- *Corresponding Author:
- Y. Zhang
Department of Anesthesiology, The Affiliated Hospital of Chengde Medical College, Chengde, Hebei Province 067000, China
E-mail: chengyi20150715@163.com
| This article was originally published in a special issue, “Transformative Discoveries in Biomedical and Pharmaceutical Research” |
| Indian J Pharm Sci 2023:85(4) Spl Issue “202-210” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the safety and analgesic effect of ketorolac tromethamine on postoperative patients with colorectal cancer. The literature search was conducted in the Chinese databases, i.e. Chinese National Knowledge Infrastructure, Wan Fang, China biology medicine and Virtual IP, and the abroad databases, i.e. PubMed, Web of Science, Embase, Cochrane Library and Joanna Briggs institute for randomized controlled trials, controlled clinical trials and case-control studies about the effects of ketorolac tromethamine on the patients with colorectal cancer after surgery from January 2010 to November 2020, in the languages of Chinese and English. We conducted literature screening and quality evaluation in strict accordance with the standards, and extracted effective data such as study type, sample size, age, intraoperative anesthesia mode and outcome indicators, which were input into RevMan 5.4 software for meta-analysis. A total of 20 literatures, including 13 randomized controlled trials, 6 controlled clinical trials and 1 case-control studies, involving 802 patients. Ketorolac tromethamine had significant lower visual analogue scores of pains at 6 h, 24 h and 48 h, shorter hospital stays and time to first feeding after operation, and lower incidences of ileus, and nausea and vomiting (all p<0.05). Ketorolac tromethamine for the colorectal neoplasms patients is better in the analgesic effect at 6 h, 24 h and 48 h after surgery, effectively shortens the hospital stay and time to first feeding after operation and reduces the incidences of ileus, and nausea and vomiting.
Keywords
Colorectal cancer, ketorolac tromethamine, analgesic effect, meta-analysis
Colorectal cancer is one of the most common malignancies in humans and is also a common tumor of the digestive system. The incidence rate of malignant tumors ranks 4th in the world, with an annual cumulative number of about 1 million and annual deaths of nearly 500 000. "2020 Global Cancer Statistics" shows that colorectal cancer has become the third most common cancer in the world[1]. Data show that the mortality rate of colorectal cancer in China is increasing year by year, with the number of newly diagnosed cases accounting for 24 % and the number of deaths accounting for 30 % of the world[2]. At present, the main treatment for colorectal cancer is surgical resection, but about 70 % of surgical patients have strong pain and discomfort after surgery, which will increase the occurrence of postoperative adverse reactions and slow down the recovery process[3,4]. And with the progress of society and the development of medical technology level, the trend of population aging is increasingly aggravated and the operation of elderly patients is gradually increasing. Colorectal cancer surgery, in particular, is highly traumatic and has a high probability of Postoperative Abdominal Pain and Cognitive Dysfunction (POCD). Postoperative pain and POCD are common complications in elderly patients after anesthesia surgery, which seriously affect the prognosis and quality of life of patients. Postoperative pain and POCD is a major problem facing anesthesiologists and surgeons today. In recent years, the incidence of postoperative cognitive dysfunction is high, which has attracted great attention from both doctors and patients. The present study showed that patients' age, postoperative pain, cytokine mediated inflammatory reaction, the choice of anesthetic and intraoperative hypotension, all have an effect on postoperative cognitive dysfunction, with the deepening of the research and the development of the clinical observation of the world, in the face of abdominal surgery patients, how to choose appropriate postoperative analgesia method to reduce the incidence of postoperative pain and POCD. It is an urgent problem to be solved in the field of anesthesia. Therefore, the selection of appropriate analgesia can allow patients to fully control the pain and avoid the occurrence of related complications. At present, both intravenous analgesia and epidural analgesia are widely used in clinical practice, but the choice of postoperative analgesia for colorectal cancer is still controversial, and the clinical application is not consistent. Ketorolac tromethamine is a novel Non- Steroidal Anti-Inflammatory Drug (NSAID), which belongs to a class of non-opioid analgesics that inhibit Cyclooxygenase (COX) activity and reduce Prostaglandin (PG) and Thromboxane A2 (TXA2) biosynthesis, to achieve anti-inflammatory, analgesic, antipyretic effects. Ketorolac tromethamine, which has been widely used during anesthesia, has COX-1 selectivity, is extensively metabolized in the liver, and is excreted through the kidney, with an elimination half-life of between 4 h and 6 h, which is moderate compared to other NSAIDs. Ketorolac tromethamine is a non-selective NSAID that can affect the formation of COX and thus reduce the production of PG. In many studies, its analgesic effect is stronger than that of other NSAIDs, such as tramadol and diclofenac. Therefore, ketorolac tromethamine has been widely used in various types of postoperative analgesia for colorectal surgery.
Therefore, this study intends to explore the impact of ketorolac tromethamine on postoperative analgesia and cognitive function in patients with colorectal cancer surgery, so as to provide a better guidance for postoperative analgesia in patients with colorectal cancer surgery. Meanwhile, good postoperative analgesia can also reduce the incidence of POCD in patients.
Materials and Methods
Inclusion and exclusion criteria for literature:
Inclusion criteria: Subjects ≥18 y old diagnosed with colorectal cancer and underwent radical resection of colorectal cancer. Research type includes Randomized Controlled Trial (RCT), semi-randomized Controlled Clinical Trials (CCT) and Case-Control Study (CCS). In group I (intervention group), 1 ml of normal saline was injected intravenously 30 min before anesthesia, then midazolam 0.05 mg/kg, sufentanil 0.5 µg/kg, propofol 1.5 mg/kg, cisatracurium benzosulfol 0.2 mg/ kg, sufentanil 0.01 µg/kg was injected intravenously 30 min before the end of surgery. After the operation, the patient-controlled intravenous analgesia pump was connected, the background infusion speed was 2 ml/h, the patient-controlled analgesia dose was 2 ml, and the locking time was 20 min. In group II (control group), 30 mg ketorolac tromethamine was injected intravenously 30 min before anesthesia, and the other operations were the same as those in group I. In outcome indicators analgesic effect, i.e., Visual Analogue Score (VAS) at 6 h, 24 h and 48 h after operation, incidence of intestinal obstruction, incidence of nausea and vomiting, time of first feeding and hospital stay; the analysis also includes the analysis of these two studies.
Exclusion criteria: Other analgesic methods excluding ketorolac tromethamine were intervention methods. The data report is incomplete, the full text cannot be obtained, or the literature is published repeatedly.
Literature search:
Four Chinese databases, Chinese National Knowledge Infrastructure (CNKI), Wanfang, China Biology Medicine (CBM) and Virtual IP (VIP), were searched comprehensively. And PubMed, Web of Science, Embase, Cochrane Library, Joanna Briggs Institute (JBI) five foreign databases on the analgesic effect of intravenous analgesia and epidural analgesia in colorectal cancer patients after surgery published studies. The literature is published from January 2010 to November 2020 in Chinese and English. The English search terms include colorectal neoplasms, colonic neoplasms, rectal neoplasms, ketorolac tromethamine and normal saline. Chinese search terms are Chinese characters translated from English. The search was carried out by combining subject terms and free words, and supplemented by tracing the references already included in the literature.
Literature screening and data extraction:
Titles and abstracts were read by both researchers for initial screening and then the full text was read to decide whether to include the literature. For questionable or poorly described literature, email can be used to contact the original paper author to resolve the problem. In case of any disagreement, it shall be settled through negotiation or third-party arbitration. The extracted data include study type, sample size, age, postoperative analgesic drugs, outcome indicators, etc. Finally, the results of screening and extraction are cross-checked.
Literature quality evaluation:
JBI's 2016 version of the authenticity assessment tool for randomized controlled studies and quasi experimental studies, the key assessment skills program (CASP), and the 2011 version of the authenticity assessment tool for case-control studies were used by two researchers to assess the risk of bias in the literature. If there is any objection, the discussion or third-party arbitration needs to be considered and settled. In addition, the evaluation results should be cross checked.
The data analysis:
The extracted valid data were input into RevMan 5.4 software for meta-analysis, and I2 and Cochrane Q test were used to test the statistical heterogeneity of the data. If I2≤50 %, it is considered that the heterogeneity among the included studies is small, and the fixed effect model is selected. Otherwise, sensitivity analysis, subgroup analysis and other methods were used to analyze the sources of heterogeneity. We chose Dersimonian Laird (D-L) method, Relative Risk (RR) and Weighted Mean Difference (WMD) as the impact indicators of qualitative and quantitative data in the calculation methods of meta-analysis. At the same time, the 95 % Confidence Interval (CI) of the effect index is given as p<0.05 indicates a statistically significant difference.
Results and Discussion
After preliminary search, 703 related articles were obtained, including 172 English articles and 531 Chinese articles. 635 articles that did not meet predetermined inclusion criteria were excluded by reading the title and abstract. Enter the bibliography into Note Express 3.2.0, the document management software. Of all the results, 21 duplicate papers were excluded by us and 20 were eventually included. Fig. 1 shows the entire document filtering process. Table 1 shows the details of the documents involved.
| Study | Type | Ketorolac tromethamine analgesia | Other analgesic methods | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Age/year | Intraoperative anesthetic | Postoperative analgesic drug | n | Age/year | Intraoperative anesthetic | Postoperative analgesic drug | ||
| Sadurni et al.[4] | RCT | 15 | 72.2±11.8 | GA+INA | Fentanyl | 15 | 74.7±13.8 | GA+INA | Fentanyl |
| Day et al.[5] | RCT | 60 | 51.0±73.0 | IA+GA | Diprivan, alfentanil | 60 | 48.0±77.0 | IA+GA | Diprivan, alfentanil |
| Wang et al.[6] | RCT | 50 | 50.2±5.0 | EA+GA | Rocuronium | 50 | 50.5±6.0 | IA+GA | Rocuronium |
| Hausken et al.[7] | RCT | 77 | 65.6 | NA | Ropivacaine | 66 | 67.1 | NA | Butorphanol tartrate |
| Radovanovic et al.[8] | RCT | 30 | 64.1±10.0 | Light GA | Diclofenac | 30 | 65.8±10.0 | GA | Ketorolac |
| Wang et al.[9] | RCT | 40 | 26.0±85.0 | INA | Levobupivacaine, fentanyl | 41 | 26.0±85.0 | INA | Morphine, fentanyl |
| Zheng et al.[10] | RCT | 81 | 59.1±12.1 | EA+GA | Lidocaine, ropivacaine | 81 | 57.6±12.1 | IA+GA | Butorphanol tartrate |
| Wu et al.[11] | RCT | 37 | 57.5±3.1 | EA+GA | Bupivacaine | 37 | 56.9±3.2 | IA | Fentanyl |
| Chang et al.[12] | RCT | 24 | 54.1±2.4 | EA+GA | Lidocaine, ropivacaine | 24 | 54.2±2.4 | IA | Butorphanol |
| Wongyingsinn et al.[13] | RCT | 31 | 61.0±15.0 | GA | Dezocine, ropivacaine | 31 | 58.0±16.0 | IA | Dezocine, fentanyl |
| Li et al.[14] | RCT | 46 | 54.7±7.2 | EA+GA | Bupivacaine, morphine | 46 | 53.9±6.8 | IA+GA | Morphine |
| Guo et al.[15] | RCT | 39 | 57.6±3.4 | EA+GA | Sufentanil, ropivacaine | 39 | 57.6±3.3 | IA+GA | Ondansetron, Sufentanil |
| Liu et al.[16] | RCT | 32 | 50.3±0.9 | INA | Lidocaine, ropivacaine | 32 | 51.1±0.8 | INA | Butorphanol |
| Ye et al.[17] | CCT | 30 | 50.2±7.3 | EA+GA | Lidocaine, ropivacaine | 30 | 50.9±7.5 | IA+GA | Butorphanol tartrate |
| Xu et al.[18] | CCT | 55 | 55.1±3.1 | EA+GA | Lidocaine, ropivacaine | 55 | 54.2±2.5 | IA+GA | Butorphanol tartrate |
| Wang et al.[19] | CCT | 27 | 54.7±7.0 | EA+GA | Bupivacaine, rphedrine | 27 | 54.7±6.7 | IA | Butorphanol tartrate |
| Ren et al.[20] | CCT | 30 | 58.6±10.2 | EA+GA | Bupivacaine, ephedrine | 31 | 59.8±9.6 | IA+GA | Butorphanol tartrate |
| Qi et al.[21] | CCT | 45 | 55.1±1.2 | IA+GA | Lidocaine, ropivacaine | 45 | 56.0±1.4 | IA+GA | Butorphanol tartrate |
| Liu et al.[22] | CCT | 24 | 56.5±4.7 | NA | Ropivacaine | 24 | 55.5±4.6 | NA | Butorphanol |
| Zhang et al.[23] | CCS | 40 | 67.6±6.2 | EA+INA | Ropivacaine | 38 | 66.1±7.1 | IA+GA | Sufentanil |
Note: GA: General Anesthesia; INA: Intravenous Anesthesia and EA: Epidural Anesthesia
Table 1: The Characteristics of the Included Studies in the Meta-Analysis
Among the included papers, 13 were RCT[5-17], 6 were CCT[18-23], and 1 was CCS[24]. There were 813 cases of epidural analgesia and 802 cases of intravenous analgesia, with a total sample size of 1615 cases. In the methodological quality evaluation, 3 papers were rated as high[6,8-9], and 17 papers were rated as medium. Although all the 13 RCTs were randomly grouped[5,7,10-24], only 5 clearly stated that the method of computer generating random numbers was adopted[7,10-13], and 4 RCTs achieved allocation hiding[5,6,8,13].
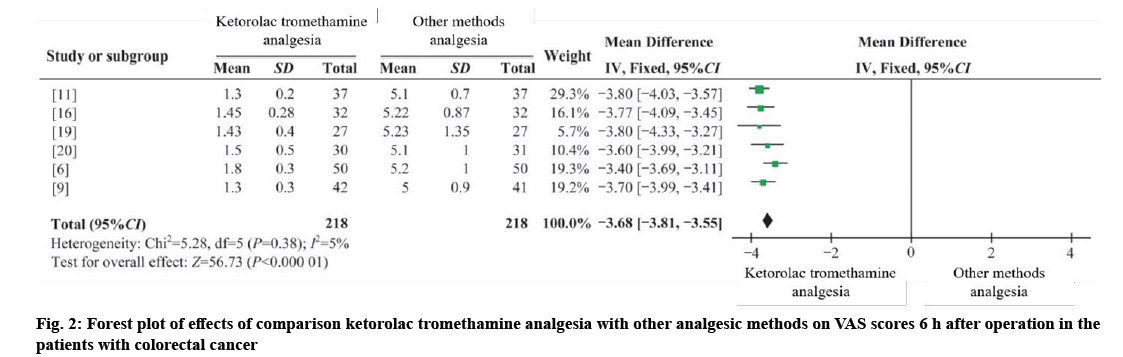
Four RCTs and two CCTs were combined[6,9,11,16], with 218 cases in the intervention group and 218 cases in the control group[19,20]. I2;=5 %, using the fixed effect model (WMD 6 h=−3.68, 95 %=−3.81 to −3.55, p=0.000). The results showed that the difference was statistically significant, and ketorolac tromethamine analgesia was better than other analgesia methods at 6 h after operation as shown in fig. 2.
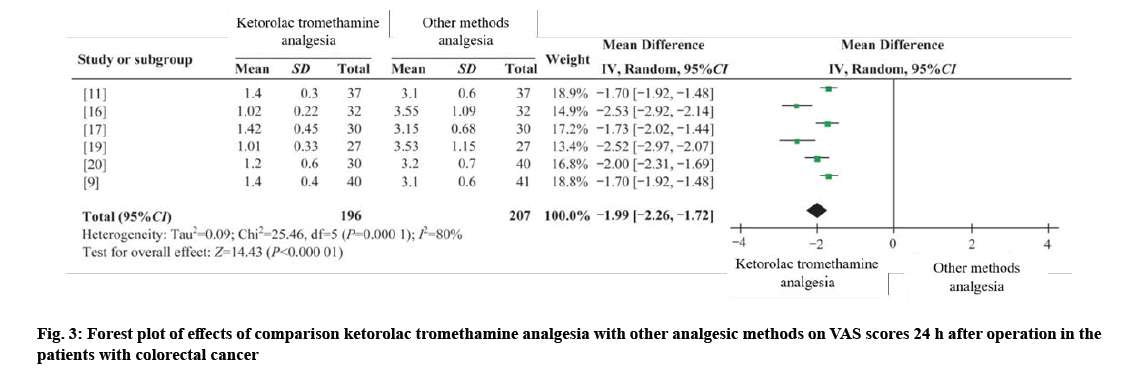
Three RCTs and three CCTs were combined[6,9,16], 196 cases in the intervention group and 207 cases in the control group[16,19,20]. I2;=80 %, using random effects model (WMD 24 h=−1.99, 95 % CI =−2.26 to −1.72, p=0.000). The results showed that the difference was statistically significant, and the analgesic effect of ketorolac tromethamine was better than that of other analgesics at 24 h after operation as shown in fig. 3.
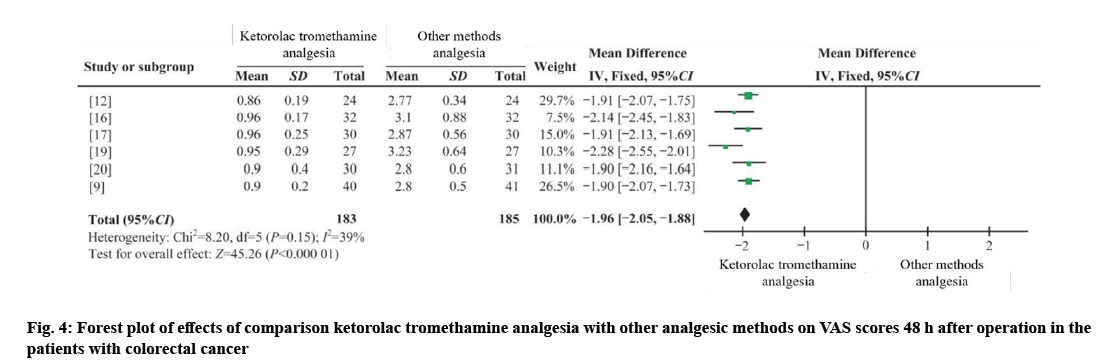
Three RCTs and three CCTs were combined[9,12,16], with 183 cases in the dry pre-treatment group and 185 cases in the control group[17,19,20]. I²=39 %, using the fixed effect model (WMD 48 h=−1.96, 95 % CI =−2.05 to −1.88, p=0.000). The results showed that the difference was statistically significant, and ketorolac tromethamine had a better analgesic effect at 48 h after operation compared with other analgesia methods as shown in fig. 4.
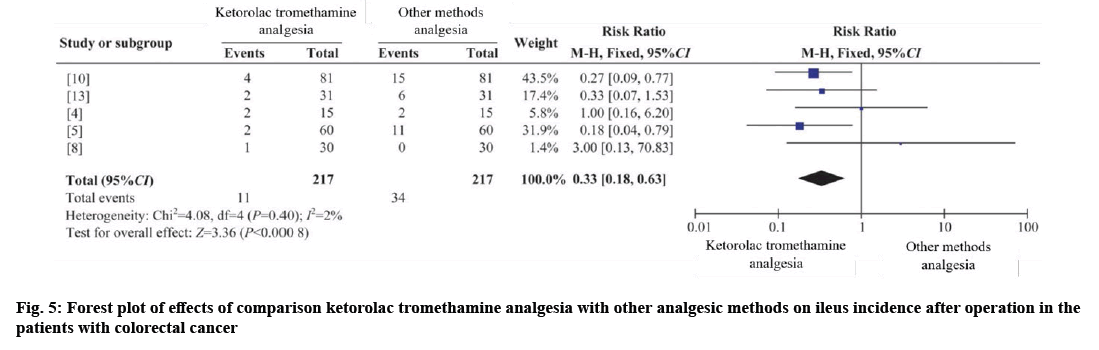
Five RCTs were combined[4,5,8,12,13], with 217 cases in the intervention group and 217 cases in the control group. I2=2 %, using fixed effects model (RR ileus=0.33, 95 % CI=0.18-0.63, p=0.001). The results showed that the difference was statistically significant, and the incidence of postoperative intestinal obstruction could be reduced by ketorolac tromethamine analgesia as shown in fig. 5.
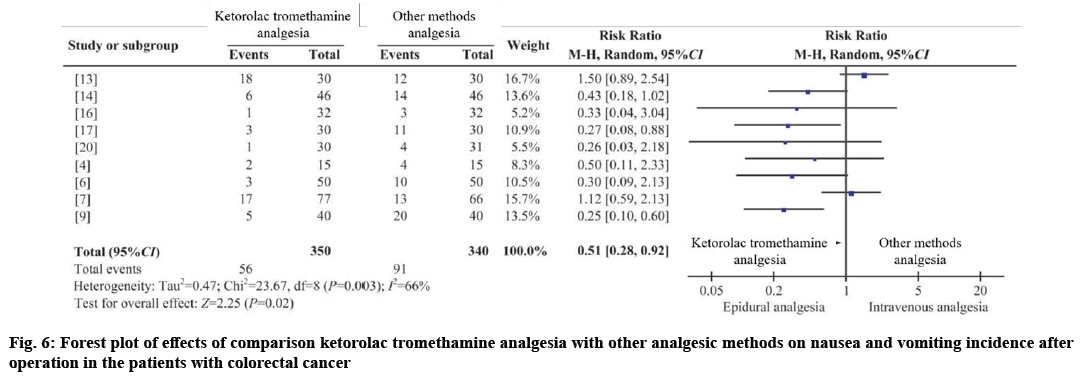
Seven RCTs and two CCTs were combined[4,6,7,9,13,14,16], with 350 cases in the intervention group and 340 cases in the control group[17,20]. I2;=66 %, using random effects model (RR nausea and vomiting=0.51, 95 % CI=0.28-0.92, p=0.020). The results showed that the difference was statistically significant, and the incidence of postoperative nausea and vomiting could be reduced by ketorolac tromethamine analgesia as shown in fig. 6.
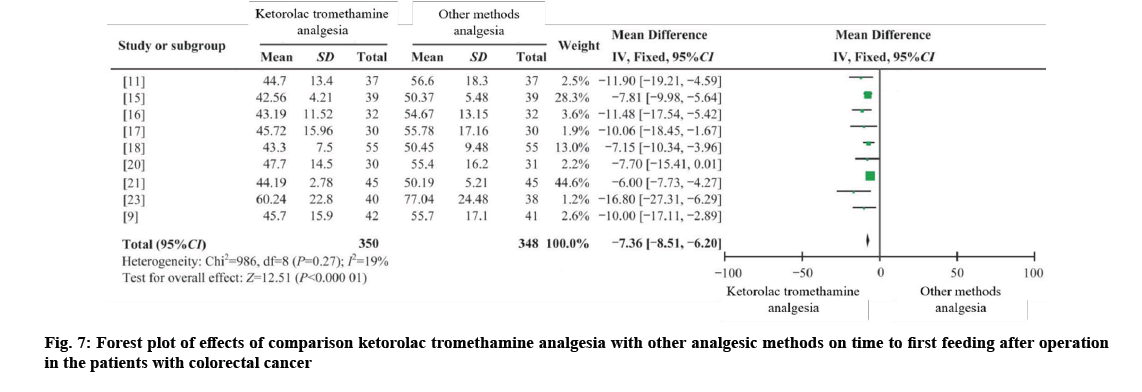
Four RCTs[9,11,15,16], four CCTs[17,18-21] and one CCS were combined, with 350 cases in the intervention group and 348 cases in the control group[23]. I2=19 %, using fixed effects model (WMD first feeding time=−7.36, 95 % CI=−8.51 to −6.20, p=0.000). The results showed that the first feeding time of postoperative patients could be shortened by ketorolac and tromethamine, and the difference was statistically significant as shown in fig. 7.
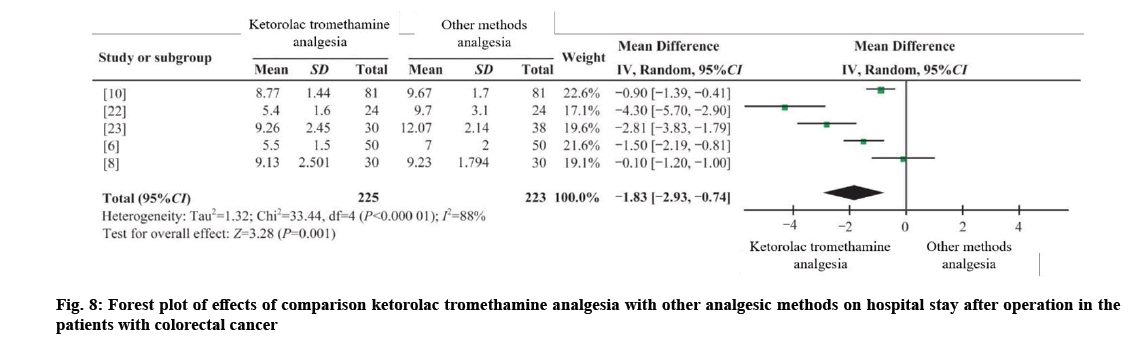
Three RCTs[6,8,10], one CCT and one CCS, were combined[22,23] with 225 cases in the intervention group and 223 cases in the control group. I2;=88 %, using random effects model (WMD hospital stay=−1.83, 95 % CI =−2.93 to −0.74, p=0.001). The results showed that the difference was statistically significant, and the postoperative hospitalization time of patients could also be shortened by ketorolac and tromethamine analgesia as shown in fig. 8.
The main post-operative stress factor is pain. Therefore, the degree of pain relief can be considered as an indicator of postoperative recovery quality. Studies have shown that ketorolac tromethamine analgesia is a kind of local nerve block anesthesia, and its analgesic effect is mainly played by blocking or inhibiting the nerve conduction pathway of specific segments, and the effect is significant and rapid[24]. Although saline alone affects the whole body through the blood circulation, the effect is slower. The conclusion that tromethamine ketorolac tromethamine has the best analgesic effect on the second postoperative day was also confirmed by Levy et al.[25]. On this basis, ketorolac tromethamine can help relieve the pain of patients 6 h after surgery, which was also effectively demonstrated in this study.
Ketorolac tromethamine can be administered according to the pain of patients, maintaining a stable concentration of analgesic drugs in vivo, with a short and lasting effect[26]. Ketorolac tromethamine analgesia can significantly relieve pain 24-48 h after operation, which is reflected in the results of Li et al.[27]. This is basically consistent with the results of this study. In addition, the results of this study indicate that VAS scores at 24 h after surgery are highly heterogeneous. After sensitivity analysis, the sources of sensitivity may include low overall quality of the included literature, different ages of patients, different intraoperative anesthesia methods and anesthetic drugs, and VAS as subjective items.
Intestinal obstruction is one of the most common postoperative complications of colorectal cancer, which is related to gender, age, history of abdominal surgery, tumor location, postoperative sympathetic allergy and other factors[28]. In addition, the mononuclear macrophages in the abdominal cavity will be stimulated and release a large number of inflammatory factors, resulting in intestinal edema and then intestinal obstruction due to the long exposure time of the patient's intestinal tube, the wide scope of lymphatic vessel dissection and the use of electric knife during the operation[29]. Studies have shown that ketorolac tromethamine injection of local anesthetics can inhibit the conduction of somatic nociceptive nerves and abdominal sympathetic nerves[30], reduce the tension of sympathetic nervous system, reduce stress response, and effectively relieve inflammatory factors in the blood. Interleukin-6 (IL-6), as one of the best indicators of surgical stress, can sensitively indicate the degree of tissue damage[31]. The release of IL-6 and the expression of inflammatory factors in patients can be reduced by ketorolac tromethamine analgesia. Compared with other methods, ketorolac tromethamine analgesia is more beneficial to reduce the incidence of postoperative intestinal obstruction.
The relaxation effect of anesthetic drugs on gastrointestinal muscles and the excitatory effect of hypoxia on the medulla oblongata vomiting center during anesthesia can lead to postoperative nausea and vomiting in patients[32]. In addition, the release of inhibitory motor neurotransmitters can be facilitated by the use of other analgesics, thus greatly increasing the incidence of postoperative nausea and vomiting. Studies have shown that tromethamine ketorolac analgesia cannot only promote the recovery of gastrointestinal function, but also reduce the use of opioids after surgery, so as to reduce the occurrence of postoperative nausea and vomiting, and other adverse reactions. This is consistent with the results of this study[33].
The main reasons why postoperative ketorolac tromethamine analgesia can shorten the time of first postoperative food intake includes; the use of ketorolac tromethamine analgesia can stimulate the vague nerve, enhance its excitability, hyperperistalsis of gastrointestinal tract, and corresponding arteriovenous dilatation, improve the postoperative blood perfusion of gastrointestinal tract, and jointly promote the recovery of gastrointestinal function, so as to shorten the first time to eat after surgery in colorectal cancer patients. Second, to achieve better analgesic effect, provide support for early walking of patients, promote early recovery of gastrointestinal function[33], and shorten the time for patients to eat for the first time after surgery can be achieved through the use of ketorolac tromethamine analgesia.
The patient's restrictive dyspnea caused by pain can be alleviated by a good analgesic effect, and the good analgesic effect can also improve the lung function, avoid lung infection, promote the patient's walking, prevent deep venous thrombosis of the lower limbs, promote the recovery of gastrointestinal function, and eat as soon as possible after the operation[34]. After injection of ketorolac tromethamine, analgesic drugs can be continuously released with strong targeting, and the effect of strong analgesia can be achieved after local anesthesia. Therefore, ketorolac tromethamine analgesia requires less drug than other analgesics, which can effectively reduce the risk of adverse reactions[16]. Therefore, epidural analgesia can significantly shorten the postoperative hospital stay of colorectal cancer patients, promote early rehabilitation of patients, and improve hospital satisfaction.
The limitations of this study include; Chinese and English literature only, but our impact on the results of meta-analysis is not clear, so we hold a conservative attitude towards the results of this study. Among the studies included in this study, the generation method of random series is clearly explained in only 9 papers, and the allocation hiding of random schemes is described, which may lead to bias and the reliability of meta-analysis results will be affected. Most of the included literature does not mention blinding, which is related to the different treatment methods. The use of ketorolac tromethamine injection requires informed consent from patients or family members, and subsequent management requires the intervention of different teams. Therefore, the obscurities of randomization and assignment need to be noted in subsequent studies, and more objective measures are adopted as far as possible, blinding outcome raters to reduce bias.
This study showed that the VAS score, the incidence of adverse reactions and the length of hospital stay of patients with colorectal cancer showed a positive effect on postoperative analgesia with ketorolac tromethamine. However, other analgesia methods are still used in clinical practice for the following reasons; the main adverse reaction of ketorolac tromethamine analgesia is hypotension, so the dosage of ephedrine is significantly more than that of intravenous analgesia[22], which will affect its promotion in clinical practice. Ketorolac tromethamine analgesia requires standardized management by professional anesthesiologists. Ordinary nurses may lack relevant knowledge and cannot deal with catheter shedding or blockage in time. However, other methods of analgesia are conducive to nurses' observation. Ketorolac tromethamine analgesia is not suitable for all patients, and its contraindications include intracranial hypertension, intracranial tumors, etc. Therefore, the knowledge of ketorolac tromethamine analgesia, the selection of appropriate analgesia methods and correct nursing interventions, and the provision of "comfortable" care for patients with colorectal cancer after surgery should be learned by medical personnel.
Acknowledgements
This work was supported by 2020 science and technology research and development program of Chengde (202006A053).
Conflict of interests
The authors declared no conflict of interests.
References
- Cao M, Chen W. Interpretation of GLOBOCAN 2020 global cancer statistics. Chin J Front Med Sci 2021;13:63-9.
- Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin Med J 2021;134(7):783-91.
[Crossref] [Google Scholar] [PubMed]
- Dong F, Li G, Meng H. Effects of dexmedetomidine multimodal analgesia on cognitive function after radical resection of colorectal cancer in elderly patients. J North Sichuan Med Coll 2021;36(2):180-3.
- Sadurni M, Beltrán de Heredia S, Dürsteler C, Pérez-Ramos A, Langohr K, Escolano F, et al. Epidural vs. intravenous fentanyl during colorectal surgery using a double-blind, double-dummy design. Acta Anaesthesiol Scand 2013;57(9):1103-10.
- Day AR, Smith RV, Scott MJ, Fawcett WJ, Rockall TA. Randomized clinical trial investigating the stress response from two different methods of analgesia after laparoscopic colorectal surgery. Br J Surg 2015;102(12):1473-9.
[Google Scholar] [PubMed]
- Wang XR, Hou JD, Wang ZG. Effect of different anesthetic and analgesic methods on postoperative outcome of laparoscopic radical resection of colorectal cancer. J Hunan Univ Tradit Chin Med 2018;38(A1):694.
- Hausken J, Fretland AA, Edwin B, Andersen MH, Dagenborg VJ, Bjørnelv GM, et al. Intravenous patient-controlled analgesia vs. thoracic epidural analgesia after open liver surgery: A prospective, randomized, controlled, noninferiority trial. Ann Surg 2019;270(2):193-9.
[Crossref] [Google Scholar] [PubMed]
- Radovanovic D, Radovanovic Z, Skoric-Jokic S, Tatic M, Mandic A, Ivkovic-Kapicl T. Thoracic epidural vs. intravenous patient-controlled analgesia after open colorectal cancer surgery. Acta Clin Croat 2017;56(2):244-54.
[Crossref] [Google Scholar] [PubMed]
- Wang F, Hu Z, Zhou Y. Effect of different anesthetic and analgesic methods on postoperative outcome of laparoscopic radical resection of colorectal cancer. J Clin Anesthesiol 2016;32(1):38-41.
- Zheng HZ, Lin ZM, Yan MF. Application of general anesthesia combined with epidural anesthesia in laparoscopic colorectal surgery. Chin J Mod Med 2017;19(9):1-4.
- Wu X, Yao L, Zhu CL. Difference of postoperative outcome in patients with laparoscopic radical resection of colorectal cancer using different anesthesia and analgesia methods. J Contemp Med 2018;24(31):8-10.
- Chang XF. Effect of different anesthetic and analgesic methods on postoperative outcome of laparoscopic radical resection of colorectal cancer. Chin J Med 2020;5:64-5.
- Wongyingsinn M, Baldini G, Charlebois P, Liberman S, Stein B, Carli F. Intravenous lidocaine vs. thoracic epidural analgesia: A randomized controlled trial in patients undergoing laparoscopic colorectal surgery using an enhanced recovery program. Reg Anesth Pain Med 2011;36(3):241-8.
[Crossref] [Google Scholar] [PubMed]
- Li RH. Study on pain control and thrombosis risk in patients with colon cancer undergoing radical resection with different analgesic methods. Colorectal Anal Surg 2017;23(2):233-6.
- Guo ZW, Wang FF. Application of different anesthesia and analgesia methods in laparoscopic colorectal cancer patients. Primary Med Forum 2020;24(16):2308-9.
- Liu Y, Chen H, Jiang CJ. Effect of different anesthetic and analgesic methods on postoperative outcome of laparoscopic radical resection of colorectal cancer. Chin J Rehab Med 2020;11(10):42-4.
- Ye D, Wang J. Effect of different anesthesia and analgesia methods on patients with laparoscopic radical resection of colorectal cancer. Mod Diagn Treat 2018;29(6):844-6.
- Xu CM, Ning QM, Ye ZQ. Comparison of postoperative outcomes of laparoscopic radical resection of colorectal cancer with different anesthetic and analgesic methods. Chin Med 2016;29(3):78-81.
- Wang F. Effect of different anesthesia and analgesia methods on postoperative outcome of laparoscopic radical resection of rectal cancer. Shenzhen J Integr Tradit Chin Western Med 2018;28(4):20-2.
- Ren D, Zhang C. Effect of different anesthesia and analgesia methods on patients with laparoscopic radical resection of colorectal cancer. Shanghai Pharm J 2019;40(15):31-96.
- Qi FL, Li MY. A comparative study of postoperative outcomes of patients undergoing laparoscopic radical resection of colorectal cancer with different anesthesia and analgesia methods. Rural Health China 2018;8(23):54-5.
- Liu K, Xu HX. Effects of different analgesic methods on early rehabilitation after radical resection of rectal cancer under enhanced recovery after surgery concept. Chin J Tradit Med 2018;8:23-7.
- Zhang X, Jiang H. Application of sevoflurane inhalation combined with epidural anesthesia in patients with colorectal cancer and its effect on postoperative perceptual function. Oncol Lett 2019;17(5):4443-8.
[Crossref] [Google Scholar] [PubMed]
- Liang L, Jiang WM. Comparison of epidural analgesia and intravenous analgesia on postoperative analgesia after spinal fusion: A meta-analysis. Chin J Spinal Cord 2014;24(5):433-9.
- Levy BF, Scott MJ, Fawcett W, Fry C, Rockall TA. Randomized clinical trial of epidural, spinal or patient-controlled analgesia for patients undergoing laparoscopic colorectal surgery. Br J Surg 2011;98(8):1068-78.
[Crossref] [Google Scholar] [PubMed]
- Chen G, Wang T, Sun H. Application of multimodal analgesia in abdominal surgery in enhanced recovery after surgery. Med Theory Pract 2019;32(12):1823-5.
- Li J, Pourrahmat M, Vasilyeva E. Response to comment on efficacy and safety of patient-controlled analgesia compared with epidural analgesia after open hepatic resection: A systematic review and meta-analysis. Ann Surg 2019;270(6):e143.
- Alfonsi P, Slim K, Chauvin M, Mariani P, Faucheron JL, Fletcher D. French guidelines for enhanced recovery after elective colorectal surgery. J Visc Surg 2014;151(1):65-79.
[Crossref] [Google Scholar] [PubMed]
- Jiang L, Wu H, He G. Incidence and influencing factors of postoperative intestinal obstruction in elderly patients with colorectal cancer undergoing laparoscopic surgery. Chin J Gerontol 2018;38(6):1338-41.
- Liu H, Li PB, Han JT. Effects of patient-controlled epidural analgesia on postoperative analgesia and interleukin-6, interleukin-10 and TNF-α in elderly patients undergoing hip replacement. Lab Med Clin 2017;14(24):3626-8.
- Wang JL, Zhang X, Ruan L. Effect of postoperative controlled epidural analgesia on IL-2 and IL-6 in patients with malignant tumor. J Mod Oncol 2009;17(7):1340-2.
- Abd El-Wahab, Abu Shanab EH, Ayaad MG, El-Dabe AA. A comparative study between combined spinal anesthesia with bilateral thoracic paravertebral block and general anesthesia in laparoscopic cholecystectomy. Tanta Med J 2018;46(1):77-82.
- Shi HX, Lkiu J, Wen ZH. Clinical application of epidural controlled analgesia in postoperative analgesia of thoracic surgery. Chin J Mult Organ Dis Elderly 2018;17(5):355-8.
- Huang G. Effect of automatic control analgesia pump on postoperative analgesia in thoracic surgery. Mod Med Health 2005;14:1831-2.