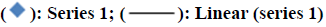- *Corresponding Author:
- G. K. Rakam
Department of Pharmaceutical Analysis,
Vignan Institute of Pharmaceutical Sciences,
Hyderabad, Telangana 508284,
India
E-mail: gopirakam@gmail.com
| Date of Received | 25 August 2020 |
| Date of Revision | 24 August 2021 |
| Date of Acceptance | 18 April 2022 |
| Indian J Pharm Sci 2022;84(2):483-492 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of the study was to develop reverse phase high performance liquid chromatography method for the determination of purity of ondansetron and pantoprazole in tablet form. A reverse phase high performance liquid chromatography method was developed for the estimation of ondansetron and Pantoprazole in tablet formulation. The current method consists of different mobile phase compositions and it is a new approach for the estimation of selected drugs in quality control laboratory, which is novel to the market and for better illusion, buffer was used in the present method. Chromatographic separation of the drug was achieved on a Phenomenex Kromosil C-18, 5 μm, column (250×4.6 mm) using a mobile phase of methanol: Acetonitrile: Potassium hydrogen ortho phosphate buffer pH 3 (40:20:40 v/v) at a flow rate of 1 ml/min. The drug eluted was monitored at 210 nm and the retention time of ondansetron and pantoprazole was found to be 2.7 and 4.l min respectively. The calibration curve was linear over the range of 20-120 μg/ml. The correlation coefficient was found to be 0.999 and 0.998 for ondansetron and pantoprazole respectively. The limit of detection and limit of quantification were found to be 0.075 μg/ml and 0.232 μg/ml for ondansetron and 0.063 and 0.219 for pantoprazole which was within the acceptance range. Forced degradation was carried out under various stress conditions to demonstrate the stabilityindicating capability of the developed reverse phase high performance liquid chromatography method. The performance of the method was validated according to International Council for Harmonization guidelines. The proposed method was found to be simple, precise, accurate and validated according to the International Council for Harmonization guidelines.
Keywords
Reverse phase high performance liquid chromatography, validation, ondansetron, pantoprazole
Pharmaceutical analysis is defined as the branch of practical chemistry which deals with the resolution, separation, identification, determination and purification of a given sample of a pharmaceutical dosage form, the detection and estimation of impurities that may be present there in is also included[1]. Analytical chemistry is the science to analyze morphologies, compositions and quantities of analytical targets. These analytical results have played critical roles from the understanding of basic science to a variety of practical applications, such as biomedical applications, environmental monitoring, quality control of industrial manufacturing and forensic science[2].
Validation is the process of establishing documentary evidence demonstrating that a procedure, process or activity carried out in testing and then production maintains the desired level of compliance at all stages[3]. The validation of the method was based on Food and Drug Administration (FDA) guidelines and on standard bioanalytical method validation recommendation[4].
The goal of equipment validation is to produce constant result with minimal variation without compromising the product and performance of the equipment.
Instrumental method is an exciting and interesting part of chemical analysis that interrelates with all areas of chemistry and with numerous other areas of pure and applied sciences. Analytical instrumentation plays an imperative role in the production and assessment of new products and in the safeguard of consumers and environment[5]. This instrumentation affords minor detection limits vital to assure safe foods, drugs, air and water. Instrumental methods are extensively used by Analytical chemists to save time, to prevent chemical separation and to acquire improved accuracy[6].
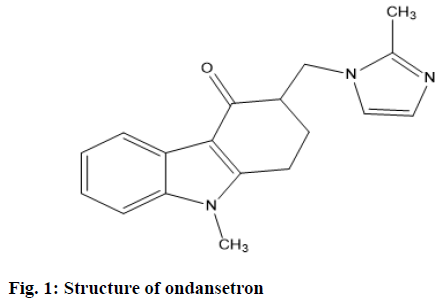
Ondansetron (OND) is chemically known as 9-methyl-3-((2-methyl-1H-imidazol-yl) methyl)- 2,3,4,9-tetrahydro-1H-carbazol-4-one hydrochloride dihydrate (fig. 1). OND, an effective and highly selective 5-HT3 receptor antagonist, it inhibits emesis following chemotherapy by antagonizing the action of 5-Hydroxytryptamine (5-HT) at 5-HT3 receptors on vagal afferent neurons that innervate the gastrointestinal tract and 5-HT3 receptors in the central vomiting system. Evidence recommends that chemotherapy prompts the release of 5-HT from enterochromaffin cells in the small intestine. This stimulates vagal afferent nerves via 5-HT3 receptors. Information is then relayed, via the vagus nerve, to the central vomiting system. 5-HT3 receptors are also found in the hindbrain vomiting system as well as the area postrema (the site of the chemoreceptor trigger zone for emesis). Consequently, following chemotherapy, 5-HT activates 5-HT3 receptors at 2 sites to induce emesis. Clinical data indicates that single doses of OND prevent acute emesis and also suggest that it is vital to block the initiation of the emetic reflex. This may prevent the recruitment of central mechanisms involving 5-HT3 receptors. OND is the subject of monograph in Indian Pharmacopoeia[7], United States Pharmacopoeia[8] and British Pharmacopoeia[9]. It is specified for the prevention of nausea and vomiting accompanying with highly emetogenic cancer chemotherapy[10].
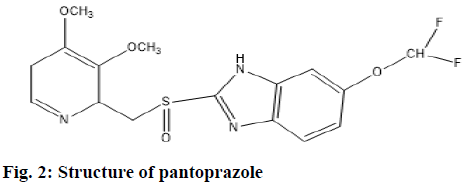
Pantoprazole is chemically known as 5-(Difluromethoxy)-2-((3,4-dimethoxy-2-pyridinyl] methyl) sulfinyl) 1H-Benzimidazole (fig. 2). It is a proton pump inhibitor, which suppresses gastric acid secretion by H+/K+ Adenosine Triphosphatase (ATPase) enzyme system at the secretary surface of the gastric parietal cell. This activity is dose-related and leads to inhibition of stimulated gastric acid secretion regardless of the stimulus[11,12]. This drug is employed for the management of gastrointestinal and esophageal ulcers. Pantoprazole is official drug in Indian Pharmacopoeia, United States Pharmacopoeia and British Pharmacopoeia. The main aim and objective of the work was to develop and validate Reverse Phase High Performance Liquid Chromatography (RP-HPLC) method for the determination of OND and pantoprazole in tablet dosage form. The developed method was validated with respect to linearity, precision, accuracy, robustness, limit of detection and limit of quantification.
Literature reveals that various Spectrophotometric[13], Colorimetric[14], thin layer chromate-graphic[15], spectrofluorimetric[16] and High Performance Liquid Chromatography (HPLC)[17] have been reported for the determination of pantoprazole in pharmaceutical preparations. Some analytical methods for the quantitative determination of OND as single or in combination with other drugs in pharmaceutical preparations are described in literature like spectrophotometric[18] and HPLC[19] their individual analysis, along with other combinations in pharmaceutical formulation and fluids. Hence, in the present communication we would like to report a simple, economic, feasible, rapid, sensitive and validated specific RP-HPLC method for the simultaneous determination of OND and pantoprazole in the tablet form.
Materials and Methods
Chemicals and reagents of OND:
An analytically pure sample of OND was acquired as gifted sample from Amazing Research Laboratories Ltd. (Dehli, India). Pharmaceutical tablet dosage form of OND (Vominil-MD) was manufactured by crescent therapeutic ltd. and label claim was found to be 4 mg. Acetonitrile (HPLC grade), methanol (HPLC grade), orthophosphoric acid, Sodium Hydroxide (NaOH), Hydrochloric Acid (HCl) and potassium dihydrogen phosphate were purchased from Merck (India). All chemicals were of analytical grade and used as received.
Chemicals and reagents of pantoprazole:
An analytically pure sample of pantoprazole was obtained as gifted sample from Amazing Research Laboratories Ltd. (Dehli, India). Pharmaceutical tablet dosage form of pantoprazole (pantoprazol) was manufactured by crescent therapeutic ltd and label claim was found to be 4mg. Acetonitrile (HPLC grade), methanol (HPLC grade), orthophosphoric acid, NaOH, HCl and potassium dihydrogen phosphate was purchased from Merck (India). All chemicals were of analytical grade and used as received.
Method development and optimization:
Initial set of chromatographic conditions were defined based on analyte molecules. Phenomenex kromosil C-18 G, 250 mm×4.6 mm, 5 μm, was selected since it has noble separation capability. Mobile phase for RP-HPLC comprise methanol:Acetonitrile:Potassium hydrogen orthophosphate buffer pH 3 (40:20:40 v/v). The overall run time was found to be 6 min. Flow rate of about 1 ml/min was selected since it was taken as optimum flow rate for the selected column conditions. Detection wavelength of 210 nm and injection volume of 20 μl was selected to elevate detection capability.
Preparation of the mobile phase: To prepare mobile phase 40 ml of methanol, 20 ml of acetonitrile and 40 ml of potassium hydrogen orthophosphate buffer were taken in 100 ml measuring cylinder and then mobile phase was filtered through 0.45 μm membrane filter and sonicated for 5 min using ultrasonicator bath and later transferred into solvent reservoir avoiding bubbles. During the preparation of buffer pH adjustment is required and the pH 3±0.02 was adjusted with orthophosphoric acid and controlled in mobile phase composition.
Preparation of buffer: 132 mg of potassium dihydrogen orthophosphate was exactly weighed, transferred in to a 100 ml volumetric flask, dissolved by addition of distilled water and finally diluted to the mark with water. During the preparation of buffer pH adjustment is required and the pH 3±0.02 was adjusted with orthophosphoric acid and controlled in mobile phase composition.
Preparation of standard solution: Standard solution was prepared by transferring 1 ml of standard stock solution in to 10 ml volumetric flask and diluted to volume with methanol:Acetonitrile:Buffer (40:20:40) and filtered through 0.45 μm membrane filter. This was marked as working standard solution (100 μg/ml).
Preparation of test solution: Seven tablets of OND were weighed accurately and crushed to get fine powder. Accurately weigh a quantity of powder equivalent to 525 mg of OND and was transferred in to 25 ml of volumetric flask and methanol was added to dilute it and later sonicated for 5 min. Filter it through 0.45 μm membrane filter and sonicate for 5 min using ultrasonicator. Three tablets of pantoprazole were weighed accurately and crushed to get fine powder of 121 mg of pantoprazole and was transferred into 25 ml of volumetric flask and methanol was added to dilute it and sonicated for 5 min and filtered through 0.45 μm membrane filter and again sonicate it for 5 min using ultrasonicator. Further dilution was prepared by pipetting 1 ml of above stock solutions, poured in to 10 ml of volumetric flask and later diluted to volume with mobile phase. Then, this solution was filtered through 0.45 μm membrane filter and sonicated for 5 min. This was marked as test solution (1000 μm).
Preparation of blank solution: In to 10 ml volumetric flask, 5 ml of Acetonitrile:Methanol:Buffer was added, sonicated for 5 min. The solution was then diluted to mark with methanol and water.
Method validation:
The method was validated as per International Council for Harmonization (ICH) guidelines[20]. The method was validated for system suitability, linearity, precision, accuracy, robustness, Limit of Detection (LOD) and Limit of Quantitation (LOQ).
System suitability test:
Under optimized chromatographic conditions, system suitability test was performed by injecting blank solution once and standard solution of 10 μg/ml test concentration six times in to stabilized HPLC system. Percentage Relative Standard Deviation (% RSD) for peak areas of OND and Pantoprazole was determined below 2 %. To establish system suitability, last peak of six injections was evaluated. System suitability was conducted by evaluating parameters like Retention Factor (k′), repeatability, Resolution (R), Tailing factor (T) and theoretical plates (N).
Linearity:
Linearity of the method was demonstrated over the concentration range of 20-120 μg/ml of OND and Pantoprazole. Each concentration was prepared in triplicate. 20 μl of each standard solution were injected at the optimized chromatographic conditions and the chromatograms were recorded. The retention time, average peak areas were recorded. Calibration curves were constructed by plotting peak area on Y-axis against concentration on X-axis and regression equation was calculated by the method of least squares. The correlation coefficient, y-intercept, slope of the regression line was submitted.
Precision:
It expresses the precision of the system. It includes analysis on system. It was performed by injecting six replicate injections of standard solution at 200 μg/ml concentration under same experimental conditions. The mean value, standard deviation and % RSD was calculated for all six replicate injections.
Method precision: It expresses the precision of the method. It was performed by injecting six replicate injections of standard solution at 150 μg/ml concentration under same experimental conditions. The mean value, standard deviation and % RSD was calculated for all six replicate injections.
Intermediate precision: It expresses the precision within laboratory variations. It includes full analysis on different days, instruments or analysts. It was performed by injecting six replicate injections of test solution at 50 μg/ml concentration on different days under same experimental conditions. Mean value, standard deviation and % RSD were calculated for six replicate injections.
Accuracy:
Accuracy of the method was established by performing recovery studies according to the ICH guidelines. It was ascertained on the basis of recovery studies by standard addition method. Recovery studies were carried out at three different levels (40 %, 80 % and 120 %) by the addition of standard drug to pre-analyzed sample solution each in triplicate. Mean percentage recovery values at three different concentrations of drug were calculated.
Robustness:
To evaluate the robustness of the developed RP-HPLC method, small deliberate variations in the optimized chromatographic conditions were done i.e., variation in flow rates (0.1±ml/min), concentration of organic phase (±2 %) and detection wavelengths (±3 nm). It was performed using 120 μg/ml.
LOD and LOQ:
The lowest concentration of analyte was detected by limit of detection and the lowest concentration of the substance was quantified by limit of quantification[21]. The LOD and LOQ were determined by using signal to noise ratio approach as defined in ICH guidelines.
Degradation studies:
As per ICH guidelines, the forced degradation studies were performed on drug substance to establish its inherent stability characteristics in order to demonstrate selectivity and stability indicating capability of the proposed method. The standard substances were exposed to forced degradation studies such as oxidation, acid degradation, alkali degradation, dry heat degradation, photo stability and neutral degradation studies.
Oxidation: 1 ml of 20 % Hydrogen Peroxide (H2O2) was added to 1 ml of stock solution (OND and pantoprazole). The solutions were kept at 60° for 30 min. For study, the resulting solution was diluted to obtain 50 ppm and 0.50 μl was injected into the system. To access the stability of sample, chromatogram was recorded.
Acid degradation: 1 ml of 2N HCN was added to 1ml of stock solution (OND and pantoprazole) and refluxed for 30 min at 60°. The resulting solution was diluted to obtain 50 ppm by using injection volume of 0.50 μl. The chromatogram was recorded to attain the stability of sample.
Alkali degradation: 1 ml of 2N NaOH was added to1 ml of stock solution (OND and pantoprazole) and refluxed for 30 min at 60°. The subsequent solution was diluted to obtain 50 ppm and 0.50 μl was injected into the system and the chromatogram was recorded to access the stability of sample.
Dry heat degradation: The standard drug solution was placed in an oven at 105° for 6 h to attain dry heat degradation. For study, the subsequent solution was diluted to 50 ppm and 0.5 μl was injected into the system and the chromatogram was recorded to gain the stability of the sample.
Photo stability studies: The photochemical stability of the drug was also studied by exposing the (500 ppm) solution to Ultraviolet (UV) light by keeping the beaker in UV Chamber for 7 d or 200Watt h/m2 in photo stability chamber. For the study, the resultant solution was diluted to obtain 50 ppm and 0.50 μl was injected into the system and the chromatogram was recorded to assess the stability of sample.
Neutral degradation studies: Stress testing under neutral conditions was studied by refluxing the drug in water for 6 h at a temperature of 60°. For our study, the resultant solution was diluted to 50 ppm solution and 0.50 μl was injected into the system and the chromatogram was recorded to assess the stability of the sample.
Assay:
Standard solution and sample solution were injected distinctly into the HPLC system from which peak area response for analyte was measured. The standard was prepared from Active Pharmaceutical Ingredient (API) and sample was prepared from formulation. Both standard and sample were analyzed by six replications. OND and pantoprazole were assessed (formulation) by taking standard as reference.
Results and Discussion
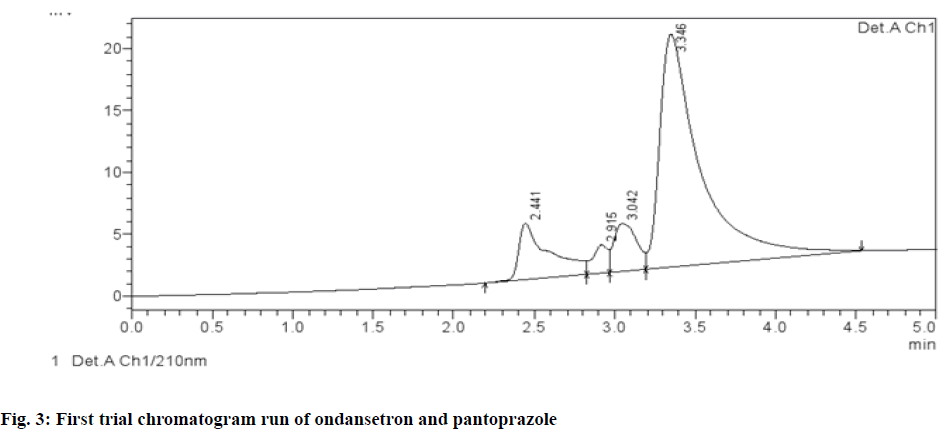
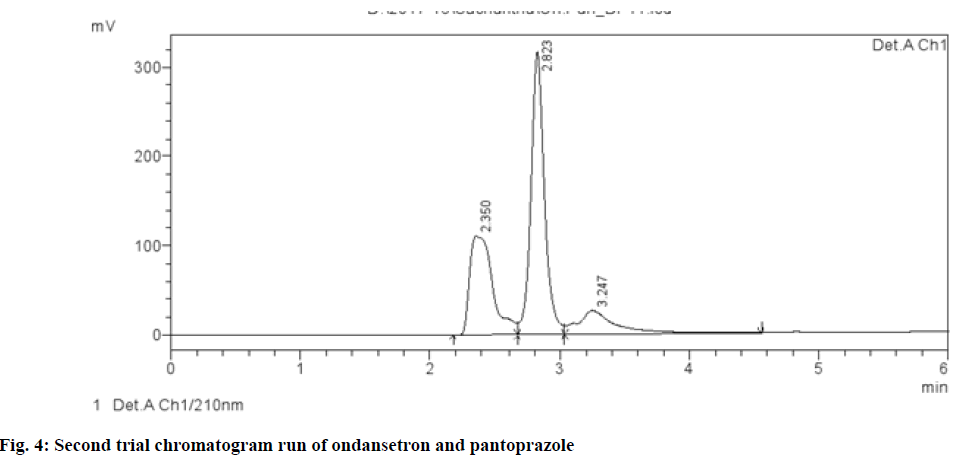
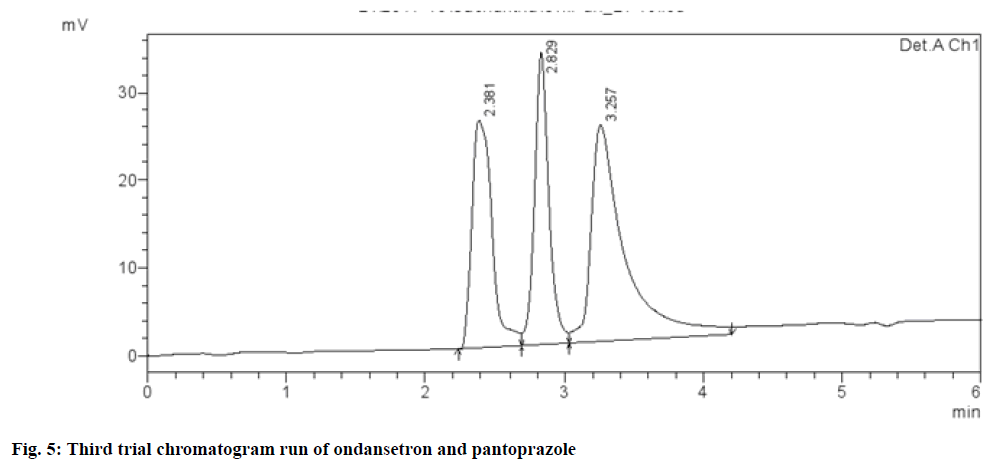
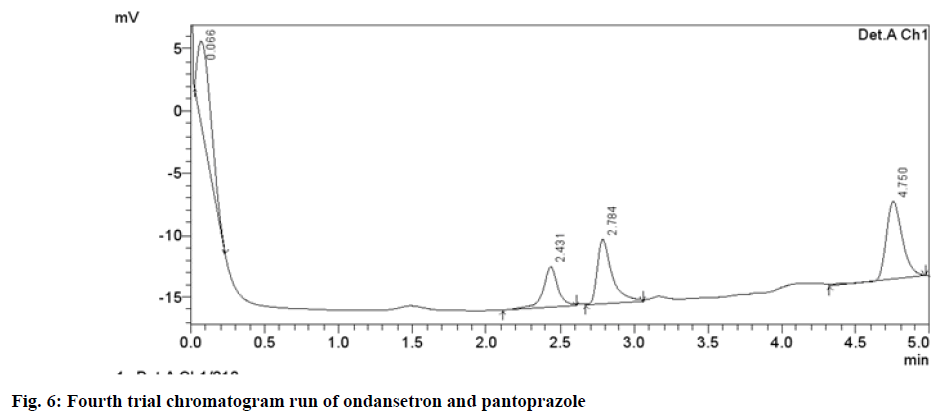
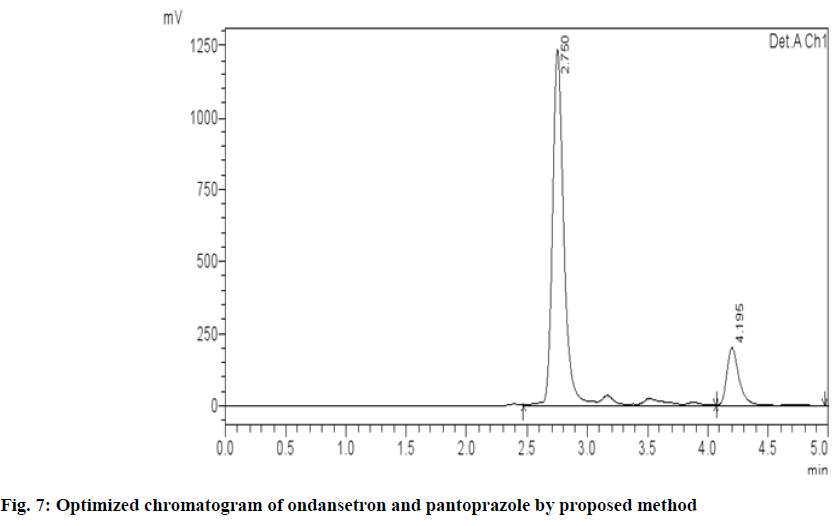
Different chromatographic trials were done to achieve the better efficacy of the chromatographic system. The trial-1 chromatogram showed base line disturbances and did not show any peaks (fig. 3). The trial-2 chromatogram has no tailing and resolution and has shown only solvent peak, so further trials were performed (fig. 4). The trial-3 chromatogram exhibited base line disturbances with no tailing factor and only one drug was determined (fig. 5). The trial-4 chromatogram displayed bad peak shape and there was no proper tailing and resolution (fig. 6). Further, trial was carried out by altering both mobile phase and column. The trial-5 chromatogram (OND and pantoprazole) eluted with good peak shape. Tailing factor was found to be <2 and theoretical plates was found to be >2000 (fig. 7). Methanol:Acetonitrile:Buffer (40:20:40) pH 3 was fixed as mobile phase. In this the peaks were well separated and meet all the system suitability parameters. The detection wavelength of OND and pantoprazole was found to be 210 nm. Detection wavelength was found to be 210 nm, as it was scanned in the UV range of 200-400 nm and the overlay spectrum with maximum absorbance was found to be 210 nm. During the preparation of buffer pH adjustment is required and the pH 3±0.02 was adjusted with orthophosphoric acid and controlled in mobile phase composition.
The method development trials were performed on the working standard at 100 % level. Several combinations of chromatographic conditions were tried but the optimum results were achieved with Phenomenex kromosil C-18 (250 mm×4.6 mm, 5 μ) column, mobile phase mixture constituting Methanol:Acetonitrile:Buffer in a ratio of 40:20:40, 1 ml/min flow rate at a detection wavelength of 210 nm. Peak of OND and pantoprazole were achieved with good resolution, peak shape and symmetry at Rt 4.1 min and 2.7 min.
The system suitability of the proposed method was accomplished from the retention factor, repeatability, resolution, tailing factor and theoretical plates of OND and pantoprazole at the optimized conditions. The parameters were recorded and tabulated (Table 1) and were found to be in compliance with the acceptance specifications. All the system suitability parameters were satisfied. The result was shown in Table 1.
| System suitability parameter | Acceptance criteria |
|---|---|
| Retention factor (k’) | The peaks of interest should be well resolved from other peaks and the void volume, generally k should be >2 |
| Repeatability | RSD of ≤2 % |
| Resolution (Rs) | R should be >2 between peak of interest and the closest eluted interferents. |
| Tailing factor (T) | T should be ≤2 |
| Theoretical plates (N) | In general N should be >2000 |
Note: % indicates percentage; > indicates greater than and ≤ indicates less than or equal to
Table 1: System Suitability Data
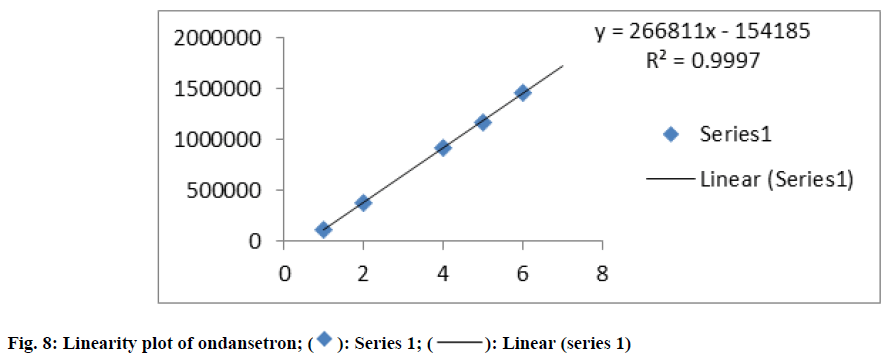
The linearity of the proposed method was accomplished from the correlation coefficients of the standard calibration curve of OND which were constructed at concentration ranges of 20-120 μg/ml. The correlation coefficient was found to be 0.999 which were in compliance with the acceptance criteria. The result was indicated in fig. 8.
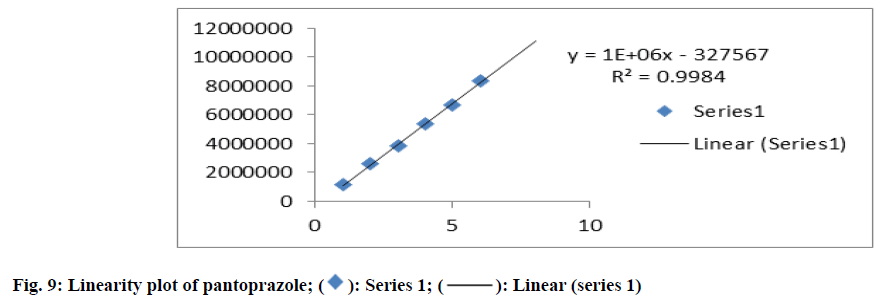
The linearity of the proposed method was accomplished from the correlation coefficients of the standard calibration curve of Pantoprazole which were constructed at concentration ranges of 20-120 μg/ml. The correlation coefficient was found to be 0.998 which were in compliance with the acceptance criteria. The result was specified in fig. 9.
The precision of the recommended method was established from the % RSDs of the percentage assays of the drugs at the levels of repeatability, intermediate precision (inter-d). The % RSDs of OND and pantoprazole at inter-d system precision were found to be 1.8 % and 1.6 % respectively. As the % RSDs were found to be within the acceptance limit (% RSD <2) at all the levels, the projected method was said to be precise. The result was designated in Table 2.
| Injections | Peak area of Ondansetron | Peak area of Pantoprazole |
|---|---|---|
| 1 | 2475007 | 232486 |
| 2 | 2461221 | 225634 |
| 3 | 2373637 | 223627 |
| 4 | 2355350 | 222362 |
| 5 | 2166768 | 209071 |
| 6 | 202122 | 19424 |
| Mean | 2005683 | 188767.3 |
| SD | 890416.8 | 83311.07 |
| % RSD | 1.8 | 1.6 |
Note: % RSD indicates percentage of relative standard deviation
Table 2: Overlain Result of System Precision
The accuracy of the proposed method was evaluated from the recovery studies, by standard addition m ethod which was performed at three levels of 40 %, 80 % and 120 % of the label claims of OND and pantoprazole. The mean percentage recoveries at each level of OND and pantoprazole were found to be within the acceptance limits of 98 %-105 % which specifies that the method was accurate and also discloses that the excipients existing in the pharmaceutical formulation were not interfering in the proposed method.
Robustness of the proposed method was demonstrated by making deliberate changes in the flow rate, wavelength and concentration of organic phase from the optimized condition of the developed method and computing the % RSD of the peak areas. The % RSD at 0.9 ml/min was found to be 0.5 % and at 1.1 ml/min was found to be 1.1 % for OND and pantoprazole respectively. At a mobile phase composition of methanol, acetonitrile and phosphate buffer in a ratio of 42:18:40, the RSD was found to be 1.0 % for OND and at a ratio of 40:20:40 of methanol, acetonitrile and buffer, it was found to be 1.2 % for pantoprazole. As the % RSDs were found to be within the acceptance limit (% RSD <2), the proposed method was said to be robust. The result was displayed in Table 3 and Table 4.
| Parameter | Concentration (150 µg/ml) | Mean peak area | % RSD |
|---|---|---|---|
| Mobile phase composition (Methanol: Acetonitrile: potassium dihydrogen orthophosphate buffer) | (40:20:40 ) v/v | 361540 | 1.2 |
| +2 % (38:22:40) | 91643 | 0.4 | |
| -2 % (42:18:40) | 3447389 | 1 | |
| Flow rate | (±0.1) 1 ml/min | 5894032 | 0.8 |
| (+0.1) 1.1 ml/min | 5847390 | 1.1 | |
| (-0.1) 0.9 ml/min | 5656880 | 0.5 | |
| Wavelength (nm) | 210 | 9350194 | 0.5 |
| 208 | 1056108 | 0.7 | |
| -212 | 3373359 | 0.9 |
Note: % RSD indicates percentage of relative standard deviation; + indicates plus; - indicates minus; v/v indicates volume by volume; nm indicates nanometers; ± indicates plus or minus
Table: 3 Robustness Study Data of Ondansetron
| Parameter | Concentration (150 µg/ml) | Mean peak area | % RSD |
|---|---|---|---|
| Mobile phase composition (Methanol: Acetonitrile: potassium dihydrogen orthophosphate buffer) | (40:20:40 ) v/v | 361540 | 1.2 |
| +2 % (38:22:40) | 91643 | 0.4 | |
| -2 % (42:18:40) | 3447389 | 1 | |
| Flow rate | (±0.1) 1 ml/min | 5894032 | 0.8 |
| (+0.1) 1.1 ml/min | 5847390 | 1.1 | |
| (-0.1) 0.9 ml/min | 5656880 | 0.5 | |
| Wavelength (nm) | 210 | 9350194 | 0.5 |
| 208 | 1056108 | 0.7 | |
| 212 | 3373359 | 0.9 |
Note: % RSD indicates percentage of relative standard deviation; + indicates plus; - indicates minus; v/v indicates volume by volume; nm indicates nanometers; ± indicates plus or minus
Table 4: Robustness Study Data of Pantoprazole
LOD and LOQ were determined by signal to noise ratio of 3:1 and 10:1 respectively. The limit of detection and limit of quantification were found to be 0.075 μg/ ml and 0.232 μg/ml for OND and 0.063 and 0.219 for pantoprazole correspondingly which was within the acceptance range. As all the validation parameters studied, complied with the acceptance criteria, the proposed method was said to be validated in accordance with ICH guidelines.
Degradation studies were performed with formulations and degraded samples. The % degradation of the drug for acid, alkali, oxidation, thermal, UV and water were found to be 4.64, 3.12, 3.68, 2.15, 1.22 and 0.76 individually for OND which was within the limit. The % degradation of the drug for acid, alkali, oxidation, thermal, UV and water were found to be 4.26, 3.22, 3.53, 2.10, 1.17 and 0.89 correspondingly for pantoprazole which was within the limit. Degradation data was mentioned in Table 5.
| S. No | Degradation Condition | % Degraded | % Degraded |
|---|---|---|---|
| 1 | Acid | 4.64 | 4.26 |
| 2 | Alkali | 3.12 | 3.22 |
| 3 | Oxidation | 3.68 | 3.53 |
| 4 | Thermal | 2.15 | 2.1 |
| 5 | UV | 1.22 | 1.17 |
| 6 | Water | 0.76 | 0.89 |
Note: % Indicates percentage; UV indicates ultra violet
Table 5: Degradation Data of Ondansetron and Pantoprazole
The assay was performed on the tablet formulation (Vominil-MD, Amazing research Laboratories Ltd) and the % drug content of OND was found to be 102.9 %, which was within the acceptance limits. As the peak of analyte was well resolved and had no interference of excipients, it was concluded that the proposed method was specific to the drug under study.
The assay was performed on the tablet formulation (pantoprazol, Amazing research laboratories Ltd) and the drug content of pantoprazole was found to be 101.41 %, which was within the acceptance limits. As the peak of analyte was well resolved and had no interference of excipients, it was concluded that the proposed method was specific to the drug under study. The results of assay were indicated in Table 6.
| Parameter | Ondansetron | Pantoprazole |
|---|---|---|
| Mean peak area | 8347007 | 1413605 |
| Wt taken (mg) | 525 | 121 |
| Label claim (mg) | 4 | 40 |
| Average. Wt (mg) | 84 | 194 |
| Amount recovered | 101 | 102 |
| % Assay | 101.40% | 102.90% |
Note: mg indicates milligram; % indicates percentage; wt indicates weight
Table 6: Results of Assay Data
Acknowledgements:
The authors are thankful to the management of Chaitanya college of Pharmacy Education and Research, Kishanpura, Hanamkonda, Warangal, Telangana, India, for providing the necessary facilities to carry out the research work. Further, authors are thankful to Vignan Institute of Pharmaceutical Sciences, Deshmukhi, Hyderabad, Telangana, India.
Conflict of interests:
The authors declared no conflict of interest.
References
- Kamboj PC. Pharmaceutical analysis. 2nd Edn.Vallabh publications; 2007.
- Erwing GW. Instrumental methods of chemical analysis. 2nd Edn, Mc Graw Hill Boo Company, Inc; 1960.
- Lavanya G, Sunil M, Eswarudu MM, Eswaraiah MC, Harisudha K, Spandana BN. Analytical method validation: An updated review. Int J Pharm Sci Res 2013;4(4):1280.
- Rote AR, Kumbhoje PA. Development and validation of HPTLC method for Simultaneous estimation of Gatifloxacin and Ornidazole in human plasma. J Chromatogr Sep Techn 2011;2:115.
- Connors KA: A text book of pharmaceutical analysis. 3rd edition, John Wiley and Sons; 1999.
- Connors KA. A text book of pharmaceutical analysis. 3rd edition. John Wiley and Sons; 2002.
- Indian Pharmacopoeia, Government of India, Ministry of health and Family Welfare. The Indian Pharmacopoeia Commission, Ghaziabad; 2010(3): 1856, 1815.
- United State Pharmacopoeia. 32nd edition. United States Pharmacopieal Convention. Inc. Rockville, Md; 2009; 3200, 3139.
- British Pharmacopoeia. Published by The Stationery Office on behalf of the Medicines and Healthcare products Regulatory Agency (MHRA); 2011(2): 1643, 1598.
- Tripathi, KD. Essential of Medical Pharmacology. 6th edition. New Delhi: J P Brothers Medical Publishers Pvt Ltd; 2010.
- Jungnickel PW. Pantoprazole: A new proton pumps inhibitor. Clin Ther 2000;22(11):1268-93.
[Crossref] [Google Scholar] [Pub Med]
- Shirazi M, Alimoradi H, Kheirandish Y, Etemad-Moghadam S, Alaeddini M, Meysamie A, et al. Pantoprazole, a proton pump inhibitor, increases orthodontic tooth movement in rats. Iran J Basic Med Sci 2014;17(6):448.
- Patel Keyur D, Raj H, Sojitra Jatin R, Nilesh K. Simultaneous estimation of levosulpiride and pantoprazole sodium by first and second derivative spectroscopic methods in capsule dosage form. Univers J Pharm 2013;2(1):160-7.
- Kalaichelv R, Rose MF, Vadivel K, Jayachandran E. Simple extractive colorimetric determination of pantoprazole sodium by aciddye complexation method in solid dosage form. Int J Chem Res 2010;1(1):6-8.
- Satish PA. High Performance Thin Layer Chromatographic method for estimation of Pantoprazole in Injection. Int Res J Pharm 2011;2(8):132-5.
- Michael PY, Mohammed M. A validated Spectrofluorimetric method for the assay of some proton inhibitors using 1, 2-Naphtoquinone-4-sulphonate. Int J Pharm Pharm Sci 2014;6(5):212-9.
- Battu PR, Reddy NK. Development and validation of RP-HPLC for the pantoprazole sodium sesquihydrate in pharmaceutical dosage forms and human plasma. Int J ChemTech Res 2009;1(2):195-8.
- Kumar PR, Krishna MM, Prakash PB, Kumar BA, Madhusudhan P. Derivative spectrophotometric estimation of ondansetron and paracetamol. E-J Chem 2006;3(3):134-6.
- Sheshala R, Darwis Y, Khan N. Development and validation of an RP–LC–UV method for the determination of ondansetron: Application to pharmaceutical dosage forms. Chromatographia 2009;70(1):75-81.
- ICH, Validation of analytical procedures: Text and Methodology Q2 (R1); 2006.
- Balan P, Kannappan N. Development and validation of stability-indicating RP-UPLC method for simultaneous estimation of thiocolchicoside and aceclofenac in combined dosage form. Int Curr Pharm J 2014;3(7):296-300.