- *Corresponding Author:
- S. K. Jain
Pharmaceutics Research Projects Laboratory, Department of Pharmaceutical Sciences, Dr. Hari Singh Gour Vishwavidyalaya, Sagar - 470 003, India.
E-mail: drskjainin@yahoo.com
| Date of Submission | 19 July 2006 |
| Date of Revision | 3 April 2007 |
| Date of Acceptance | 5 July 2007 |
| Indian J Pharm Sci 2007, 69 (4): 498-504 |
Abstract
Novel mucoadhesive chitosan microspheres were developed to explore the possibilities of non invasive delivery of insulin. The mucoadhesive chitosan microspheres were prepared by emulsification method. Formulations were characterized for various physiochemical attributes, shape, surface morphology, size and size distribution, drug payload, swelling ability and mucoadhesion. Mucoadhesive chitosan microspheres bearing insulin were evaluated for in vitro drug release and in vitro drug permeation through mucosal membrane. In vivo performance was studied on blood plasma level of glucose. Glutaraldehyde cross-linked microspheres showed better reduction of blood glucose level than citric acid cross-linked microspheres. The in vivo performance of mucoadhesive microspheres showed prolonged and controlled release of drug as compared with the conventional dosage form.
Keywords
Mucoadhesion, chitosan microspheres, insulin delivery
Parenteral administration of proteins/peptides requires repeated injections because of their extremely short biological half-life. Daily multiple injections are highly risky and require close medical supervision. Therefore, the delivery of proteins and peptides by routes other than parenteral route has gained much attention in recent years. As a drug delivery route, nasal cavity offers several possible advantages. The nasal epithelium is highly vascularized and offers a relatively large surface area for drug absorption. In addition the porous endothelial basement membrane and the direct transport of absorbed drugs into the systemic circulation, avoids the hepatic first-pass effect present in peroral administration. Further since the nasal membrane posses lower enzymatic activity compared with the gastrointestinal tract and the liver [1].
In the present project mucoadhesive chitosan microspheres were prepared for non-invasive delivery of insulin. Chitosan is a widely used mucoadhesive polymer. It is a cationic hydrophilic polysaccharide comprising copolymers of glycosamine and Nacetylglucosamine and can be derived by partial deacetylation of chitin from crustacean shells [2]. Chitosan microspheres have been reported to provide controlled release of many drugs and these microspheres are being investigated for both for parenteral and oral drug delivery [3].
Therefore, it was aimed to exploit the mucoadhesive property of natural polysaccharide, chitosan and higher permeability, less hostile environment and extended surface area of nasal mucosa to develop mucoadhesive microspheres bearing insulin for the painless delivery of insulin. Insulin has been taken as model peptide. As insulin represents a polar, hydrophilic peptide of a comparatively high molecular mass, its nasal delivery is a challenging task.
Materials and Methods
Chitosan (purified viscosity grade 50) was obtained from Central Institute of Fisheries Technology, Cochin, India. Insulin (98-102% pure) was received as a kind gift from Cadila Healthcare Ltd., Ahemdabad, India. Span 80 and sodium taurocholate were procured from Sigma Chemicals, St. Louis, MO, USA. Liquid paraffin (viscosity 90 cp at 30°) was supplied from S. D. Fine Chemicals, Mumbai, India. All other solvents and reagents were of analytical grade.
Preparation of microspheres
The chitosan microspheres were prepared by modified emulsification technique reported by Thanoo et al. [4]. A 4% w/v solution of chitosan was prepared in 5% aqueous acetic acid. This solution (6.5 g) was dispersed in 37.5 ml of liquid paraffin (1:1mixture of light and heavy) containing 0.15 g of Span 80 in a 100 ml beaker. The dispersion was stirred using a stainless steel half-moon paddle stirrer at various speeds (1000-4000 rpm) for 2 min and glutaraldehyde saturated toluene solution (1-4 ml) or 1 ml of aqueous solution of citric acid (1-4%) was added and stirring was continued (1-4 h). After the stipulated stirring time, the microspheres were centrifuged, washed several times with hexane, methanol and finally with acetone. The microspheres were then dried at 50°. Formulations having varying compositions were prepared in order to optimize the process parameters (Table 1).
| Formulation code | Quantity of cross linking agent (ml) | Drug payload (%) | Swellability | Muco adhesion (%) | Average particle diameter |
|---|---|---|---|---|---|
| (µm) | |||||
| DG1E | 1** | 74.76 ± 1.27 | 1.1762 ± 2.11 | 83.10 ± 4.02 | 12.80 ± 0.62 |
| DG2E | 2 | 70.51 ± 0.74 | 1.1172 ± 2.32 | 81.56 ± 4.08 | 13.56 ± 0.71 |
| DG3E | 3 | 67.13 ± 1.05 | 0.9715 ± 0.21 | 78.32 ± 3.98 | 14.38 ± 0.74 |
| DG4E | 4 | 62.87 ± 1.14 | 0.7544 ± 0.09 | 75.59 ± 3.74 | 15.34 ± 0.68 |
| DC1E | 1** | 71.28 ± 0.38 | 1.1437 ± 0.24 | 82.76 ± 4.34 | 13.06 ± 0.68 |
| DC2E | 2 | 68.53 ± 1.45 | 1.0587 ± 0.20 | 80.17 ± 4.10 | 13.65 ± 0.59 |
| DC3E | 3 | 64.90 ± 1.19 | 0.8915 ± 0.09 | 76.06 ± 3.92 | 14.52 ± 0.54 |
| DC4E | 4 | 60.43 ± 1.33 | 0.7106 ± 0.21 | 74.67 ± 3.84 | 15.42 ± 0.60 |
| DGE* | 80.41 ± 1.26 | 1.2106 ± 0.06 | 84.10 ± 4.20 | 12.19 ± 0.60 | |
| DCE* | 77.59 ± 3.87 | 1.1820 ± 0.06 | 83.04 ± 4.15 | 12.60 ± 0.61 | |
Table 1: Various microsphere formulations showing effect of cross linking agent on Physicochemical attributes
Drug loading was carried out by the method reported by Bjork and Edman [5]. Preformed microspheres (100 mg) were incubated with 100 IU of insulin. The gel was freeze dried overnight till after which an orange, pale red to brown powder was recovered. It was then, passed through a 63 μm sieve and stored in dry place at 4° to 8°. The insulin-loaded microspheres were subjected to scanning electron microscopy (SEM). During loading, 1.0% sodium taurocholate (permeation enhancer) and 0.1% aprotinin (protease inhibitor) were mixed in the drug solution so that they could also be loaded in the preformed microspheres.
Characterization of microspheres
Shape and surface morphology of microspheres was determined by scanning electron microscopy (Leo435 VP, Cambridge, UK). Size of microspheres was determined using laser diffraction particle size analyzer (1064 L, Cilas, Orleans, France). Effect of drug concentration, cross linking agent concentration and permeation enhancer on the average particle size was studied.
A definite quantity (100 mg) of insulin-loaded microspheres was weighed and digested overnight by suspending in 5 ml of 0.5 M acetic acid and 5 ml of 0.5 M sodium hydroxide at 10°. The pH was adjusted to 5.0 by further addition of acetic acid. The digested homogenate was diluted with PBS pH 6.4 and centrifuged at 3000 rpm for 15 min. The supernatant was taken and the drug content was determined spectrophotometrically at 270 nm. Effect of glutaraldehyde concentration and citric acid concentration on drug payload was observed.
Degree of swelling
The swell ability of microspheres in physiological media was determined by swelling them in the PBS pH 6.4. Accurately weighed amount of microspheres was immersed in little excess of PBS pH 6.4 for 24 h and washed. The degree of swelling was calculated using following formula, α=(Ws-Wo)/Wo, where α is the degree of swelling, Wo is the weight of microspheres before swelling and Ws is the weight of microspheres after swelling.
Mucoadhesion
Mucoadhesion of microspheres was assessed using the method reported by Mumtaz and Ching [6] with little modifications. Briefly, a strip of she goat intestinal mucosa was mounted on a glass slide and accurately weighed microspheres were placed on the mucosa of the intestine. This glass slide was incubated for 15 min in desiccators at 90% relative humidity to allow the polymer to interact with the membrane and finally placed in the cell that was attached to the outer assembly at an angle of 45°. Phosphate buffered saline (pH 6.4) previously warmed to 37±0.5° was circulated all over the microspheres and membrane at the rate of 1ml/min with the help of peristaltic pump. Washings were collected at different time intervals and microspheres were collected by centrifugation followed by drying at 50°. The weight of washed out microspheres was determined and percent mucoadhesion was calculated by the method given by Takeuchi et al. [7]. Percentage mucoadhesion= (Wa- W1)×100/Wa, where Wa is the weight of microspheres applied and W1 is the weight of microspheres leached out.
In vitro drug release studies
The drug release from different formulations was determined using treated cellophane membrane and Franz diffusion cell (Crown Glass Company, USA). The treated cellophane membrane was fixed between the donor and receptor compartment of the diffusion cell to support the microspheres. Microspheres (100 mg) were placed on the cellophane membrane in the donor compartment contained PBS (pH 6.4) maintained at 37±1°. The samples were withdrawn periodically for 24 h and replaced with fresh PBS (pH 6.4). The samples were analyzed spectrophotometrically for drug content at 270 nm.
Drug permeation through mucosal membrane
Drug permeation through mucosal membrane was assessed using she goat intestinal mucosa for ensuring in vivo drug absorption. The mucosal membrane was placed between the donor and receptor compartment of the Franz diffusion cell. Weighed quantity of microspheres suspended in PBS (pH 6.4) was kept in the donor compartment, while the receptor compartment was filled with PBS (pH 7.4) at 37±1°. Samples were withdrawn at regular time intervals for 24 h and replaced immediately with fresh PBS (pH 7.4). These samples were analyzed spectrophotometrically at 270 nm for drug content.
In vivo performance evaluation
The in vivo performance of mucoadhesive microspheres was evaluated on albino rabbits in which diabetes were induced. Twelve rabbits of either sex weighing between 1.5 to 2.0 kg were made diabetic by administering intravenous injection of streptozotocine solution in citrate buffer pH 5.0 (65 mg/kg) for 4 to 5 days. The animals that showed fasting blood glucose level more than 300 mg/dl confirmed induction of diabetes were taken for study. These rabbits were divided into 3 groups of 4 rabbits in each group and their blood glucose level after overnight fasting was determined. Animals of the first group received placebo microspheres whereas rabbits of second and third group were administered with drug containing glutaraldehyde cross linked and citric acid cross linked microspheres respectively. The microspheres were administered (equivalent to 10 IU/kg body weight) into each nostril of the rabbit with the help of nasal spray. During the experiment, the body temperature of the rabbits was maintained at 37o with the help of a heating pad. The blood samples were collected by an indwelling catheter from the marginal ear vein. The serum was separated from the blood samples by centrifugation at 3000 rpm for 10 min and then blood glucose level was estimated using glucose assay kit (Autopak, Bayer Diagnostics) on a blood Auto Analyzer (Technicorn RA-50, Italy). Percent reduction in blood glucose level was calculated using the formula, % Reduction in blood glucose level = [Blood glucose level (before drug administration-after drug administration)/ blood glucose level before drug administration]×100
Result and Discussion
Over the last few decades, the use of mucoadhesive polymers as a non-toxic alternative to enhancer systems has been investigated. Chitosan is such an excellent mucoadhesive and biodegradable polymer and also reported to have penetration enhancing ability. Microspheres were cross-linked with chemical cross linking agents to control the release of drug from the particulate system. Microspheres were cross-linked covalently and ionically with glutaraldehyde saturated toluene solution and 1% of aqueous solution of citric acid respectively to observe the effect of cross-linking mechanism on the various evaluation parameters of formulations. Glutaraldehyde cross-linking of chitosan is an instantaneous reaction. The aldehyde groups form covalent imine bonds with the amino group of chitosan, due to the resonance established with adjacent double ethylene bonds [8,9] via a Schiff reaction and the links with hydroxyl group of chitosan is also possible [10]. Glutaraldehyde saturated toluene solution was used instead of aqueous glutaraldehyde solution to obtain good spherical geometry and surface smoothness of chitosan microspheres. In case of citric acid cross-linking, ionic bridges are formed between the positively charged amine group of chitosan and negatively charged carboxylate ion of acid. Ionic interaction between the negative charges of cross-linking agent and positively charged groups of chitosan are main interaction inside the network [11]. Other additional interactions such as hydroxyl groups could also react with cross linker [12] and/or formation of interchain hydrogen bonds could also be possible [13]. The insulin was loaded in preformed microspheres to avoid the effect of process variable by in situ loading technique with little modifications [5].
SEM showed smooth and cross-linked surface of the particles. Nearly smooth surface of the placebo microspheres is observed under SEM while surface morphologies changed significantly after incorporation of drug in to chitosan microspheres. Several pores were seen on surface along with roughness and deficiencies in insulin loaded microspheres (figs. 1 and 2). Surface of citric acid cross-linked microspheres was found rougher than glutaraldehyde cross linked microspheres. This could be due to the loss of ionic interaction between citric acid and chitosan present on microsphere surface. Whereas, glutaraldehyde crosslinked microspheres have comparatively smoother surface with few pores this could be due to formation relatively stronger covalent bond between aldehyde and amine group of chitosan with the presence of small channels.
The average particle size of the microspheres prepared using different concentrations of glutaraldehyde was found to be in the range of 12.80±0.62 to 15.34±0.68 μm. Similarly, the average particle size of the citric acid cross-linked microspheres was found in the range of 13.06±0.68 to 15.42±0.60 µm (Table 1). Above data revealed that increasing the concentration of the cross linking agent caused a slight increase in the particle size of the formulation. This could be due to adherence of excess cross- linking agents on the surface of microspheres. The slight increased size of citric acid cross-linked microspheres than glutaraldehyde cross-linked microspheres could be due to adherence of excess citrate anion on the positively charged surface of chitosan.
Similarly, the effect of cross linking agent concentration on drug payload was studied and found that on increasing the concentration of the cross linking agent the drug loading efficiency of the microsphere was decreased (Table 1). The decreased drug payload by increasing the cross linking of the polymer could be due to increased rigidity of polymer surface, more dense structural arrangement of crosslinked polymer and lesser polymer swellability. The drug payload was observed slightly higher in case of glutaraldehyde cross-linked microspheres, which could be explained on the basis of formation of covalent network. The conformational change of chitosan with glutaraldehyde leads to the formation of a permanent network due to the stronger covalent bond between the two reactive groups of glutaraldehyde and chitosan polymer chains [11]. Therefore, glutaraldehyde cross-linking leads to development of organized free diffusion passages, which could facilitate the entry (diffusion) of drug. In case of citric acid cross linking the network formed is unorganized and further the passage of drug could be hindered by the development of secondary attraction between the charged polymer groups and anions of citric acid molecule.
Further the, swellability is an indicative parameter for rapid availability of drug solution for diffusion with greater flux. It was found that degree of swelling decreases with increasing the concentrations of cross-linking agents (Table 1). On increasing the glutaraldehyde concentration from 1ml to 4ml, the degree of swelling of microspheres was found to decrease from 1.1762±0.11 to 0.7544±0.09, whereas on increasing the citric acid concentration from 1 to 4%, the degree of swelling of various microspheres was decreased from 1.1437±0.24 to 0.7106±0.21, respectively. The higher concentration of cross linking agents increase the rigidity of the polymer surface and also decrease the hydrophilic groups available in the chitosan molecule that are responsible for swelling. Swelling ability of glutaraldehyde cross linked microspheres was found to be pronounced than citric acid cross linked microspheres, which could be due to the reasons explained in above paragraph.
Percent mucoadhesion was found to decrease from 83.10±4.02 to 75.59±3.74% with gradual increase in the concentration of glutaraldehyde from 1 to 4 ml. Similarly, on increasing the concentration of the citric acid from 1 to 4%, the percent mucoadhesion of the microsphere was decreased from 82.76±4.34 to 74.67±3.84% (Table 1). The cross linked microspheres are more rigid as compared to non cross linked microspheres and availability of the less number of sites for mucoadhesion, results in reduction in the mucoadhesive properties. In case of citric acid cross-linked microspheres the lesser value of percent mucoadhesion observed, which might be due to partial neutralization of free amino group of polymer surface by anionic cross linker.
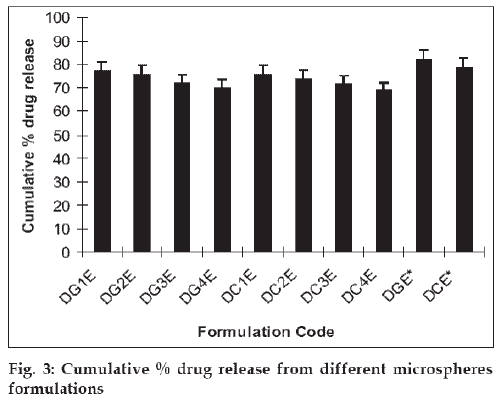
The mucoadhesive microspheres were assessed to establish the release kinetics of insulin. The in vitro drug release studies were performed in phosphate buffered saline (pH 6.4), as the pH of the nasal fluids is around 6 to 7. The results revealed that increase in the concentration of cross-linking agents cause the reduction in percentage drug release. Cumulative drug release after 24 h was decreased from 77.06±3.85% to 70.03±3.50% with increasing the glutaraldehyde concentration from 1.0 ml (DG1E) to 4.0 ml (DG4E). Similarly, on increasing the citric acid concentration from 1% (DC1C) to 4% (DC4C), cumulative drug release was deceased from 75.59±3.77% to 68.94±3.44%. In this case drug release was seen to decrease in a more sustained manner in comparison to glutaraldehyde cross linking (fig. 3). It could be due to the establishment of ionic equilibrium within the citric acid cross linked polymer. Another reason could be the degree of ionization of amine group, which decreases greatly when the solution pH increased above 6.0 (around the pKa of chitosan 6.3) [14] and at pH higher than 7.5 usually less than 10% of amine groups are ionized [15]. At pH 6.4, sufficient charge density at amine groups could be available to hold anionic cross linker as citric acid (a weak acid) attributes significant charge density at this pH. These ionic attractions along with secondary attractions i.e. hydrogen binding etc. provide firmness to polymer network, which results in more sustained drug release.
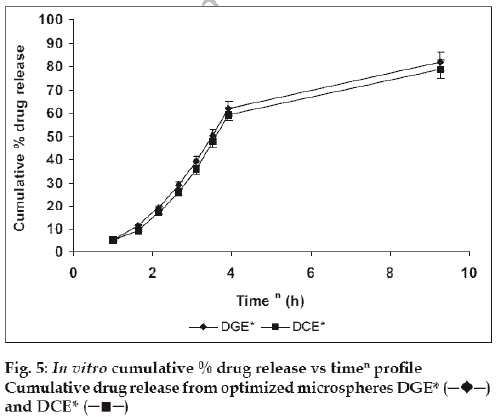
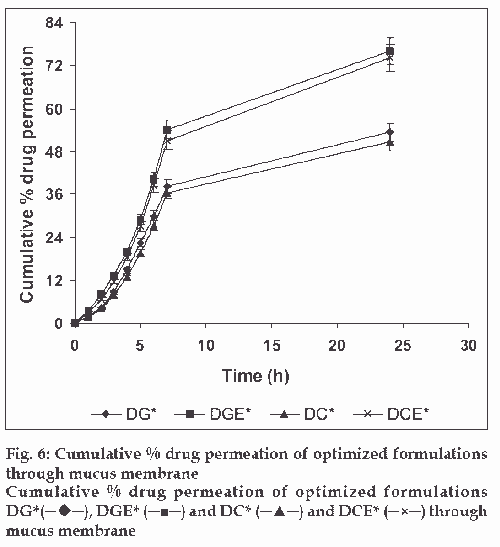
The in vitro drug release kinetics from the microspheres was established by determining the diffusion release exponent (η) from the plot of log cumulative drug release vs log time (fig. 4). The slope of the straight line (0.68-0.72) was recorded as the value of diffusion release exponent (η) [16]. It indicates that the release rate of drug from the microspheres follows non-Fickian diffusion pattern. Hence, a linear relationship was obtained between cumulative percent of drug release versus timeη (fig. 5). Further, for in vitro drug permeation study through mucosal membrane, she goat intestinal mucosa was used. For this study only optimized formulations (i.e. DG* and DC*), which exhibited maximum in vitro drug release were chosen. These optimized formulations were incubated with permeation enhancer (sodium taurocholate) and 0.1% aprotinin (protease inhibitor) during in situ drug loading in microspheres. It was observed that addition of permeation enhancer and protease inhibitor in the permissible limits cause significant increase in drug permeation. It was seen that the optimized formulations DG* and DC* (without permeation enhancer and protease inhibitor) exhibited 53.29±2.66% and 50.76±2.53% drug permeation, respectively in 24 h, whereas the similar formulations with permeation enhancer and protease inhibitor i.e. DGE* and DCE* showed increased permeation values of 75.96±3.72% and 74.19±3.36%, respectively (fig. 6). Despite much lower in vitro drug release through citric acid cross linked microspheres, the drug permeation study revealed no major difference between permeation of drug from two types of microspheres (DGE* and DCE*) this could be due to penetration enhancer property of citric acid [9].
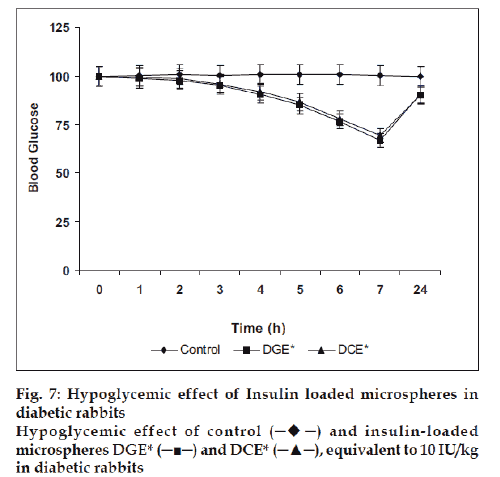
Finally, the microspheres bearing insulin were studied for their in vivo performance evaluation. The optimized microspheres (DGE* and DCE*) were assessed by periodic measurement of blood glucose level in streptozotocin induced diabetic rabbits after nasal administration of formulations (equivalent to 10 IU/Kg). It was observed that the glutaraldehyde cross linked microspheres (DGE*) showed better reduction of blood glucose level than citric acid cross linked microspheres (DCE*). After administration, the maximum blood glucose reduction (66.78±3.33%) was observed in 7 h with glutaraldehyde cross linked microspheres whereas with citric acid cross linked microspheres exhibited 69.37±3.46% of the initial blood glucose level after 7 h (fig. 7).
In conclusion, it can be concluded that with mucoadhesive microspheres containing protease inhibitors and permeability enhancer can improve the bioavailability of the protein/peptide drugs in a much better way. This system showed a better response in replacing the need for parental administration of protein/peptide drugs. However, exhaustive clinical trials must be studied before introducing this system in the market.
Acknowledgements
The authors are grateful to Head, Department of Pharmaceutical Sciences, Dr. H. S. Gour University, Sagar, India and AIIMS, New-Delhi, India.
References
- Ugwoke MI, Verbeke N, Kinget R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J Pharm Pharmacol 2001;53:3.
- Jain SK, Chourasia MK, Jain AK, Jain RK. Development and characterization of mucoadhesive microspheres bearing salbutamol for nasal delivery. Drug Del 2004;11:113.
- Sinha VR, Singla AK, Wadhawan S, Kaushic R, Kumria R, Bansal K, et al . Chitosan microspheres as a potential carrier for drugs. Int J Pharm 2004;274:1.
- Thanoo BC, Sunny MC, Jayakrishnan A. Crosslinked chitosan microspheres preparation and evaluation as a matrix for the controlled release of pharmaceutics. J Pharm Pharmcol 1992;44:283. Back to cited text no. 4
- Bjork E, Edman P. Degradable starch microspheres as a nasal delivery system for insulin. Int J Pharm 1988;47:233.
- Mumtaz AH, Ching H. Design of a dissolution apparatus suitable for in situ release study of triamcinolone acetonide from bioadhesive buccal tablets. Int J Pharm 1995;121:129.
- Takeuchi H, Yamamoto H, Niwa T, Hini T, Kawashima Y. Enteral Absorption of Insulin in Rats from Mucoadhesive Chitosan-Coated Liposomes. Pharm Res 1996;13:896.
- Monteiro OAC, Airoldi C. Some studies of crosslinking chitosan-glutaraldehyde interaction in a homogeneous system. Int J Biol Macromol 1999;26:119.
- Yao KD, Yin YJ, Xu MX, Wang YF. Investigation of pH-sensitive drug delivery system of chitosan/gelatin hybrid polymer network. Poly Int 1995;38:77.
- Dumitriu S, Vidal PF, Chornet E. Hydrogels based on polysaccharides. In : Dumitriu S, editor. Polysaccharides in Medicinal Applications. Marcel Dekker: Vol. 1, New York; 1999. p. 125.
- Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm 2004;57(1):19.
- Mi FL, Chen CT, Tseng YC, Kuan CY, Shyu SS. Iron(III)-carboxymethylchitin microsphere for the pH-sensitive release of 6-mercaptopurine. J Control Rel 1997;44:19.
- Barck HP, Tirmizi SA, Risen WM. A spectroscopic and viscometric study of the metal ion-induced gelation of the biopolymer chitosan. Polymer 1997;38::2351.
- Yalpam M, Hall LD. Some chemical and analytical aspects of polysaccharide modifications. III. Formation of branched-chain, soluble chitosan derivatives. Macromolecules 1984;17:272.
- Shu XZ, Zhu KJ, Song W. Novel pH-sensitive citrate cross-linked chitosan film for drug controlled release. Int J Pharm 2001;212:19.
- Langer MA, Peppas NA. Present and future applications of biomaterials in controlled drug delivery systems. Biomaterials 1981;2:201.


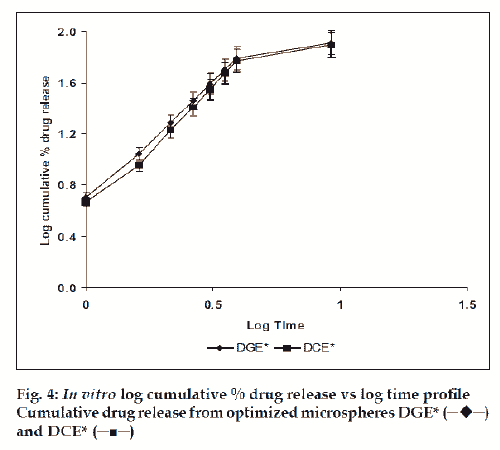
 and DCE* (─■─)
and DCE* (─■─)