- *Corresponding Author:
- Purnima Amin
Department of Pharmaceutical Sciences and Technology, Institute of Chemical Technology, Matunga, Mumbai‑400 019, India
E-mail: purnima.amin@yahoo.co.in
| Date of Submission | 18 September 2012 |
| Date of Revision | 28 May 2013 |
| Date of Acceptance | 31 May 2013 |
| Indian J Pharm Sci, 2013;75(4):450-456 |
Abstract
In the current research work an attempt was made to develop ''Melt in mouth pellets'' (Meltlets® ) containing 40% herbal extract of soy isoflavones that served to provide antioxidants activity in menopausal women. The process of extrusion-spheronization was optimized for extruder speed, extruder screen size, spheronization speed, and time. While doing so the herbal extract incorporated in the pellet matrix was subjected to various processing conditions such as the effect of the presence of other excipients, mixing or kneading to prepare wet mass, heat generated during the process of extrusion, spheronization, and drying. Thus, the work further investigates the effect of these processing parameters on the antioxidant activity of the soy isoflavone herbal extract incorporated in the formula. Thereby, the antioxidant activity of the soya bean herbal extract, Meltlets® and of the placebo pellets was evaluated using DPPH free radical scavenging assay and total reduction capacity.
Keywords
Antioxidant activity, DPPH, extrusion, excipients, fluid–bed, granulation, spheronization, mixing, soy isoflavones, Oyaizu method
Extrusion-spheronization is widely used in the pharmaceutical and other sectors for manufacturing dense granules with controlled and high sphericity. It is a multistep process involving number of parameters that have a final bearing on the characteristics of the obtained pellets. A novel extension of pellets as delivery system is the development of ‘‘Melt in mouth pellets’’ or Meltlets®. Meltlets® are pleasantly flavored fast disintegrating pellets of the drug or nutraceutical filled in single‑serve sachets. The contents of the sachet are to be emptied directly into the mouth which disintegrates to form a soft mass that can be swallowed easily without the aid of water. A wide variety of drugs and nutraceuticals can be incorporated in these mouth dissolving pellets thereby providing advantages of enhanced patient compliance and also provide a competitive advantage when a therapeutic category is crowded with similar products [1-4]. Isoflavones have phenolic structure, similar to that of animal estrogens thereby exhibiting weak estrogenic properties and are termed as phytoestrogens. Thus, they offer many benefits to women in menopausal stage. Soybeans contain a number of important phenolic compounds, including free phenolic acid, phenolic acid esters, isoflavones, genistein and daidzein, their glycosides genistin and daidzin and coumesterol [5,6]. Moreover, the antioxidant activity may be related to polyphenol content since it has been reported that these phenolic compounds can act breaking the chain reaction of lipid by scavenging several reactive oxygen species (ROS) [7], and inhibiting chemiluminescence reactions [8]. Thus, Meltlets® containing 40% herbal extract of soy isoflavones (Label Claim: Each 1 g sachet contains 100 mg of extract) were formulated which served to provide antioxidant activity in menopausal women.
Appropriate selection of highly hydrophilic excipients, disintegrating agents and maximizing porous structure of the pellet matrix are the prerequisites for the development of Meltlets® so that they disintegrate or dissolve in oral cavity with saliva within 15 to 60 s without the aid of water. The objective of the present work was to optimize the extrusion–spheronization process parameters for the development of Meltlets® of soy isoflavones. While doing so the herbal extract incorporated in the pellet matrix is subjected to various processing conditions such as the effect of the presence of other excipients, mixing or kneading to prepare wet mass, heat generated during the process of extrusion, spheronization and drying. Thus, the work further investigates the effect of these processing parameters on the antioxidant activity of the soy isoflavone herbal extract incorporated in the formula.
Materials and Methods
Solgen® 40 soybean extract containing 40% total isoflavones was obtained as a gift sample from, Solbar Plant Extract, Ashdod, Israel. Microcrystalline cellulose (Avicel® PH 101), sodium carboxymethyl cellulose (Avicel® RC591) of FMC Corp., USA were gifted by Signet Chemical Corporation, Mumbai, India. Ion exchange resin (Indion® 414, Ion Exchange, Mumbai, India) was used as received without further purification. 2,2‑Diphenyl‑1‑picrylhydrazyl (DPPH) were obtained from Sigma Chemical Co., St. Louis, MO, USA. All other reagents were of the highest grade commercially available. The amount of solvent used had no effects on the assays.
Characterization of solgen® 40 soybean extract
Dry soya bean extract containing 40% soy isoflavones was characterized for various physical properties pertaining to formulation development, such as determination of particle size, density, flow properties, moisture content, and microbial load. Further total polyphenolic content of the extract was determined using Folin–Ciocalteau colorimetric method [9].
The total phenolic content in the extract were determined by using three different solvents viz. methanol, methanol–water mixture (1:1), and plain water. A total of 100 mg of extract was stirred with 100 ml of each of the above mentioned solvents for 30 min to ensure complete extraction of the phenols in the respective solvents. The suspensions were then centrifuged at 1660×g for 10 min and aliquots of supernatant fraction were withdrawn and were analyzed for total polyphenolic content using the following method. In this methodology, 100 μl of the extract solution was mixed with 900 μl of distilled water. To each of these solutions 2 ml of distilled water was added followed by the addition of 1 ml of Folin‑Ciocalteu reagent that was diluted in the ratio of 1:1 with distilled water and 1 ml of sodium carbonate (Na2CO3, 2 g/l). The final volume was made up to 10 ml with distilled water and the solutions were cyclomixed on a vortex mixer, incubated at 40º for 30 min and its absorbance was recorded at 760 nm. The content of total polyphenols in the extract was expressed as mg/g (Gallic acid equivalents).
Preparation of Meltlets® by extrusion– spheronization
The extrusion–spheronization process parameters were optimized in terms of granulation fluid level, wet mass mixing time, feed rate, die opening diameter, spheronization residence time, disk speed, and drying (use of fluidized bed dryer, tray dryer and oven dryer). The formulation variables like soy extract:Avicel® PH 101:Avicel® RC 591 ratio, concentration levels of disintegrating agent (Ion exchange resin Indion® 414), flavor, sweetener and granulation fluid composition, that is, sugar solution concentration so as to produce sweet and pleasantly tasting pellets with good mouth dispersion, were also optimized.
All the powders were sieved through 40 mesh sieve and were blended together in a Hobart planetary mixer for 5 min. Sugar solution at 5% concentration level was added slowly and blended with the powder mass so as to form wet mass for extrusion. The wet mass was further mixed for 1 min and then passed through axial single screw extruder (Naomi Enterprise, Mumbai, India) equipped with 0.8 mm aperture diameter screen with L/R ratio of 1.7, where L is thickness of screen and R is radius of screen aperture. The extruder rate was maintained at 50 rpm. The extrudates were collected on butcher paper and were further spheronized at the spheronizer rate of 1200 rpm for 3 min followed by 800 rpm for 5 min in a spheronizer (R. R. Enterprises, Thane, India) fitted with a cross hatched plate with the length of each groove of 2 mm. The pellets were emptied from the spheronizer, were dried in an aromatic fluidized bed dryer (S. B. Panchal and Sons, Mumbai, India) at 50° for 90 min.
Size and shape of the pellets
Sphericity of the pellets was determined by assigning a rank score, with 1.0 being very spherical and 12 being a cylinder. When two different scores were obtained, rank scores were averaged. Further the pellets were characterized using Leica Galen™ III image analyzer (USA) which consisted of a computer system connected to a camera mounted on a stereo microscope. The magnification was fixed at ×4. Projected area and perimeter were determined using Biovis image plus software. Prior to processing of the images, care was taken to assure that all pellets were detected as single entities. All the measurements were performed on 400±50 pellets. Aspect ratio is the ratio of maximum diameter and the diameter perpendicular to the maximum diameter. Ideally spherical pellets will have value 1, but pellets with aspect ratio less than 1.2 are considered as spherical. The aspect ratio that describes the elongation of the pellets was calculated using: Aspect ratio= [major axis/minor axis ro (length/breadth)], where the minor axis was the shortest distance perpendicular to the major axis. Sphericity/circularity emphasizes the spherical shape of the pellets using the equation: Sphericity=4πA/ P2, where A is the projected area of the individual pellet and P is the perimeter of that image, a perfectly spherical pellet would yield a sphericity of 1. Any value less than 1 indicates a less spherical image [10].
Friability test
Friability test (n=3) was performed using Roche friabilator (Labindia, Mumbai, India). A preweighed sample (5 g, 16/25 mesh fraction) was placed in the friabilator along with 25 steel balls, each 2 mm in diameter. After 100 revolutions at 25 rpm, the mass retained on 25 mesh sieve was weighed, and friability was calculated as the percentage loss of mass between the initial and final weights of each bead sample [11].
Flow properties
Flow behavior was assessed in terms of parameters like angle of repose, bulk density, tapped density, Carr’s index, and Hausner’s ratio as per USP 30.
Gustatory sensation test
Gustatory sensation test was performed by modifying a previously described method [12]. In brief, quinine hydrochloride solution 1 mM was considered as standard for bitterness with a bitterness score of 5 and purified water as 0. The human volunteer study was done according to ethical guidelines for biomedical research on human subjects by Indian Council of Medical Research (www.icmr.nic.in/ethical.pdf). The protocol for the test was approved by institutional ethical committee. The test was performed with ten well‑trained volunteers. The developed pellets were rated between 0 and 5 depending upon intensity of bitterness, from tasteless to very bitter. After tasting each sample, volunteers gargled well with water and waited for at least 20 min before tasting the next sample. Also, the volunteers rated the pellets for mouth feel and dispersion in mouth (how fast the pellets disperse in the saliva) on the scale of 1 to 5.
Evaluation of antioxidant activity of Meltlets®
The antioxidant activity of soybean extract and of Meltlets® added with this extract were evaluated using DPPH free radical scavenging assay and total reduction capacity by Oyaizu Method. The antioxidant activity of the Meltlets® was then compared to that of the soybean extract in the same final concentration and with the placebo pellets.
DPPH free radical scavenging assay
For radical scavenging measurements, appropriate aliquots of the solution of soybean extract, Meltlets® and of the placebo pellets dissolved in methanol were withdrawn so as to make solutions with concentrations ranging between 50 and 500 μg/ml and were further diluted with methanol to make final volume of 1 ml. Two such sets were prepared and to one of this set 1 ml of methanol solution of DPPH (200 μM) was added while to the other set 1 ml of methanol was added. Control solution was prepared by mixing 1 ml of plain methanol to 1 ml of methanol solution of DPPH (200 μM). The samples were incubated at room temperature for 20 min and the absorbance was recorded at 517 nm. The percent radical scavenging activity was determined from the difference in absorbance (A) of DPPH between the control and samples using the following formula: Radical Scavenging (%)=((OD of Control‑OD of Sample)/OD of Control)×100, where OD is optical density. Inhibitory concentration (IC50) was used as a measure for comparison of antioxidant activity of different extracts. It is calculated from the plot of percent DPPH inhibition V/S Concentration [13].
Total reduction capacity by Oyaizu method
The total reduction capacity of soya bean extract and Meltlets® was determined by diluting an appropriate aliquot of the test extract dissolved in different solvents (viz. methanol, methanol:water (1:1) and water) with concentration of 1500 μg/ml with water to make the final volume of 1 ml. To this 2.5 ml of phosphate buffer (0.2M, pH 6.6) and 2.5 ml of potassium ferricyanide (K3Fe(CN)6; 10 g/l) were added. The mixture was incubated at 50º for 30 min. After incubation, 2.5 ml of trichloroacetic acid (100 g/l) was added and the mixture was centrifuged at 1650 g for 10 min. Finally, 2.5 ml of the supernatant solution was mixed with 2.5 ml of distilled water and 0.5 ml of ferric chloride (FeCl3; 1 g/l). The absorbance of the solution was measured at 700 nm. High absorbance indicates high reducing power [14].
Statistical analysis
Data were expressed as means±standard error determined of triplicate analysis. The concentration which caused 50% of inhibition of the systems assessed (IC50) by the soy isoflavones in Meltlets® was determined using GraphPad Prism® software. Data were statistically analyzed by one‑way ANOVA, followed by Bonferroni’s multiple comparisons t‑test for evaluation of the formulation influence in the antioxidant activity assays. Results were presented as means±SEM (standard error mean) and considered significantly different when P<0.001 was obtained.
Results and Discussion
The extract showed fair flow behavior as indicated by the Carr’s index, Hausners ratio, and angle of repose values all falling within the range of fair flow as per USP 29. The moisture content and microbial load of the extract were found to be within limit. Amongst the different solvents used for determining total polyphenolic content by Folin‑Ciocalteau method calculated in terms of gallic acid equivalent (GAE) it was found that the extract had maximum methanol soluble polyphenols while minimum water soluble polyphenols. Results are summarized in Table 1.
| Parameters | Observation |
|---|---|
| Description | Light brown colored |
| powder with sweet odor | |
| pH of 1% solution | 4.3 |
| Particle size determination (nm) | 180–250 |
| Bulk density (g/ml) | 0.4348 |
| Tap density (g/ml) | 0.5263 |
| Carr’s index (%) | 17.38 |
| Hausner’s ratio | 1.21 |
| Angle of repose (º) | 15.52 |
| % LOD: (105° for 10 min) (%) | 4.02 |
| Microbial limit test (IP) | |
| Limit test for E. coli | Absent |
| Limit test for Salmonella | Absent |
| Limit test for Pseudomonas | Absent |
| Limit test for S. aureus | Absent |
| Total bacterial count ( CFU/g) | 60 |
| Total fungal count | Nil |
| Total polyphenolic content | |
| Extraction solvent | % total polyphenol |
| Methanol | 62.96 |
| Methanol:Water (1:1) | 33.74 |
| Water | 21.82 |
| LOD=Loss on drying, CFU=Colony forming units |
Table 1: Characterization of Soybean Extract
During initial optimization batches the desired sphericity was obtained when Avicel® PH 101 was incorporated at 22% concentration level. But for the pellets to exhibit melt in mouth effect they should possess porous matrix, which was not obtained with Avicel® PH 101 alone. Hence, some part of Avicel® PH 101 was replaced by Avicel® RC 591, which is microcrystalline cellulose in combination with sodium carboxymethyl cellulose. Thus, the pellets dissolved immediately in oral cavity forming smooth, soft mass unlike Avicel® PH 101 the pellets of which remains intact and has to be chewed into a soft mass.
The ratio of soy extract:Avicel® PH 101:Avicel® RC 591 was optimized in order to improve sphericity of Meltlets®. It was observed that Avicel® PH 101 at concentration level of 12% and Avicel® RC 591 at concentration level of 10% were required to obtain maximum sphericity. The concentration of ion exchange resin Indion® 414 as super disintegrant [15] in the formulation was optimized to 5% concentration level such that at this concentration the Meltlets® dissolved in oral cavity within 15-60 s. Combination of aspartame and monoamine glycyrrhizic acid (MAG) was used as sweetener in the ratio of 2:1. Sugar solution at 5% concentration level was used as granulating fluid. Xylitol was used as diluent.
Moisture content is an extremely important parameter in the extrusion–spheronization process. It is necessary to give the powder mass its plasticity so that it can be extruded and shaped afterwards. The extent of moisture content also influences the mechanical strength, friability, internal porosity, and the particle size distribution of pellets [2]. Water when used alone as granulating fluid in the formation of Meltlets® resulted in hard pellets. Hence, sugar solution was further tried instead of water. On drying the pellets prepared using sugar solution, sugar gets deposited in the pellet matrix making it porous and thereby aiding in the overall mouth dissolve effect and better taste masking.
The amount of granulating fluid was also optimized (0.16 ml/g of dry blend) in terms of desirable extrudate characteristics. Granulating fluid acts as a lubricant at the extrudate and die interface a lubricant and facilitated the movement of the extrudates through the die, which was evident from lesser extrusion force required when granulating fluid level was increased. The extrudates produced were plastic enough to deform and did not adhere to each other when collected or rolled in the spheronizer. The surface impairments of extrudates like roughness and shark skinning have been reported to increase at a higher extrusion speed as well as when the stress at the wall of the die perforations increases with increasing thickness (i.e., higher L/R ratio) of the extrusion screen or as the granulating fluid content of the formulation decreases [16‑20]. It was indeed found to be the situation and therefore the lower L/R ratio (1.7) screen was found to be suitable for getting extrudates with desirable properties, which are mentioned above. Further, employing extrusion speed of 50 rpm and granulating fluid content of 0.16 ml/g of dry blend produced extrudates with no surface roughness as well as shark skin effect. The wet mass mixing time was optimized in terms of desirable properties of extrudates (like length, reduced shark‑skin effect, and low extrusion force) and was found to be 2 min.
Pellet quality is also dependent on the type of dryer used. Ibuprofen pellets that were dried either by tray drying or fluidized bed drying showed that the drying technique has a quantifiable effect on the diametral crushing strength and elasticity of the pellets [21,22]. Thus, in the current research work it was found that pellets obtained by fluidized bed drying were most porous and exhibited fast and better dispersion in oral cavity as compared to pellets obtained by other drying techniques. The optimized processing parameters are summarized in Table 2.
| Processing parameters | Observations |
|---|---|
| Granulating aid used | 5% sugar syrup at the level of 16% of the |
| and its concentration | total blend |
| Extrusion | Single screw extruder, manual, 0.8 mm |
| screen | |
| Spheronization | Spheroniser S -150, 2 mm plate, 1200 rpm |
| for 3 minutes followed by 800 rpm for 5 min | |
| Drying | 50° for 90 min in a Fluidized bed dryer |
| Fractionation | 22#-40# |
Table 2: Optimized Process Parameters
Sphericity is one of the most important properties of the pellet and is also related to flow properties [23]. Visual inspection of Meltlets® indicated that they were spherical in shape. This was further confirmed by the aspect ratio of 1.12. Conducting spheronization at different speeds for varying time span aided in achieving good spherical pellets. It was observed that the initial high speed spheronization (at 1200 rpm) brought about quick cutting and rough rounding of the extrudates, while subsequent spheronization at slower speed (at 800 rpm) helped in better rounding of the pellets thereby increasing the overall sphericity. Friability of pellets is influenced by spheronization residence time and speed [24]. The optimized formulation showed the friability value of 1.87% which is significantly lower than those that have been reported abundantly in the literature [25‑28] and which may also be attributed to the presence of Avicel® RC 591 having strong binding property. In this study, the flow properties as evident from the values of Carr ’s index, Hausner’s ratio, and angle of repose indicated that flow behavior of the optimized formulation is good. The results of the evaluation of Meltlets® are depicted in Table 3.
| Parameters | Observation |
|---|---|
| Appearance | Brown colored, spherical, uniform pellets |
| Taste | Chocolate flavored, sweet, pleasant tasting |
| Mouth dispersion | Forms a soft mass with no palpable core |
| Disintegration time (s) | 45 |
| Swelling index | 1.6 |
| Moisture content (%) | 3.47 |
| Bulk density (g/ml) | 0.678 |
| Tap density (g/ml) | 0.726 |
| Hausner’s ratio | 1.07 |
| Carr’s index (%) | 6.61 |
| Angle of repose (°) | 23.73 |
| Porosity (%) | 76 |
| Friability (%) | 1.87±0.04 |
| Sphericity (n=50) | 1.52±0.24 |
| Aspect ratio | 1.12 |
| Assay (%) | 107.13±1.17 of total Isoflavones |
Table 3: Evaluation Of Meltlets®
In the current study, the mean bitterness score of 0.2 of the pellets was obtained which was not significant (P<0.001). The significant bitterness score is 5 when tested with quinine hydrochloride. Further, the Meltlets® disintegrated easily into a soft mass and were easy to swallow without the aid of water. No bitter taste of the extract or after bitter taste was perceived.
Different methods were used to evaluate the antioxidant activity since the oxidative stress depends on the type of generated ROS, how it is generated, where it is generated, and the oxidative target evaluated. Furthermore, the total antioxidant activities of herbal extracts cannot be evaluated by any single method, due to the complex nature of phytochemicals. Two or more methods should always be employed in order to evaluate the total antioxidant effects of herbal extracts [29].
To ensure whether the soy isoflavones present in the extract have retained their antioxidant activity after being subjected to various processing conditions of extrusion–spheronization and drying, the Meltlets® were evaluated for their antioxidant activity using DPPH free radical scavenging assay and total reduction capacity by Oyaizu method. The activity was compared to the original soyabean extract that was used to formulate the pellets and with placebo pellets (all excipients only) to study the possible interference of the excipients with respect to antioxidant activity.
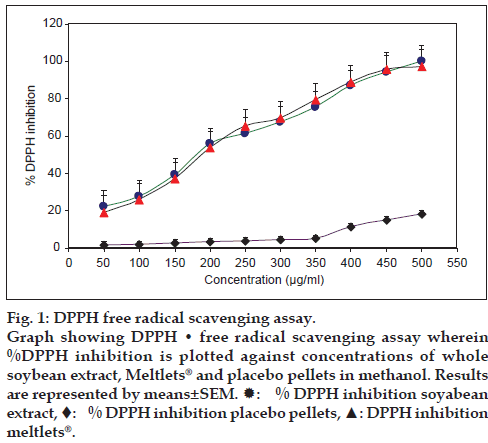
The DPPH assay is widely used for the measurement of free radical scavenging capacity in phyto technology, food technology, and pharmacology/ toxicology. The radical scavenging capacity (RSC) of pure compounds or plant extracts can be assessed by the trapping of this synthetic and stable radical in methanol at room temperature. Antioxidants on interaction with DPPH, transfer either electron or hydrogen atom to DPPH and thus neutralize its free radical character. In this method, the capacity of extracts to scavenge the lipid soluble DPPH radical, which results in the bleaching of the purple color exhibited by the stable DPPH radical is monitored at wavelength of 517 nm. This bleaching occurs due to the reduction of the antioxidants and lower absorbance of the reaction mixture thereby indicating higher free radical scavenging activity [13]. Gallic acid was taken as the standard antioxidant. About 175 μg/ml of soybean extract was able to inhibit 50% of DPPH in the assay. The Meltlets® showed similar IC50 value while placebo pellets failed to inhibit DPPH at all the given concentrations. The results are indicated in fig. 1.
Figure 1: DPPH free radical scavenging assay.
Graph showing DPPH • free radical scavenging assay wherein %DPPH inhibition is plotted against concentrations of whole soybean extract, Meltlets® and placebo pellets in methanol. Results are represented by means±SEM.  : % DPPH inhibition soyabean extract, ♦: % DPPH inhibition placebo pellets, ▲: DPPH inhibition meltlets®.
: % DPPH inhibition soyabean extract, ♦: % DPPH inhibition placebo pellets, ▲: DPPH inhibition meltlets®.
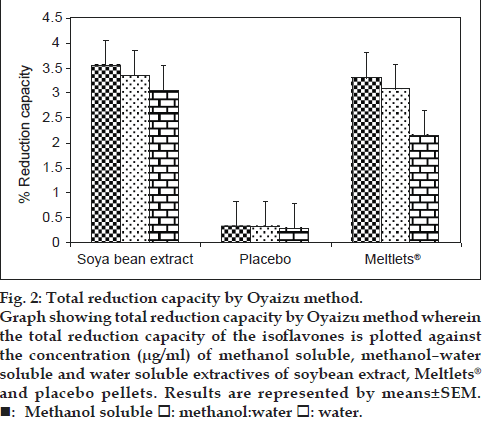
Different studies have indicated that the electron donation capacity (reflecting the reducing power) of bioactive compounds is associated with antioxidant activity. Thus, in case of Oyaizu method the ability of extracts to reduce the ferric‑ferricyanide (Fe+3) complex to the ferrous–ferricyanide (Fe+2) complex of Prussian blue was determined by recording the absorbance at 700 nm after incubation and compared to that of gallic acid which is a known reducing agent [14]. The results of the total reduction capacity by Oyaizu method were consistent with those of the DPPH free radical scavenging assay with Meltlets® showing total reduction capacity value similar to that of the original soybean extract in all the three extraction solvents. The total reduction capacity value for the placebo pellets was found to be almost negligible as indicated in fig. 2.
Figure 2: Total reduction capacity by Oyaizu method.
Graph showing total reduction capacity by Oyaizu method wherein the total reduction capacity of the isoflavones is plotted against the concentration (μg/ml) of methanol soluble, methanol–water
soluble and water soluble extractives of soybean extract, Meltlets® and placebo pellets. Results are represented by means±SEM. : Methanol soluble
: Methanol soluble  : methanol:water
: methanol:water  : water
: water
Thereby, from the results it was confirmed that the antioxidant activity of the pellets was not altered after being subjected to various processing parameters used during extrusion–spheronization, drying at 50°or due to the presence of various excipients used to formulate Meltlets®.
Thus, to conclude the research work, the process parameters for the development of Meltlets® of soy isoflavones were optimized so as to afford a melt in mouth dosage form that dissolves within 60 s without the aid of water. The pellets complied with various parameters of evaluation. Further, the effect of the processing parameters on the antioxidant activity of the extract was investigated using DPPH free radical scavenging assay and total reduction capacity by Oyaizu method. It was found that the antioxidant activity of the Meltlets® was comparable with the original extract thereby indicating that the antioxidant activity of the extract was not altered after being subjected to harsh processing conditions and that the excipients used did not contribute to the antioxidant activity of the formulation.
Acknowledgements
We wish to thank the Technical Education Quality Improvement Programme (TEQIP) for funding this research work. We also thank the Solbar Plant Extractives Ltd, Israel for providing generous gift samples of Soy isoflavone herbal extract.
References
- Mascia S, Seiler C, Fitzpatrick S, Wilsona DI. Extrusion–spheronization of microcrystalline cellulose pastes using anon‑aqueous liquid binder. Int J Pharm 2010;389:1-9.
- Gandhi R, Kaul CL, Panchagnula R. Extrusion and spheronization in the development of oral controlled–release dosage forms. Pharm SciTechnol Today 1999;2:160-70.
- Gruber P. Easy to swallow oral medicament composition. US Patent No. 6,709,678, 2004.
- Augello M, Vladyka RS, Dell SM. Taste masked pharmaceutical compositions. US Patent No. 5,904,937, 1999.
- Georgetti SR, Casagrande R, Vicentini FT, Verri WA, Vieira Fonseca MJ. Evaluation of the antioxidant activity of soybean extractby different in vitro methods and investigation of this activity after its incorporationin topical formulations. Eur J Pharm Biopharm 2006;64:99-106.
- Seo A, Morr CV. Improved high‑performance liquid chromatographic analysis of phenolic and isoflavonoids from soybean protein products. J Agric Food Chem 1984;32:530-3.
- Marquele FD, Di Mambro VM, Georgetti SR, Casagrande R, Valim YM, Fonseca MJ. Assessment of the antioxidant activities of Brazilian extracts of propolis alone and in topical pharmaceutical formulations. J Pharm Biomed 2005;39:455-62
- Georgetti SR, Casagrande R, Di Mambro VM, Azzolini AE, Fonseca MJ. Evaluation of the antioxidant activity of different flavonoids by thechemiluminescence method. AAPS PharmSciTech 2003;5:1-7.
- Kumazawa S, Hamasaka T, Nakayama T. Antioxidant activity of propolis of various geographic origins. Food Chem 2004;84:329-39.
- Liew CV, Josephine LG, Soh LP, Heng PW. Functionality of cross‑linked polyvinyl pyrrolidone as a spheronization aid: A promising alternative to microcrystalline cellulose. Pharm Res 2005;22:1387-98.
- Howard MA, Neau SH, Sack MJ. PEO and MPEG in high drug load extruded and spheronised beads those are devoid of MCC. Int J Pharm 2006;307:66-76.
- Tokuyama E, Shibasaki T, Kawabe H, Mukai J, Okada S, Uchida T. Bitterness suppression of BCAA solutions by L‑ornithine. Chem Pharm Bull 2006;54:1288-92.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958;26:1199-200.
- Kadriye IB, Kubilay G, İzzet T, Birsen D, Reşat A. Total antioxidant capacity assay using optimized ferricyanide/prussian blue method. Food Anal Methods 2010;3:154-68.
- Amin P, Prabhu N, Wadhwani A. Indion 414 as superdisintegrant in formulation of mouth dissolve tablets. Indian J Pharm Sci 2006;68:117-9.
- Ostuka M, Gao J, Matsuda Y. Effect of amount of added water during extrusion‑spheronization process on pharmaceutical properties of granules. Drug DevInd Pharm 1994;20:2977-92.
- Harrison PJ, Newton JM, Rowe RC. The characterization of wet powder masses suitable for extrusion/spheronization. J Pharm Pharmacol 1985;37:686-91.
- Harrison PJ, Newton JM, Rowe RC. Flow defects in wet powder mass extrusion. J Pharm Pharmacol 1985;37:81-3.
- Harrison PJ, Newton JM, Rowe RC. Convergent flow analysis in the extrusion of wet powder masses. J Pharm Pharmacol 1984;36:796-8.
- O’Conner RE, Schwartz JB. Extrusion and spheronization technology. In: Ghebre‑Sellasie I, editor. Pharmaceutical Pelletization Technology. New York: Marcel Dekker; 1989. p. 187-216.
- Bataille B, Ligarski K, Jacob N, Thohas C, Duru C. Study of the influence of spheronization and drying conditions on the physico‑mechanical properties of neutral spheroids containing avicel PH 101 and lactose. Drug DevInd Pharm 1993;19:653-71.
- Dyer AM, Khan KA, Aulton ME. Effect of the drying method on the mechanical and drug release properties of pellets prepared by extrusion‑spheronization. Drug DevInd Pharm 1994;20:3045-68.
- Woodruff CW, Nuessle NO. Effect of processing variables on particles obtained by extrusion‑spheronisation processing. J Pharm Sci 1972;61:787-90.
- Erkoboni DF. Extrusion/spheronization. In: Ghebre‑Sellasie I, Martin C, editors. Pharmaceutical Extrusion Technology. New York: Marcel Dekker; 2003. p. 277-322.
- Bianchini R, Bruni G, Gazzaniga A, Vecchio C. Influence of extrusion‑spheronization processing on the physical properties of d‑indobufen pellets containing pH adjusters. Drug DevInd Pharm 1992;18:1485-515.
- Malinowski HJ, Smith WE. Use of factorial design to evaluate granulations prepared by spheronization. J Pharm Sci 1975;64:1688-92.
- Mesiha MS, Valles J. A screening study of lubricants in wet powder masses suitable for extrusion‑spheronization. Drug DevInd Pharm 1993;19:943-59.
- Zhang G, Schwartz JB, Schnaare RL, Wigent RJ, Sugita ET. Bead coating: II. Effect of spheronization technique on dissolution from coated spheres. Drug DevInd Pharm 1991;17:817-30.
- Nuutila AM, Puupponem‑Pimia R, Aarni M, Oksman‑Caldentey KM. Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chem 2003;83:485-93.