone receptor and estradiol expression horizontals in premature ovarian failure were reduced than control group, the expression horizontals of follicle stimulating hormone receptor and estradiol in thymopentin+premature ovarian failure group were raised than premature ovarian failure group (p<0.05), and the horizontal of follicle stimulating hormone in premature ovarian failure group was raised than control group (p<0.05). The horizontal of follicle stimulating hormone in thymopentin+premature ovarian failure group was lower than that in premature ovarian failure. Premature ovarian failure atretic follicle rate was higher than control group, atretic follicle rate in thymopentin+premature ovarian failure was lower than premature ovarian failure, and the ratio of original follicle to total follicle number in premature ovarian failure group was lower than control group. Thymopentin can reduce endoplasmic reticulum stress and apoptosis of granulosa cells induced by cisplatin, increase the number of follicles, prevent follicle atretic, and participate in the protection of cisplatin induced premature ovarian failure, which provides the basis for clinical drug treatment of premature ovarian failure.
Keywords
Thymopentin, granulosa cell, premature ovarian failure, gonadotropin, cisplatin
Premature Ovarian Failure (POF) is the loss of ovarian function before the age of 40, and the number of follicles in the ovary decreases rapidly or there are few remaining follicles[1]. The proliferation and differentiation of Granulosa Cell (GC) in follicles adjust the maturation of oocytes, so severe endoplasmic reticulum stress in GCs can bring about ovarian dysfunction[2,3]. The hyperplasias of GCs are closely going hand in hand endoplasmic reticulum stress. Endoplasmic reticulum plays an important role in albumen folding, transportation, but the microenvironment of endoplasmic reticulum should be interfered by endoplasmic reticulum stress by Calcium ions (Ca2+) deficiency, hypoxia, which is in the company of excessive accumulation of unfolded in endoplasmic reticulum. Therefore, the endoplasmic reticulum unfolded protein reaction is initiated to remittence endoplasmic reticulum stress. The role of endoplasmic reticulum stress, which involves many physiological processes[4]. In addition, studies have shown that endoplasmic reticulum stress is participating in many processes of female reproduction. And the apoptosis pathway mediated by endoplasmic reticulum stress can trigger ovarian atresia[5]. Thymopentin (TP5) is a synthetic thymogenesis, which has similar biological avidity to thymopoietin. In this study, the protective function of TP5 on follicles and GCs was discussed by creating POF and treating primary cultured GCs with cisplatin. TP5 can save ovarian function by relieving endoplasmic reticulum stress.
Materials and Methods
Materials:
Experimental animals: The experimental animal center provided 2 mo old mice for the study, 24°±2° light condition, and cisplatin was injected intraperitoneally for 10 d to establish POF model. Animal procedures strictly adhered to the guidelines set forth by the National Institutes of Health.
Isolation and culture of primary ovarian GC: Ovarian follicles dissociated primary GC cultured according to the previous method. After injecting 20 U Pregnant Mare Serum Gonadotropin (PMSG) for 48 h, GCs was placed in a Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) 1X petri dish containing 10 % (v/v) Fetal Bovine Serum (FBS) (Gibraltar, Stahly Island), and educated for 48 h at 37° and 5 % Carbon dioxide (CO2). According to the experimental requirements, after counting with a hemocytometer, the corresponding amount of GC was coated on the culture plate. The POF model induced by GCs for 24 h was treated with cisplatin (10 μM).
Experimental grouping: According to the purpose of the experiment, mice were divided into control group (healthy mice raised under normal conditions were injected with normal saline as a blank control, n=20), POF group (as mentioned above, mice were injected with cisplatin at 5.0 mg/kg intraperitoneally to establish POF model, n=20), and thymopentin+POF group (TP5 with a certain concentration was given on the basis of POF mice, n=20). The isolated and cultured GC cells were beaked into the following groups; GCs group (GC incubated in conventional culture medium as experimental control), cisplatin-induced group (cells were treated with 10 μM cisplatin for 24 h to guide POF model), and thymopentin group (GC cells were pretreated with 4 μg/ml TP5 before POF model was induced by cisplatin).
Methods:
Immunohistochemistry and immunofluorescence: Images were obtained by NIKON 80i. The primary antibodies involved Glucose-Regulated Protein 78 (GRP78), X-Box Binding Protein 1 (XBP1), C/EBP Homologous Protein (CHOP) and Bcl-2-Associated X Protein (BAX), etc., Elabscience provided Horseradish Peroxidase (HRP)-binding secondary antibodies.
Real-time quantitative Polymerase Chain Reaction (PCR): TRIzol reagent extracted total messenger Ribonucleic Acid (mRNA) from tissue or cell samples. According to PrimeScript RT kit, transcript mRNA was reversed to obtain complementary Deoxyribonucleic Acid (cDNA), and then subsequent testing was performed.
Preparation of mitochondrial suspension and detection of mitochondrial function: The cells were harvested in a pre-cooling medium including Tris-Hydrochloride (HCl), sucrose and Ethylenediaminetetraacetic Acid (EDTA). The mitochondrial microspheres were resuspended in the above 5 ml buffer and centrifuged. High purity mitochondrial microspheres were resuspended in sucrose, Potassium Phosphate monobasic (KH2PO4), Magnesium chloride (MgCl2), Potassium chloride (KCl), 5 mm 4-(2-Hydroxyethyl)-1-Piperazineethanesulfonic Acid (HEPES) and EDTA. The albumen concentration was determined by Bicinchoninic Acid (BCA) protein determination kit, and the protein concentration was adjusted to 100-1000 μg/ml. Metalloproteinase (MMP) (18) and 1-Methyl-4-Phenyl-1, 2, 3,6-Tetrahydropyridine (mPTP) were measured by mitochondrial suspension.
Enzyme-Linked Immunosorbent Assay (ELISA): Cells were collected, and the horizontal of GRP78, XBP1s and CHOP in cell samples were detected using ELISA kit. Serum was collected; the eyeball vein blood sample was taken and centrifuged at 3000 rpm for 15 min. The horizontal of FSH or Estradiol (E2) in the sample was detected by ELISA kit.
Hematoxylin and Eosin (HE) staining and follicular counting: 4 % paraformaldehyde fixed the ovaries with tissues stained with HE solutions, and the pathological structure of ovaries was observed and the amount of follicles was calculated. Follicle counting was carried out. Each group randomly selected 5 consecutive slices with the largest cross section in the center of ovary, and each slice took 5 non-repeated slices for statistical unpack, and the equally of 5 slices was taken. Primitive follicle is an undeveloped follicle composed of oocytes, which are partially wrapped by flat squamous preadipocytes. In the atresia follicle, the oocyte chromosomes were dissolved compactly, the nucleus was atrophied, and the GCs on the surface of the follicle were condensed. Granular cells from atresia follicles break away from the membrane, float in follicular fluid.
Statistical analysis:
The data were expressed as mean±standard deviation, and were statistically analyzed by SPSS software (Version 23.0). One-way Analysis of Variance (ANOVA) and Fisher exact test were used to analyze the statistical differences among the groups. p<0.05 was statistically significant.
Results and Discussion
The results showed that the expressions horizontal of GRP78 and XBP1s in cisplatin-induced group were lower than GCs group, while GRP78 and XBP1s expressions horizontal in thymopentin were higher than cisplatin induction, and CHOP expression horizontal in cisplatin induction was higher than GCs (p<0.05) as shown in Table 1.
| Group | GRP78 (pg/ml) | XBP1s (pg/ml) | CHOP (pg/ml) |
|---|---|---|---|
| GCs | 1.94±0.15 | 1.95±0.16 | 1.03±0.03 |
| Cisplatin induction | 1.03±0.02 | 1.08±0.04 | 1.92±0.17 |
| Thymic pentapeptide | 1.95±0.15 | 1.89±0.14 | 1.14±0.08 |
| Variance ratio | 8.332 | 13.419 | 10.628 |
| p | 0.004 | 0.017 | 0.024 |
Table 1: Elisa Analysis of Endoplasmic Reticulum Stress-Specific Protein Expression (x̄±s).
The expression horizontal of Cell Cycle Progression Gene 1 (CCPG1) and Light Chain 3 (LC3II) protein in cisplatin-induced group was higher than that in GCs group (p<0.05), while the expression horizontal of CCPG1 and LC3II protein in thymopentin group was lower than that in cisplatin-induced group (p<0.05) as shown in Table 2.
| Group | CCPG1 | LC3II |
|---|---|---|
| GCs | 1.12±0.04 | 1.05±0.03 |
| Cisplatin inducion | 1.95±0.16 | 1.99±0.20 |
| Thymic pentapeptide | 1.08±0.05 | 1.07±0.04 |
| Variance ratio | 12.773 | 9.065 |
| p | 0.002 | 0.031 |
Table 2: Western Blot Analysis (x̄±s).
The expression of BAX and caspase 3 mRNA in cisplatin-induced group was higher than GCs group (p<0.05), while the expression of BAX and caspase 3 mRNA in thymopentin group was lower than cisplatin-induced group (p<0.05) as shown in Table 3.
| Group | BAX | Caspase 3 |
|---|---|---|
| GCs | 1.04±0.03 | 1.06±0.05 |
| Cisplatin inducion | 1.92±0.15 | 2.05±0.18 |
| Thymic pentapeptide | 1.13±0.06 | 1.15±0.07 |
| Variance ratio | 9.553 | 12.187 |
| p | 0.005 | 0.012 |
Table 3: Real-Time Quantitative PCR Analysis (x̄±s).
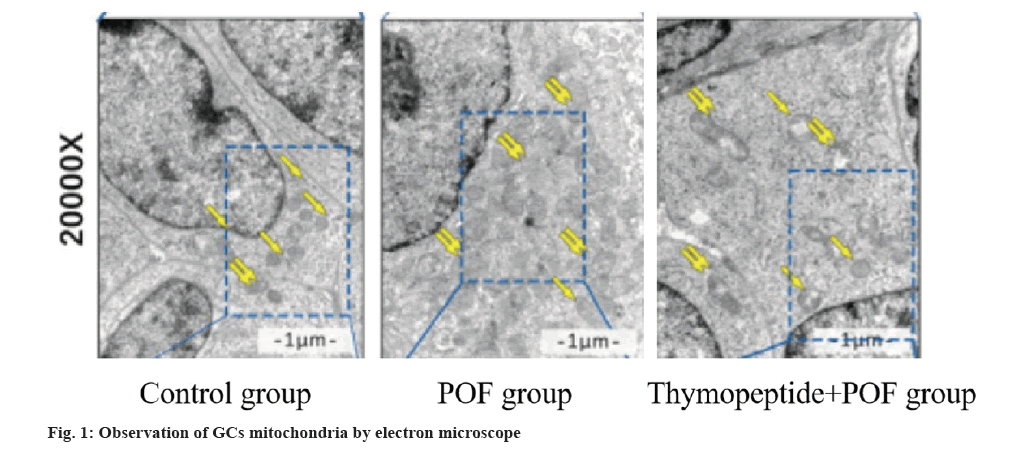
MMP and mPTP were measured by mitochondrial suspension. MPP in cisplatin-induced group was lower than GCs group (p<0.05), MPP in thymopentin group was higher than cisplatin-induced group (p<0.05), mPTP in cisplatin-induced group was higher than GCs group (p<0.05), and mPTP in thymopentin group was lower than cisplatin-induced group (p<0.05). The function of mitochondria was observed by electron microscope. The results suggested that the mitochondria in cisplatin-induced group were intact, but the mitochondria were damaged, and the membrane was swollen and ruptured. The mitochondrial damage rate in cisplatin-induced group was higher than control group (p<0.05), and thymopentin significantly improved the mitochondrial damage induced by cisplatin (p<0.05) as shown in fig. 1 and Table 4.
| Group | MMP (red/green) | mPTP (%) |
|---|---|---|
| GCs | 1.58±0.32 | 0.21±0.02 |
| Cisplatin induction | 1.02±0.14 | 0.62±0.05 |
| Thymic pentapeptide | 1.53±0.28 | 0.37±0.03 |
| Variance ratio | 11.533 | 13.416 |
| p | 0.002 | 0.013 |
Table 4: Analysis of GCs Mitochondrial Function (x̄±s).
The expression of ovarian GC-specific protein Follicle Stimulating Hormone Receptor (FSHR) was detected by immunofluorescence, and the concentrations of FSH and E2 in the samples were checked by ELISA kit. The expression levels of FSHR and E2 in POF were lower, while those in thymopentin +POF were higher, and the FSH level in POF was higher (p<0.05) as shown in Table 5.
| Group | FSHR | E2 (pg/ml) | FSH (ng/ml) |
|---|---|---|---|
| Control | 1.83±0.15 | 23.46±3.76 | 5.33±0.62 |
| POF | 1.15±0.06 | 15.42±1.95 | 13.49±1.88 |
| Thymopeptide+POF | 1.92±0.18 | 20.73±2.04 | 7.68±1.14 |
| Variance ratio | 12.056 | 11.491 | 10.674 |
| p | 0.012 | 0.006 | 0.026 |
Table 5: Analysis of POF Ovarian Function (x̄±s).
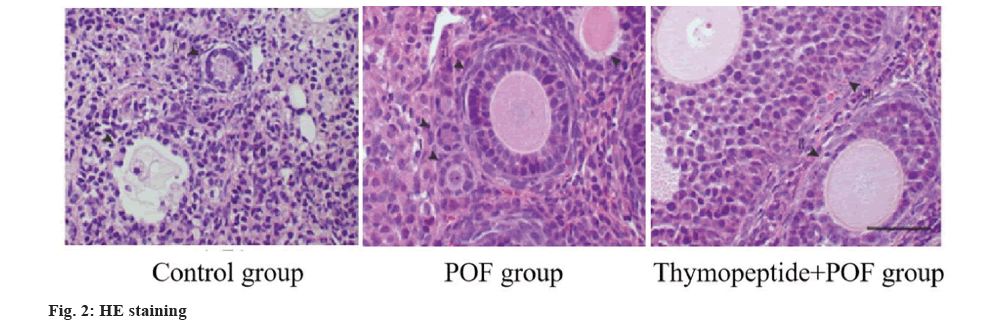
By counting and analyzing the follicles by HE staining, the ratio of atresia follicles in POF group was higher, while that in thymopentin+POF was lower than POF, and the ratio of primordial follicles to total follicles in POF group was lower than control group (p<0.05) as shown in fig. 2 and Table 6.
| Group | Atresia follicular rate | Primordial follicle/total follicle number |
|---|---|---|
| Control | 28.37±3.18 | 37.29±4.67 |
| POF | 42.64±5.52 | 18.45±2.35 |
| Thymopeptide+POF | 30.65±3.49 | 32.67±4.05 |
| Variance ratio | 14.026 | 9.775 |
| p | 0.002 | 0.017 |
Table 6: He Staining and Follicle Count (x̄±s).
POF is used to represent women younger than 40 y old, which is characterized by amenorrhea, hypergonadism. Clinical manifestations are varied, and many different illness can make for POF[6].
Endoplasmic reticulum is the primary organelle[7,8]. However, many pathological requirement, eventually bring about endoplasmic reticulum stress. Endoplasmic reticulum stress is a pathological state of endoplasmic reticulum. As a key organelle for albumen, endoplasmic reticulum’s homeostasis also effects normal function maintenance[9-11]. Once the dynamic equilibrium, notably the dynamic equilibrium of protein synthesis, it will induce endoplasmic reticulum stress, and if it cannot be relieved quickly, it will also lead to unfolded protein reaction, leading to many illness. In addition, many studies in this field in recent years have pointed out that the induction and development of endoplasmic reticulum pressure in ovarian GC go hand in hand in the ubiquitination course of specific molecules in cells[12]. GC cells play a vital role in biological course, but the role of TP5 in GC has not been thoroughly studied[13,14]. In this study, we found that TP5 was helpful to maintain the steady state of GCs endoplasmic reticulum and provide a stationary internal environment for follicular advancement. The stress-specific molecules of endoplasmic reticulum were tested in the POF model of GC cells, and the horizontals of GRP78 and XBP1s reduced. CHOP expression activated the apoptosis pathway. It has been reported that endoplasmic reticulum stress can guide endoplasmic reticulum molecular chaperones expression to perform protective action. Therefore, cisplatin-induced POF framework was through endoplasmic reticulum pressure.
Mitochondrial dysfunction can induce apoptosis of GC[15]. The aging of GC are the reasons for the reduce of ovarian reserve function in POF patients[16]. Granular cell injury is usually related to mitochondrial dysfunction of POF. Mitochondrial apoptosis signaling pathway plays a critical role in endoplasmic reticulum stress, and organ damage can guide mitochondrial dysfunction[17,18]. In this study, endoplasmic reticulum stress was related to mitochondrial morphological changes (swelling or membrane rupture) and mitochondrial function (mitochondrial membrane potential and mitochondrial permeability), but TP5 protected mitochondria by restraining endoplasmic reticulum stress.
Endoplasmic reticulum stress-induced autophagy and apoptosis are common consequences caused by endoplasmic reticulum stress, which are usually related to calcium ions and their activated kinases[19]. Persistent endoplasmic reticulum stress can activate reticular phagocytosis and key functions of endoplasmic reticulum[20]. Our results showed that ovarian follicular atresia get at apoptosis mediated by endoplasmic reticulum stress, which eventually led to POF, and the role were accompanied by endoplasmic reticulum stress activation and reticular phagocytosis. Consistent with this, endoplasmic reticulum stress triggered GC apoptosis to participate in ovarian follicular atresia, ovarian aging and POF. Endoplasmic reticulum stress interferes with endoplasmic reticulum function, further initiates autophagy related to endoplasmic reticulum stress, and reestablishes endoplasmic reticulum balance[21,22]. Otherwise, unresolved endoplasmic reticulum stress will inevitably lead to cell death by inducing apoptosis. Our results showed that the ovarian follicular atresia induced by endoplasmic reticulum stress initiated selective autophagy-reticular phagocytosis in GC apoptosis in vitro. Endoplasmic reticulum stress activates reticular phagocytosis in GC apoptosis and follicular atresia. CCPG1 and LC3II are atypical autophagy cargo receptors, which are very important for reticular phagocytosis and endoplasmic reticulum protein homeostasis. CCPG1 is a transmembrane endoplasmic reticulum receptor, which can respond to endoplasmic reticulum stress signals. Endoplasmic reticulum stress is a short dynamic procedure, easily influenced by other element[23]. In order to avoid stimulating endoplasmic reticulum homeostasis in the process of primary GCs separation, normal GCs received cisplatin instead of others. After cisplatin treatment, cells accumulated many amounts of misfolded albumen, which led to endoplasmic reticulum stress. Unfolded Protein Response (UPR) pathway activation relieved the transient endoplasmic reticulum pressure[24,25]. This study confirmed that cisplatin induction GCs cell apoptosis and increased BAX and caspase 3 activities by inducing severe endoplasmic reticulum stress. In addition, studies have proved that TP5 plays a protective part in endoplasmic reticulum stress. In GCs-POF and mouse POF models, TP5 can relieve cisplatin-induced endoplasmic reticulum stress, regulate hormone balance, debase the number of atresia follicles and improve ovarian function.
To sum up, this study shows that TP5 can debase the stress and apoptosis of GCs induced by cisplatin, increase the number of follicles, prevent follicular atresia, and participate in the protection of POF induced by cisplatin, which supply a basis for clinical drug treatment of POF.
Funding:
The research is supported by Baoding Science and Technology Bureau, Clinical Study on Improving Ovarian Function in Patients with POF by Activating the Expression of YY2/Lin28A (No. 2341ZF093).
Conflict of interests:
The authors declared no conflict of interests.
References
- Naz S, Shah FA, Nadeem H, Sarwar S, Tan Z, Imran M, et al. Amino acid conjugates of aminothiazole and aminopyridine as potential anticancer agents: Synthesis, molecular docking and in vitro evaluation. Drug Des Devel Ther 2021:1459-76.
[Crossref] [Google Scholar] [PubMed]
- Del Castillo LM, Buigues A, Rossi V, Soriano MJ, Martinez J, de Felici M, et al. The cyto-protective effects of LH on ovarian reserve and female fertility during exposure to gonadotoxic alkylating agents in an adult mouse model. Hum Reproduct 2021;36(9):2514-28.
[Crossref] [Google Scholar] [PubMed]
- Alonso A, Liauw W, Kennedy H, Alzahrani NA, Morris DL. Sodium thiosulfate during cisplatin-based hyperthermic intraperitoneal chemotherapy is associated with transient hypernatraemia without clinical sequelae. Pleura Peritoneum 2022;7(2):87-93.
[Crossref] [Google Scholar] [PubMed]
- Di-Battista A, Moysés-Oliveira M, Melaragno MI. Genetics of premature ovarian insufficiency and the association with X-autosome translocations. Reproduction 2020;160(4):R55-64.
[Crossref] [Google Scholar] [PubMed]
- Zhao LQ, Gao W, Zhang P, Zhang YL, Fang CY, Shou HF. Surgery in platinum-resistant recurrent epithelial ovarian carcinoma. World J Clin Cases 2022;10(12):3739-53.
[Crossref] [Google Scholar] [PubMed]
- Gouveia BB, Barberino RD, dos Santos Silva RL, Lins TL, da Silva Guimarães V, do Monte AP, et al. Involvement of PTEN and FOXO3a proteins in the protective activity of protocatechuic acid against cisplatin-induced ovarian toxicity in mice. Reproduct Sci 2021;28:865-76.
[Crossref] [Google Scholar] [PubMed]
- Li H, Lei Y, Li S, Li F, Lei J. microRNA-20a-5p inhibits the autophagy and cisplatin resistance in ovarian cancer via regulating DNMT3B-mediated DNA methylation of RBP1. Reproduct Toxicol 2022;109:93-100.
[Crossref] [Google Scholar] [PubMed]
- Fuller EA, Sominsky L, Sutherland JM, Redgrove KA, Harms L, McLaughlin EA, et al. Neonatal immune activation depletes the ovarian follicle reserve and alters ovarian acute inflammatory mediators in neonatal rats. Biol Reproduct 2017;97(5):719-30.
[Crossref] [Google Scholar] [PubMed]
- Wu YY, Liang CY, Liu TT, Liang YM, Li SJ, Lu YY, et al. Protective roles and mechanisms of polysaccharides from Dendrobium officinal on natural aging-induced premature ovarian failure. Biomed Pharmacother 2018;101:953-60.
[Crossref] [Google Scholar] [PubMed]
- Berkel C, Cacan E. In silico analysis of DYNLL1 expression in ovarian cancer chemoresistance. Cell Biol Int 2020;44(8):1598-605.
[Crossref] [Google Scholar] [PubMed]
- Zhang Q, Wang W, Gao Q. β-TRCP-mediated AEBP2 ubiquitination and destruction controls cisplatin resistance in ovarian cancer. Biochem Biophys Res Commun 2019;523(1):274-9.
[Crossref] [Google Scholar] [PubMed]
- Luo X, Xu J, Zhao R, Qin J, Wang X, Yan Y, et al. The role of inactivated NF-κB in premature ovarian failure. Am J Pathol 2022;192(3):468-83.
[Crossref] [Google Scholar] [PubMed]
- Huang J, Shan W, Li N, Zhou B, Guo E, Xia M, et al. Melatonin provides protection against cisplatin-induced ovarian damage and loss of fertility in mice. Reprod Biomed Online 2021;42(3):505-19.
[Crossref] [Google Scholar] [PubMed]
- Biyik I, Ozatik FY, Albayrak M, Ozatik O, Teksen Y, Ari NS, et al. The effects of recombinant klotho in cisplatin-induced ovarian failure in mice. J Obstetr Gynaecol Res 2021;47(5):1817-24.
[Crossref] [Google Scholar] [PubMed]
- Li H, Zhao W, Wang L, Luo Q, Yin N, Lu X, et al. Human placenta-derived mesenchymal stem cells inhibit apoptosis of granulosa cells induced by IRE1α pathway in autoimmune POF mice. Cell Biol Int 2019;43(8):899-909.
[Crossref] [Google Scholar] [PubMed]
- Sun Z, Zhang H, Wang X, Wang QC, Zhang C, Wang JQ, et al. TMCO1 is essential for ovarian follicle development by regulating ER Ca2+ store of granulosa cells. Cell Death Differ 2018;25(9):1686-701.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Yin H, Jiang H, Du X, Yang Z. The protective effects of human umbilical cord mesenchymal stem cell-derived extracellular vesicles on cisplatin-damaged granulosa cells. Taiwan J Obstet Gynecol 2020;59(4):527-33.
[Crossref] [Google Scholar] [PubMed]
- Qin K, Zhang F, Wang H, Wang N, Qiu H, Jia X, et al. circRNA circSnx12 confers cisplatin chemoresistance to ovarian cancer by inhibiting ferroptosis through a miR-194-5p/SLC7A11 axis. BMB Rep 2023;56(3):184-9.
[Crossref] [Google Scholar] [PubMed]
- Dinc K, Ozyurt R, Coban TA, Yazici GN, Suleyman Z, Yavuzer B, et al. The effect of carvacrol on the proinflammatory cytokines, histology, and fertility outcome of cisplatin-related ovarian change in a rat model. Taiwan J Obstet Gynecol 2023;62(2):256-63.
[Crossref] [Google Scholar] [PubMed]
- Zou J, Jian L. Inhibition of ceramide kinase is effective against cisplatin-resistant ovarian cancer cells by regulating ceramide and C1P levels. Gynecol Obstet Invest 2023;88(1):61-70.
[Crossref] [Google Scholar] [PubMed]
- Jafari S, Bakhshaei A, Eskandani M, Molavi O. Silibinin-loaded nanostructured lipid carriers for growth inhibition of cisplatin-resistant ovarian cancer cells. Assay Drug Devel Technol 2022;20(8):339-48.
[Crossref] [Google Scholar] [PubMed]
- Xiang Y, Chen YJ, Yan YB, Liu Y, Qiu J, Tan RQ, et al. miR-186 bidirectionally regulates cisplatin sensitivity of ovarian cancer cells via suppressing targets PIK3R3 and PTEN and upregulating APAF1 expression. J Cancer 2020;11(12):3446.
[Crossref] [Google Scholar] [PubMed]
- Calvo JA, Fritchman B, Hernandez D, Persky NS, Johannessen CM, Piccioni F, et al. Comprehensive mutational analysis of the BRCA1-associated DNA helicase and tumor-suppressor FANCJ/BACH1/BRIP1. Mol Cancer Res 2021;19(6):1015-25.
[Crossref] [Google Scholar] [PubMed]
- Wang S, Li MY, Liu Y, Vlantis AC, Chan JY, Xue L, et al. The role of microRNA in cisplatin resistance or sensitivity. Exp Opin Ther Targets 2020;24(9):885-97.
[Crossref] [Google Scholar] [PubMed]
- Han Y, You J, Han Y, Liu Y, Huang M, Lu X, et al. LINC00184 promotes ovarian cancer cells proliferation and cisplatin resistance by elevating CNTN1 expression via sponging miR-1305. Onco Targets Ther 2021;14:2711-26.
[Crossref] [Google Scholar] [PubMed]