- *Corresponding Author:
- S.S. Moselhy
Experimental Biochemistry Unit, King Fahd Medical Research Center (KAU), Egypt
E-mail: moselhy6@hotmail.com
| Date of Submission | 23 December 2016 |
| Date of Revision | 23 April 2017 |
| Date of Acceptance | 04 March 2018 |
| Indian J Pharm Sci 2018;80(3): 400-411 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Curcumin is chemically known as diferuloylmethane, which is an important nutraceutical obtained from the yellow spice Curcuma longa. It has been used traditionally as a folklore medicine in countries like India, China and Thailand for nearly 2000 years without any prior knowledge of the mechanism of action. Cell-based studies and clinical trials that have been reported so far have provided evidence that curcumin could be effectively used as antimicrobial, anticancer, antidiabetic, antiinflammatory, antimalarial and antioxidant. There are more than 9400 articles published on this “magical molecule” till date. Furthermore, nearly 100 clinical trials have been done using curcumin, which demonstrated safety, efficacy and prophylactic activity of curcumin. On the molecular level, curcumin has shown to modulate several crucial and significantly interlinked pathways in rendering an optimistic outcome against life-threatening diseases like cancer, diabetes, lupus nephritis, and other autoimmune and neurodegenerative diseases. In this communication, the chemical structure, biological properties and possible molecular targets of curcumin have been reviewed along with some recent developments in drug delivery systems of curcumin.
Keywords
Curcumin, Curcuma longa, turmeric, antimicrobial, anticancerous, antiinflammatory, antioxidative, curcumin-delivery systems
Natural products and their derivatives have been the centre of attraction for researchers globally for the past few decades due to their wide therapeutic and clinical applications. This concept may seem recent to us but our ancestors have been benefiting from them since the time immemorial without any prior knowledge of the mechanisms involved or the bioactive components of the derivatives. There has been a spurt in the production of synthetic drugs and pharmaceuticals addressing many health disorders and diseases but the side effects and inefficiency caused due to them has craved the researchers to explore the properties of natural products, which are much more effective with minimized side effects than their synthetic counterparts.
Curcumin (CUR) is extracted from the rhizomes of a perennial herb, Curcuma longa (Figure 1). CUR has gained immense interest in the scientific community due to its antibacterial [1], antifungal [2] antiviral [3], anticancer [4], antiinflammatory [5], antimalarial [6], antioxidant [7] and wound healing [8] properties. Asian countries like India and China have used turmeric as a traditional medicine for nearly 2000 y in the form of a paste or in oral form to treat various ailments of skin and illnesses successfully until the modern medicine found it prominent in the last two centuries. CUR was isolated in crystalline form in the year 1870 and the complete structure was elucidated in 1910.
Physiochemical Properties of Cur
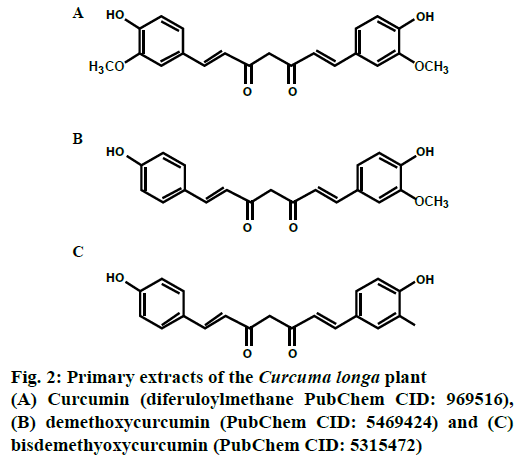
Primary extracts from C. longa yielded 3 curcuminoids namely curcumin (PubChem CID: 969516), demethoxycurcumin (PubChem CID: 5469424) and bisdemethoxycurcumin (PubChem CID: 5315472) as depicted in Figure 2, all of which are polyphenols, wherein the phenolic groups are inter-connected with unsaturated carbonyl groups.
CUR, chemically 1,7-Bis(4-hydroxy-3- methoxyphenyl)-1,6-heptadiene-3,5-dione is the most widely studied and active component, comprising of monocyclic sesquiterpenes and oxygenated derivatives with a melting point of 183° [9]. CUR doesn’t dissolve readily in water but dissolves in acetone, methanol and dimethyl sulfoxide. The absorption coefficient of CUR is 415-420 nm and 430 nm in acetone and methanol, respectively [10]. In addition to the above-mentioned curcuminoids, turmeric also contains some volatile oils like tumerone, zingiberone, atlantone, proteins, sugars and resins but except CUR, no other constituent of turmeric was found to be antiproliferative and antiinflammatory.
Cur, a Strong Antioxidant
The lipophilic nature of CUR made it an active antioxidant in radical scavenging of superoxide anions and DPPH. The antioxidant property of CUR is by virtue of its perfectly arranged structure, wherein the two α, β unsaturated carbonyl groups arranged in stable enol form, interconnects the two methoxylated phenols. The bioactivity of CUR is attributed to its phenolic O-H and the C-H groups. CUR is also involved in the inhibition of lipid peroxidation using a polyunsaturated fatty acid-linoleate, which is later oxidized to form a fatty acid radical. CUR is also involved in the neutralization of lipid radicals and participate in the intermolecular Diels-Alder reaction by breaking the chain at the 3’ position [11]. Apart from lipid peroxidation inhibition, free radical scavenging activity of CUR has been demonstrated in in vitro and in vivo models by using peritoneal macrophages of a rat model [12]. Macrophages produce various reactive oxygen species (ROS) like hydrogen peroxide, nitrite radicals and superoxide anions, which are actively scavenged by CUR.
The oxidative stress induced by the body due to the insults from the entry of pathogens or the other factors leads to the upregulation of inducible nitric oxide synthase (iNOS), which is commonly found in macrophages. iNOS produced large amounts of nitric oxide (NO), which further react with superoxide radicals in the surrounding oxidative environment to form peroxynitrite as a toxic product, which was found to be quite lethal to cells. CUR is reported to downregulate the activity of iNOS and reduces the levels of ROS in the cells. Additional studies pertaining to the microglial cells have proved that this antioxidant spice could protect the neural cells against oxidative damage by reducing the output of NO generation and also could reduce the neuroinflammation caused due to some chronic neurodegenerative disorders like Alzheimer’s disease [13].
Cur Exerts Considerable Antiinflammatory Effect
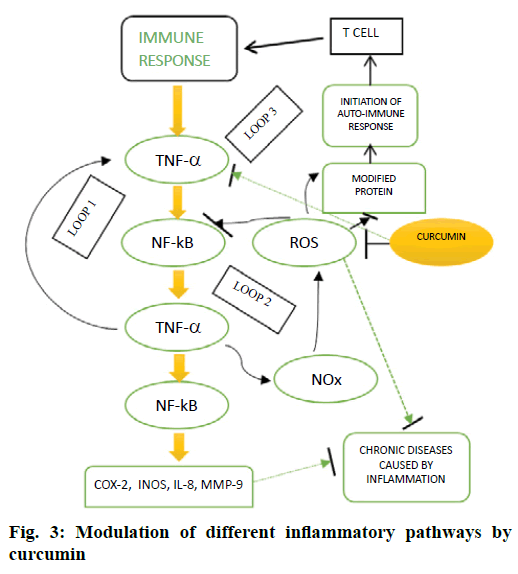
The role of CUR as a potent natural antiinflammatory agent has been investigated in many chronic diseases like arthritis, diabetes, inflammatory bowel disease, cardiovascular diseases and Alzheimer’s disease. CUR inhibited many inflammatory mediators as depicted in Figure 3. Further, it modified the expression of tumour necrosis factor-α (TNF-α) by inhibiting p300/CREBspecific acetyltransferase, which in turn caused the repression of acetylation of histone or non-histone proteins resulting in the inhibition of transcription [14]. Additionally, this lipophilic polyphenol also affected the methylation pattern of the TNF-α promoter [15] and hence regulating the expression of TNF-α. The lipopolysaccharide (LPS)-mediated induction of cyclooxygenase-2 (COX-2) expression is inhibited by the surface application of CUR, which reduced the formation of prostaglandin synthase E2. However, in macrophages, CUR raised the levels of COX-2, which was independent of LPS-mediated induction. It has also been demonstrated that CUR downregulated the secretion of inflammatory cytokines [16] and hence minimized the toxic effects in adipocytes and exerted a cytoprotective effect in a dose-dependent manner. CUR also eased the inflammatory responses associated with asthma by down-regulation of expression of interleukin-1B (IL-1B), IL-6 and TNF-α by activating NrF2/HO-1 [16].
This antiinflammatory phytochemical also inhibited the expression of IL-6 and IL-1B, through many signalling pathways like mitogen-activated protein kinase and nuclear factor kappa-B (NF-ĸB) pathways in TNF-α treated HaCaT cells. It has also been found that CUR downregulated the expression of IL-8, IL-1B, MMP-8, TNF-α and acute phase proteins and hence protecting rats from paracetamol-induced cytotoxicity [17]. Two novel synthetic analogues of curcumin-C66 and B06 have been reported recently, which prevented the activation of JNK/NF-ĸB signalling by downregulating the mRNA levels of COX-2, TNF-α, IL-6, IL-1 and iNOS, therefore reducing the production of NO and TNF-α in primary peritoneal macrophages stimulated by high glucose [18,19].
Bacteriocidal and Bacteriostatic Effects of Cur
Bacterial infections and diseases have given a tough time to the researchers worldwide and kept them on their toes to constantly develop new drugs to combat these dreadful diseases. However, after an extensive research of nearly 50 y, new antimicrobial drugs have been successfully isolated from different sources. Despite all the technological developments, the need to find new alternative sources still remains a necessity due to the rapid development of multi-drug resistant bacteria (MDR). Different extracts of CUR have demonstrated to exert a bacteriostatic or a bactericidal effect on many strains of bacterial species at different concentrations. The aqueous extract of C. longa was proven to be quite effective in the antibacterial study against Klebsiella pneumonia ATCC 10031, Staphylococcus epidermis ATCC 12228, S. aureus ATCC 25923 and Escherichia coli ATCC 25922 with an minimum bactericidal concentration value of 16 to 32 g/l and minimum inhibitory concentration (MIC) value of 4 to 16 g/l [20]. The highest antimicrobial activity was evidenced with ethanol and hexane extract of CUR against 24 pathogenic bacteria, isolated from shrimps and chicken [21].
In a different study, methanol extract of CUR was evaluated against Bacillus subtilis and S. aureus and it was concluded that the extract was quite effective even at minimal concentrations [22]. In a separate study, bactericidal effects were observed with hexane and methanol extracts of C. longa against 13 species of bacteria, which included B. subtilis, B. cereus, Vibrio cholera, V. harveyi, V. vulnificus, V. parahaemolyticus, V. alginolyticus, Aeromonas hydrophilia, Streptococcus agalactia, Staphylococcus aureus, S. epidermidis, S. intermedius and Edwardsiella tarda [23]. It has also been reported that CUR exhibited poor antimicrobial activity (5.9 %) in the elimination of Helicobacter pylori infection in patients as compared with OAM (omeprazole, amoxicillin and metronidazole) treatment (78.9 %) [24]. The inflammation of gastric tissue due to H. pylori infection of the stomach was reduced by CUR due to the apparent blockage of NF-ҡB pathway activation and as a consequence, the release of IL-8 was inhibited, accompanied by the prevention of degradation of IB [25].
Additionally, in vitro studies were carried out with three new compounds of CUR namely indium curcumin, diacetyl curcumin and indium diacetyl curcumin against E. coli, P. aeruginosa, S. aureus and S. epidermis. It was reported that the antibacterial activity of indium curcumin was far better than that of curcumin and diacetyl curcumin was found to be ineffective [26]. To overcome the incidences of the development of drug resistance by different microbial strains, synergistic effects of the existing drugs with the combination of plant derivatives are being explored currently.
For instance, the resistance of S. aureus to penicillin is on the rise accompanied by adverse side effects like anaphylactic reactions and hyper sensitivity [27]. Therefore, the synergistic studies of curcuminoids with ampicillin has resulted in a drastic reduction of MIC against S. aureus ATCC 25923 strain. Now with the development of nanotechnology, the use of strongly bound metal complexes and silver nanocomposite films against several strains of pathogenic species of bacteria has opened the gates of new avenues with high antibacterial effects in treating MDR infections.
Cur: A Potent Antimycotic Chemopreventive
With the rise in the mortality and morbidity due to the rise in the fungal infections, there is an urgent need to explore alternative and new sources of drugs, which can inhibit the growth of these deadly pathogens. The resistance of pathogenic strains of fungus and ineffectiveness of the available drugs remains a problem that needs to be addressed. Many phytochemicals extracted from different natural sources have been tested successfully in in vitro and in vivo studies during the recent years. In a study conducted on the inhibitory effect of ethyl acetate extract of C. longa, it was observed that at a concentration of 0.001 g/ml, it inhibited the growth of Puccinia recondite, P. infestans, Rhizoctonia solani and Botrytis cinerea. At the same concentration of 0.001 g/ml, hexane extract of C. longa was found to be effective against some species of Erysiphe graminis, Phytophthora infestans and R. solani [28]. However, the methanol extract showed appreciable antifungal activity at MIC values of 2.56 and 128 g/ml against Candida albicans and Cryptococcus neoformans, respectively [22].
Turmeric oil has been proved to be effective in combating many plant fungal pathogens like Helminthosporium oryzae and Fusarium solani. In an interesting study conducted, the most effective inhibition was demonstrated at an IC50 of 12.7 and 19.73 g/ml, respectively against H. oryzae and F. solani [29]. Trichophyton rubrum infected guinea pigs were chosen as the animal models for in vivo study to examine the healing effect of the topically applied turmeric oil. At a dilution rate of 1:80 it effectively cured the wounds within a week. The mechanism by which this antifungal effect is exerted is quite simple and involved the production of ROS leading to cell death due to rapid accumulation of biosynthetic precursors of ergosterol, via down-regulation of its production due to decreased turnover of Δ5,6-desaturase (ERG3) enzyme [30]. Apart from this, some other possible factors like changes in the membrane bound ATPase activity and reduction in the secretion of proteinase could be anticipated. Many species of Candida have become resistant to the excessive use of the existing drugs. Hence, finding new drugs with inhibitory effects against these fungal species have become the biggest challenge for the researchers.
In a clinical study, CUR has demonstrated to be an effective fungicide against 14 strains of candida including 4 ATCC strains with MIC values ranging from 250 to 2000 g/ml [31]. In an intriguing study, the antifungal activity with CUR was effectual against some fluconazole resistant strains [32]. The basis of the mechanism of action of CUR on candida is speculated to be intracellular acidification via inhibition of H+ extrusion leading to cell death [32]. To enhance the efficacy of the existing fungicidal drugs, many combinations of CUR have been tried and tested successfully with an appreciable reduction in MIC values. The synergistic studies of CUR along with, itraconazole, voriconazole, miconazole, ketoconazole, fluconazole, nystatin and amphotericin B have shown a 10-35 fold reduction in the MIC values of the drugs against 21 clinical isolates of C. albicans [33]. Indeed, the systemic fungal infections like candidemia and candidosis can be effectively treated with the different synergistic combinations of CUR with these fungicides saving the time and resources.
Cur Can Act as an Antitumour Agent
During recent years, many researchers have reported affirmative results of CUR as an antitumour agent in in vitro and in vivo experiments against different tumours [3,34]. The basis of the chemopreventive and anticarcinogenic effect of the CUR is attributed to its selective targeting of growth factors, transcription factors, apoptotic genes, adhesion molecules, angiogenesis regulators and cellular signalling molecules. These different pathways modified by curcumin in different tumours are described in Table 1 [35-45].
| Molecular targets | Cell line/cancer | Reference |
|---|---|---|
| Apoptosis via PI3K/Akt pathway | SKOV3 | [35] |
| Inhibition of NFkB pathway | Colorectal cancer | [36] |
| Modulation of cell cycle | Ehrlich's ascites carcinoma (EAC) | [37] |
| Inhibition of EGF-mediated tyrosine kinase activity | A431 | [38] |
| Increase in caspase-3 and caspase-9 activities | MCF-7 | [39] |
| STAT-3 mediated down-regulation of Bcl-XL and survivin | NCI-H446 and NCI-1688 | [40] |
| Induction of autophagy | SCC | [41] |
| G0/G1 Cell cycle arrest |
SK-MEL-37, MCF-7, Rh1, Rh30, PC-3, DU145 and HeLa | [3,42,43] |
| Inhibition of VEGF, c-jun-p, and MMP-2/-9 |
A549 cells | [44] |
| Inhibition of microtubule polymerization | B16-F10 skin cells | [45] |
Table 1: Molecular Targets and the Pathways Modified by Curcumin in Various Tumors
CUR is reported to exert its antitumour effect by initiating either intrinsic (mitochondrial) or extrinsic (receptor-mediated) apoptotic pathways. The tumour suppressor p53 played a pivotal role in initiating a caspase cascade through the immediate activation of pro-apoptotic proteins of B-cell lymphoma 2 (Bcl-2) family like Bcl-2 associated X protein (Bax) and Bcl-2 homologous antagonist killer (Bak). These Bax and Bak perforate the mitochondrial membrane to release cytochrome c into the cytoplasm promoting mitochondria-mediated apoptosis. CUR also exhibited the ability to inhibit antiapoptotic proteins, Bcl-2 and B-cell lymphoma extra-large (Bcl-xL). Molecular docking studies reported by Luthra et al. confirmed the inhibition of Bcl-2 by direct binding of CUR to the second cavity of the protein, through the intermolecular interactions of amino acids [46].
Guo et al. demonstrated that CUR inhibited the growth of colorectal carcinoma LoVo cells by initiating a caspase cascade at a significant concentration of 0-30 μM. Moreover, the nuclear staining by Annexin V/PI was found to be positive, confirming the activation of apoptotic machinery [47]. Recently, several reports have confirmed the antiproliferative action of CUR in cancers of breast, pancreas and lung through the down-regulation of epidermal growth factor receptor (EGFR), which is usually highly upregulated in tumour circumstances [48].
The cell cycle is tightly regulated by a group of cell cycle regulatory proteins called cyclins and cyclin-dependent kinases (CDK), which regulate the proliferation of cells. But in tumours, these regulatory proteins are upregulated, leading to the rapid proliferation of cells. However, CUR exerted its inhibitory effect on these regulatory proteins and inhibited the proliferation eventually [49].
Accumulated evidence showed that CUR not only checked the proliferation of cancerous cells or inhibited ROS, but also promoted apoptosis via induction of expression of different secondary messengers like NO synthase, COX-2, matrix metalloproteinase-2 and matrix metalloproteinase-9 in tumour cells [50]. Sethi and colleagues have reported that abnormal inflammatory signalling pathways also played a pivotal role in the advancement and growth of several cancers [51]. NF-κB is one such important inducible transcription factor, which can significantly regulate the inflammatory cytokines like IL-1β, IL-6, IL-8 and TNF-α [52]. This NF-κB signalling pathway is activated in most of the cancers and recently CUR has shown to inhibit this pathway gathering further interest of researchers towards the exploitation of this pathway in treating tumours [53].
Additionally, several studies have reported that CUR acted as a chemosensitizer for tumour cells without affecting the normal cells at the molecular level. Global Clinical studies were performed on CUR to prove its efficacy in the treatment of patients with pre-invasive malignant or high-risk pre-malignant conditions. There are many studies, which showed that CUR has been effective against the cancers of breast, colon, stomach, pancreas, skin and oral cavity.
Pharmacodynamic studies were conducted by Garcea et al. [54] with 12 patients suffering from colorectal cancer of different stages. It was concluded that after administration of varying doses of CUR from 450-3600 mg per day for seven days, there was a decrease in M1G levels from 4.8±2.9 adducts per 107 nucleotides in malignant colorectal tissue to 2.0±1.8 adducts per 107 nucleotides, without any change in the levels of COX-2 protein levels. Hence, they suggested a safe dosage of 3.6 g per day without any adverse effects [54]. Braumann et al. presented a case report stating that good partial remission was observed in a 75-y-old patient with colon cancer metastatic to the liver, after administration of leucovorin, 5-fluorouracil and oxaliplatin in combination with 5 g of CUR daily, for five months [55]. Hence, it can be conjectured from the on-going discussion that CUR can serve as an effective anticancer chemopreventive agent against different tumours.
Can these Bioavailability issues of cur be resolved?
In a joint report released by the World Health Organization and Food and Agriculture Organization on food additives, it was recommended that the maximum daily intake of CUR without any possible adverse effects to be 0-1 mg/kg. An interesting chronic toxicity study has reported that the maximum daily dose of CUR can be up to 8 g when administered orally [56]. In the in vivo studies conducted on rats, it was described that after administration of an oral dose of 1 g/kg, nearly 75 % of CUR was excreted in faeces and a considerable amount was seen in urine [57].
While human pharmacokinetic studies have reported that the plasma levels of free CUR remained at 25 nmol when CUR complex was administered orally at a dose of 3.6-12 g daily for a week or longer [58]. Whatsoever be the activity and the effect of a compound in in vivo system, the bioavailability always played a crucial role in determining the efficacy of the drug. While examining the properties of CUR, it was contemplated that the ineffectiveness of the CUR is exclusively due to low water solubility and poor intestinal permeability. Curcuminoids are insoluble in water and are excessively sensitive to pH, light, oxygen levels and also to the solvent system employed to dissolve them.
Aggarwal and colleagues reported in their research that the maximum solubility of CUR was as low as 11 ng/ml in an aqueous buffer with a pH of 5.0 [59]. While in a different study reported by Bernabé- Pineda et al., it was concluded that, at high pH values (>11.7), there was an improvement in the stability of CUR [60]. At a pH of 7.4, there was a rapid degradation of CUR. There was a steep decrease in the absorbance at 426 nm, from 50 % in 5 min to just 10 % after 10 min. New absorption coefficients were obtained at 210 and 262 nm. Moreover, it was demonstrated that the final resulting solution was colourless, suggesting that the yellow conjugated system did not exist anymore in the degradation products. In addition, poor intestinal permeability has been a major obstacle in retaining the drug in the system for an extended period. In an interesting study, Ravindran and Chandrasekhara assessed that the poor permeability of CUR and the apparent permeability coefficients (Papp) using a Caco-2 cell line [61]. For the basolateral-apical study, Papp was found to be 2.55±0.02×10-6 cm/s and for apical-basolateral study, it was calculated to be 2.93±0.94×10-6 cm/s. When further studies were done on lysed cells, it was revealed that more than 20 % of CUR was accumulated in the cells and nearly 11.78 % was metabolized during transport. Thus, it was concluded that the intracellular accumulation and first-pass metabolism in the intestine played a major role in decreasing the efficacy of CUR.
D’Souza and Devarajan suggested that the bioavailability largely depended on the stability of the drug in the gastrointestinal tract, which increased the absorption manifold. Hence, galectin-mediated absorption using galactose polysaccharides like arabinogalactan and kappa-carrageenan have been quite promising to enhance the bioavailability to nearly 25 % [62].
Cur Delivery Systems
The retention of the drug for an extended period of time in the biological system and bypassing the first-pass metabolism is crucial in making any drug effective. To achieve this target, researchers have developed novel delivery systems, which aim at enhancing the bioavailability of CUR. With the increase in the pace of development of technology and incorporation of modern techniques in research, the use of solid CURloaded nanoparticles, hydrogels, microemulsions, transdermal delivery systems and implantable devices have gained momentum. Though there are many delivery systems in use, but in the following section, we have explored selected delivery systems of CUR, which are widely used currently.
Liposomes
Liposomes are bilayered spherical vesicles possessing an aqueous interior, formed by the self-association of ampiphilic phospholipids and cholesterol molecules. The lipophilic part of CUR can be successfully trapped in the lipophilic core of the liposomes, hence, enhancing the solubility significantly. Kusano et al. demonstrated that the oral administration of liposome-encapsulated CUR showed increased bioavailability, compared to nascent CUR [63]. In an another study, it was reported that silica-coated liposomes loaded with CUR and N-trimethyl chitosan chloride-coated CUR liposomes exhibited increased bioavailability compared to uncoated liposomes or suspension of CUR [64].
In an another distinguished study, Li and co-workers reported that a liposomal formulation of CUR using dimyristoyl-sn-glycero-3-phosphocholine (DGPC) was tested on human pancreatic cancer cells for the modification of pathways like apoptosis, angiogenesis, and proliferation. When this formulation was administered at 40 mg/kg for 3 times a week, it arrested the proliferation of MiaPaCa2 and BXPC3 tumours in a xenograft murine model demonstrating the efficacy of liposomes as an effective delivery system [65]. However, this delivery system has some disadvantages as well, like difficulty in sterilization, low drug loading, stability and poor batch to batch reproducibility [66].
Microemulsions/self-emulsifying emulsions
These are dynamic microstructures formed spontaneously by the combination of hydrophilic and lipophilic excipients in the presence of appropriate surfactants. These formulations are thermodynamically stable, transparent and optically isotropic and are regarded as ideal delivery systems with a faster rate of drug diffusion and high solubilisation capacity. Due to the lipophilic nature of CUR, it can not only pass through the cell membrane but also pass through the skin as reported by Teichmann et al., that the oil in water microemulsions of CUR can penetrate up to the follicular infundibula via stratum corneum [67].
Self-micro emulsions are more effective than emulsions when administrated orally as they can form microemulsions with particle sizes of less than 100 nm under suitable gastrointestinal conditions. Selfmicro emulsions are obtained by blending surfactants, co-surfactants, oil and the drug molecule to yield an isotropic mixture without a water phase, which renders it more stable.
When these microemulsions or self-micro emulsions are loaded with CUR, they deliver it in an absorbable form, enhancing the absorption and penetration to reach epithelial cells. Moreover, it has been demonstrated that the microemulsion droplets can be taken up by lymphatic tissues from the perfusion of the self-microemulsion. Bergonzi et al. formulated an o/w emulsion to enhance the stability and solubility by using food grade components [68]. They investigated the absorption potential of the oral CUR emulsion using parallel artificial membrane permeability assay in in vitro studies. This technique instantly measures the ability of the compound to passively diffuse through an artificial membrane. It was demonstrated that the permeation amount of the optimized microemulsion through the artificial membrane was 17.44 μg after 6 h and about 120.12 μg after 24 h with a solubility of 14.57 mg/ml. Whereas, the free curcuminoids dissolved in free PBS diffused at much lower speeds. But if buffer solution was used, the permeation amount was just 0.17 μg at 6 h and 1.66 μg after 24 h. It has been reported in various studies that the new self-microemulsifying systems in pellet and liquid forms are more stable and hence are being exploited by the researchers worldwide yielding promising results.
Setthacheewakul et al. studied the development and formulation of self-microemulsifying drug delivery systems (SMEDDS) in both pellet and liquid forms, which resulted in an increased solubility and stability in both in vitro and in vivo models [69]. An optimized formulation of SMEDDS was prepared which comprised of 70 % mixtures of two surfactants, Labrasol and Cremophor EL (1:1) and 30 % mixtures of oil Capryol 90 and Labrafac PG (1:1) in both pellet and liquid forms with a particle size of 29.6-32.8 and 25.8-28.8 nm, respectively. These formulations were quite stable up to 6 mo under optimum and accelerated conditions. In vivo studies done on rats show that the pelleted forms and the liquid forms showed enhanced absorption, which was nearly 10- and 14-fold compared to the aqueous solutions of curcuminoids, respectively. Hence, self-microemulsifying systems hold immense potential in enhancing the bioavailability of CUR.
Polymeric micelles
In the recent past, the use of mixed micelles has gained attention, which is formed by the mixing of two different surface active agents to yield better results than a single surfactant. Duan et al. performed a study using CUR-loaded phospholipid-sodium deoxycholate mixed micelles prepared by thin-film dispersion method followed by the optimization of central composite design-response surface method [70]. It was concluded that the resultant micelles formed had a negatively charged colloidal surface, were smaller in size and spherical in shape. Moreover, the low critical micellar concentration of PC and SDC facilitated the integrity of the micelles when administered parenterally. Further, the circulation of theses micelles in blood was prolonged and the cytotoxicity on MCF-7 was higher when compared to free CUR. Due to the smaller particle size of the micelles, which varies from 20-100 nm in aqueous solution, it can be successfully absorbed in the intact form through endocytosis to the intestinal cells.
Letchford et al. prepared a polymeric micellar formulation of CUR-containing methoxy poly(ethyleneglycol)-block-polycaprolactone diblock copolymers (MePEG-b-PCL), which enhanced its solubility to nearly 13×105 fold [71]. This was later verified by the pharmacokinetic studies conducted by Ma et al. that compared to free CUR the biological half-life of polymeric micelles was 60-fold higher in rats [72]. All these findings suggest that the use of micelles as a successful drug delivery system for CUR is quite economic and can be exploited.
Transdermal drug delivery system (TDDS)
TDDS refers to the superficial or topical application of the drug on the stratum corneum layer of the intact skin for localized or systemic treatment. This delivery system has an advantage over the oral and parenteral routes of drug administration due to enhanced penetration, prolonged plasma circulation levels, better retention and bypassing the first-pass metabolism to address the problems of poor bioavailability. There are several well-documented studies, which have reported the use of hydroxy propyl methyl cellulose K4M (HPMC K4M) and ethyl cellulose (EC) for controlled release of drugs [73,74]. In one such intriguing research, significant results were observed in antiinflammatory studies performed with different transdermal films prepared by incorporating CUR in the matrix of K4M (HPMC K4M) and EC with oleic acid as a permeation enhancer [75].
The use of microneedle injections is sophisticated yet expensive; microneedle approach and other nanocarriers are already currently in use. However, due to limitations and disadvantages, there remains an indispensable demand to fill up the void by the development of novel strategies. Patra et al. reported the preparation of a new generation polyarginine containing nano-liposomes incorporated with carbon dots, which had better penetration ability than the conventional liposomes, owing to its small size and enhanced stability. This TDDS was effective in killing cancer cells when loaded with CUR and had better permeation capacity which was confirmed by confocal fluorescence microscopic analysis [76]. Further, many of the researchers have used the distinct form of carriers like ethosomes for TDDS. Ethosomes are spherical, malleable, soft vesicles comprising generally of phospholipids (phosphatidylserine, phosphatidylcholine and phosphatidic acid) accompanied by a high concentration of ethanol and water. These can be designed for the incorporation of active agents according to the requisite of the study involved.
In antiinflammatory studies reported by Madhavi et al. it was demonstrated that ethosomes incorporated with curcumin-β-cyclodextrin complex showed enhanced penetration (78.01±0.22 %) compared to the aqueous (5.61±0.263 %), ethanol (62.31± 0.263 %) and liposomal (59.3±0.44 %) preparations, which could be attributed to the increased solubility of CUR in β-cyclodextrin complex [77]. This approach of TDDS could be a hopeful strategy in the near future due to its increased bioavailability and efficacy in treating topical and systemic diseases.
Nanoparticle approach
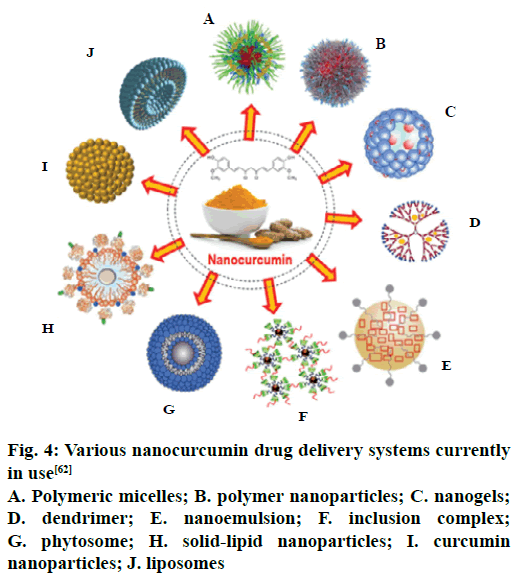
With the advancement in the frontiers of nanotechnology, several researchers have developed interest towards it owing to the prompt and effective results. The different nanodelivery systems are depicted in Figure 4. Mohanty and Sahoo designed a delivery system of nanoparticles by loading CUR in glycerol monooleate/Pluronic F-127 particles using an emulsification technique by the successive addition of 0.5 % w/v polyvinyl alcohol. The resultant particles were spherically shaped with an average diameter of 192+7 nm with an encapsulation efficiency of 90+3 %, as determined by HPLC. The initial drug release experiments performed in in vitro model showed that nearly 46 % of the drug was released in 24 h and moreover, the remaining was released slowly up to a period of 10 d. The researchers found that the drug uptake was exclusively concentration-dependant; demonstrating better uptake and antiproliferative activity at low concentrations of CUR-loaded nanoparticles, than the unmodified CUR [78].
Figure 4: Various nanocurcumin drug delivery systems currently in use [62]
A. Polymeric micelles; B. polymer nanoparticles; C. nanogels; D. dendrimer; E. nanoemulsion; F. inclusion complex; G. phytosome; H. solid-lipid nanoparticles; I. curcumin nanoparticles; J. liposomes
In a unique study Sindhu et al. synthesized spherical gold nanoparticles using only CUR as a reducing agent. The average size of the particles was nearly 58 nm with a zeta potential of –23 mv. The particles were quite stable for nearly 6 mo at room temperature and no toxicity was reported in the in vitro studies so far [79].
Many authors have demonstrated that the CUR-based nanoparticles are more effective in the chemotherapy for the treatment than the unmodified CUR due to its effective delivery to the target sites. Recently, animal studies were carried out using CUR-loaded magnetic nanoparticles on mice induced with pancreatic cancer. It was demonstrated that the formulation inhibited cancer effectively and moreover there was a 2.5 fold increase in the bioavailability as compared to native CUR. Hence, it can be speculated that the nanoparticle approach holds the solution to the treatment of cancer [80].
Many studies have reported that the stability and solubility of CUR-loaded dextrin nano gels were much higher than the unmodified CUR. Cell culture studies were done successfully on Hela cell line to prove the efficacy of these formulations, which could be used in cancer therapy in near future [81]. It has also been demonstrated in in vitro studies performed by using polymer-based CUR nanoparticles, that these forms of drug delivery systems are even effective to treat multiple brain tumours. A similar study by Lim et al. reported that these polymer-based nanoparticles could inhibit brain tumours arising from embryonal tumourderived lines DAOY, D283Med and the glioblastoma neurosphere lines HSR-GBM1 and JHH-GBM14 [82]. However, the choice of delivery system depends on many factors like the site of action, economic perspective, stability and safety with regard to the patient compliance.
In summary, curcumin has been used in traditional and complementary medicine for centuries and could be a promising drug candidate in the near future provided that it is delivered using an effective drug delivery system. The question that whether curcumin can be used as a drug alone or in a suitable formulation with an additional drug, which could enhance its potential on the frontiers of chemotherapeutic strategies is yet to be addressed. However, further studies and data are needed to understand the more precise and detailed mechanisms to enhance the antimicrobial, anticancer, antiangiogenic, antiinflammatory properties. Further, poor pharmacokinetic properties, poor solubility and low bioavailability still remain an area of research, which could be improved by employing emerging techniques bearing the economical aspect on the front. The new formulations in the form of transdermal implants, nanoparticles, nanoliposomes and nano gels are on the rise and efforts are being made to make them available within the reach of a commoner. The dictum “there is always room for improvement” is precisely in agreement with the pace of the ongoing developments to make curcumin as an effective drug candidate.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant no. (87-130-35-HiCi). The authors, therefore, appreciate the efforts of DSR for technical and financial support.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Bhat SV, Amin T, Nazir S. Biological activities of turmeric (Curcuma longa Linn.)-an overview. BMR Microbiol 2015;1:1-5.
- Chen J, He ZM, Wang FL, Zhang ZS, Liu XZ, Zhai DD, et al. Curcumin and its promise as an anticancer drug: An analysis of its anticancer and antifungal effects in cancer and associated complications from invasive fungal infections. Eur J Pharmacol 2016:33-42.
- Prasad S, Tyagi AK, Granich RM, Gilks CF, Dye C, Cock KM De, et al. Curcumin and its analogues: a potential natural compound against HIV infection and AIDS. Food Funct 2015;6:3412-9.
- Pröhl M, Schubert US, Weigand W, Gottschaldt M. Metal complexes of curcumin and curcumin derivatives for molecular imaging and anticancer therapy. Coord Chem Rev 2016:32-41.
- Pulido-Moran M, Moreno-Fernandez J, Ramirez-Tortosa C, Ramirez-Tortosa MC. Curcumin and health. Molecules 2016;21:264.
- Padmanaban G, Rangarajan PN. Curcumin as an adjunct drug for infectious diseases. Trends Pharmacol Sci 2016;37:1-3.
- Liu GM, Xu K, Li J, Luo YG. Curcumin upregulates S100 expression and improves regeneration of the sciatic nerve following its complete amputation in mice. Neural Regen Res 2016;11:1304-11.
- Rahimi HR, Nedaeinia R, Shamloo AS, Nikdoust S, Oskuee RK. Novel delivery system for natural products: Nano-curcumin formulations. Avicenna J Phytomed 2016;6:383-98.
- Salem M, Rohani S, Gillies ER. Curcumin, a promising anticancer therapeutic: a review of its chemical properties, bioactivity and approaches to cancer cell delivery. RSC Adv 2014;4:10815.
- Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv 2014;32:1053-64.
- Masuda T, Maekawa T, Hidaka K, Bando H, Takeda Y, Yamaguchi H. Chemical studies on antioxidant mechanism of curcumin: analysis of oxidative coupling products from curcumin and linoleate. J Agric Food Chem 2001;49:2539-47.
- Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr 2004;44:97-111.
- He L, Chen HJ, Qian L, Chen G, Buzby JS. Curcumin protects pre-oligodendrocytes from activated microglia in vitro and in vivo. Brain Res 2010;1339:60-9.
- Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U, et al. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem 2004;279:51163-71.
- Reuter S, Gupta SC, Park B, Goel A, Aggarwal BB. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr 2011;6:93-108.
- Priyanka A, Anusree SS, Nisha VM, Raghu KG. Curcumin improves hypoxia induced dysfunctions in 3T3-L1 adipocytes by protecting mitochondria and down regulating inflammation. Biofactors 2014;40:513-23.
- Soliman MM, Abdo Nassan M, Ismail TA. Immunohistochemical and molecular study on the protective effect of curcumin against hepatic toxicity induced by paracetamol in Wistar rats. BMC Complement Altern Med 2014;14:457.
- Pan Y, Wang Y, Cai L, Cai Y, Hu J, Yu C, et al. Inhibition of high glucose-induced inflammatory response and macrophage infiltration by a novel curcumin derivative prevents renal injury in diabetic rats. Br J Pharmacol 2012;166:1169-82.
- Pan Y, Zhu G, Wang Y, Cai L, Cai Y, Hu J, et al. Attenuation of high-glucose-induced inflammatory response by a novel curcumin derivative B06 contributes to its protection from diabetic pathogenic changes in rat kidney and heart. J Nutr Biochem 2013;24:146-55.
- Niamsa N, Sittiwet C. Antimicrobial Activity of Curcuma longa Aqueous Extract. J Pharmacol Toxicol 2009;4:173-7.
- Zorofchian MS, Abdul Kadir H, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int 2014;2014:186864.
- Ungphaiboon S, Supavita T, Singchangchai P, Sungkarak S, Rattanasuwan P, Itharat A. Study on antioxidant and antimicrobial activities of turmeric clear liquid soap for wound treatment of HIV patients. Songklanakarin J Sci Technol 2005;27:569-78.
- Lawhavinit OA, Kongkathip N, Kongkathip B. Antimicrobial Activity of Curcuminoids from Curcuma longa L. on Pathogenic Bacteria of Shrimp and Chicken. Kasetsart J Nat Sci 2010;44:364-71.
- Koosirirat C, Linpisarn S, Changsom D, Chawansuntati K, Wipasa J. Investigation of the antiinflammatory effect of Curcuma longa in Helicobacter pylori-infected patients. Int Immunopharmacol 2010;10:815-8.
- Foryst-Ludwig A, Neumann M, Schneider-Brachert W, Naumann M. Curcumin blocks NF-ҡB and the motogenic response in Helicobacter pylori-infected epithelial cells. Biochem Biophys Res Commun 2004;316:1065-72.
- Tajbakhsh S, Mohammadi K, Deilami I, Zandi K, Fouladvand M, Ramedani E, et al. Antibacterial activity of indium curcumin and indium diacetylcurcumin. African J Biotechnol 2008;7:3832-5.
- Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 2003;52:1.
- Kim M key, Choi G ja, Lee HS. Fungicidal property of Curcuma longa L. rhizome-derived curcumin against phytopathogenic fungi in a greenhouse. J Agric Food Chem 2003;51:1578-81.
- Chowdhury H, Banerjee T, Walia S. In vitro screening of Curcuma longa L. and its derivatives as antifungal agents against Helminthosporium oryzae and Fusarium solani. Pestic Res J 2008;20:6-9.
- Sharma M, Manoharlal R, Puri N, Prasad R. Antifungal curcumin induces reactive oxygen species and triggers an early apoptosis but prevents hyphae development by targeting the global repressor TUP1 in Candida albicans. Biosci Rep 2010;30:391-404.
- Neelofar K, Shreaz S, Rimple B, Muralidhar S, Nikhat M, Khan LA. Curcumin as a promising anticandidal of clinical interest. Can J Microbiol 2011;57:204-10.
- Khan N, Shreaz S, Bhatia R, Ahmad SI, Muralidhar S, Manzoor N, et al. Anticandidal activity of curcumin and methyl cinnamaldehyde. Fitoterapia 2012;83:434-40.
- Sharma M, Manoharlal R, Negi AS, Prasad R. Synergistic anticandidal activity of pure polyphenol curcumin in combination with azoles and polyenes generates reactive oxygen species leading to apoptosis. FEMS Yeast Res 2010;10:570-8.
- Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol Adv 2014;32(6):1053-64.
- Yu Z, Wan Y, Liu Y, Yang J, Li L, Zhang W. Curcumin induced apoptosis via PI3K/Akt-signalling pathways in SKOV3 cells. Pharm Biol 2016;54:2026-32.
- Uzzan B, Benamouzig R. Is Curcumin a Chemopreventive Agent for Colorectal Cancer? Curr Colorectal Cancer Rep 2016;12:35-41.
- Ibrahim ES. The Curcumin as an Antioxidant Natural Herb, with Emphasize on its Effects against Some Diseases. Int J Appl Biol Pharm 2016;1:26-41.
- Starok M, Preira P, Vayssade M, Haupt K, Salomé L, Rossi C. EGFR inhibition by curcumin in cancer cells: A dual mode of action. Biomacromolecules 2015;16(5):1634-42.
- Li HQ, Jin LJ, Wu FF, Li XY, You JS, Cao ZH, et al. Effect of curcumin on proliferation, cell cycle, and caspases and MCF-7 cells. Afr J Pharm Pharmacol 2012;6(12):864-70.
- Yang CL, Liu YY, Ma YG, Xue YX, Liu DG, Ren Y, et al. Curcumin Blocks Small Cell Lung Cancer Cells Migration, Invasion, Angiogenesis, Cell Cycle and Neoplasia through Janus Kinase-STAT3 Signalling Pathway. PLoS One 2012;7(5):e37960.
- Kim JY, Cho TJ, Woo BH, Choi KU, Lee CH, Ryu MH, et al. Curcumin-induced autophagy contributes to the decreased survival of oral cancer cells. Arch Oral Biol 2012;57(8):1018-25.
- Carneiro LB, Porfírio EP, Otake AH, Chammas R, Báo SN, Guillo LA. Morphological alterations and G0/G1 cell cycle arrest induced by curcumin in human SK-MEL-37 melanoma cells. Braz Arch Biol Technol 2010;53:343-52.
- Kumar G, Mittal S, Sak K, Tuli HS. Molecular mechanisms underlying chemopreventive potential of curcumin: Current challenges and future perspectives. Life Sci 2016;148:313-28.
- Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A. Suppression of NF-kappa B activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem Pharmacol 2007;73(9):1434-45.
- Ruan BF, Lu X, Li TT, Tang JF, Wei Y, Wang XL, et al. Synthesis, biological evaluation and molecular docking studies of resveratrol derivatives possessing curcumin moiety as potent antitubulin agents. Bioorg Med Chem 2012;20(2):1113-21.
- Luthra PM, Kumar R, Prakash A. Demethoxycurcumin induces Bcl-2 mediated G2/M arrest and apoptosis in human glioma U87 cells. Biochem Biophys Res Commun 2009;384:420-25.
- Guo L, Chen XJ, Hu YH, Yu ZJ, Wang D, Liu JZ. Curcumin inhibits proliferation and induces apoptosis of human colorectal cancer cells by activating the mitochondria apoptotic pathway. Phytother Res 2013;27:422-30.
- Somers-Edgar TJ, Taurin S, Larsen L, Chandramouli A, Nelson MA, Rosengren RJ. Mechanisms for the activity of heterocyclic cyclohexanone curcumin derivatives in estrogen receptor negative human breast cancer cell lines. Invest New Drugs 2011;29:87-97.
- Shao H, Tan Y, Eton D, Yang Z, Uberti MG, Li S, et al. Statin and stromal cell-derived factor-1 additively promote angiogenesis by enhancement of progenitor cells incorporation into new vessels. Stem Cells 2008;26:1376-84.
- Park W, Ruhul Amin ARM, Chen ZG, Shin DM. New perspectives of curcumin in cancer prevention. Cancer Prev Res 2013;6:387-400.
- Sethi G, Shanmugam MK, Ramachandran L, Kumar AP, Tergaonkar V. Multifaceted link between cancer and inflammation. Biosci Rep 2012;32:1-15.
- Farazuddin M, Dua B, Zia Q, Khan AA, Joshi B, Owais M. Chemotherapeutic potential of curcumin-bearing microcells against hepatocellular carcinoma in model animals. Int J Nanomedicine 2014;9:1139-52.
- Shanmugam MK, Rane G, Kanchi MM, Arfuso F, Chinnathambi A, Zayed ME, et al. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015;20:2728-69.
- Garcea G, Berry DP, Jones DJL, Singh R, Dennison AR, Farmer PB, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev 2005;14:120-5.
- Braumann C, Guenther N, Loeffler LM, Dubiel W. Liver metastases after colonic carcinoma-palliative chemotherapy plus curcumin. Int J Colorectal Dis 2009;24:85960.
- Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, et al. Phase I clinical trial of oral curcumin : Biomarkers of systemic activity and compliance phase I clinical trial of oral curcumin : Biomarkers of systemic activity and compliance. Clin Cancer Res 2004;10:6847-54.
- Wahlström B, Blennow G. A Study on the Fate of Curcumin in the Rat. Acta Pharmacol Toxicol 1978;43:86-92.
- Villegas I, Sánchez-Fidalgo S, Alarcón de la Lastra C. New mechanisms and therapeutic potential of curcumin for colorectal cancer. Mol Nutr Food Res 2008;52:1040-61.
- Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res 2003;23:363-98.
- Bernabé-Pineda M, Ramírez-Silva MT, Romero-Romo M, González-Vergara E, Rojas-Hernández A. Determination of acidity constants of curcumin in aqueous solution and apparent rate constant of its decomposition. Spectrochim Acta A Mol Biomol Spectrosc 2004;60:1091-7.
- Ravindranath V, Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology 1980;16:259-65.
- D’Souza AA, Devarajan PV. Bioenhanced oral curcumin nanoparticles: Role of carbohydrates. Carbohydr Polym 2016;136:1251-8.
- Kusano T, Berberich T, Tateda C, Takahashi Y. Polyamines: Essential factors for growth and survival. Planta 2008;228:367-81.
- Chen X, Zou LQ, Niu J, Liu W, Peng SF, Liu CM. The stability, sustained release and cellular antioxidant activity of curcumin nanoliposomes. Molecules 2015;20:14293-311.
- Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: In vitro and in vivo effects on proliferation, apoptosis, signalling, and angiogenesis. Cancer 2005;104:1322-31.
- Campbell RB, Ying B, Kuesters GM, Hemphill R. Fighting cancer: From the bench to bedside using second generation cationic liposomal therapeutics. J Pharm Sci 2009;98:411-29.
- Teichmann A, Heuschkel S, Jacobi U, Presse G, Neubert RHH, Sterry W, et al. Comparison of stratum corneum penetration and localization of a lipophilic model drug applied in an o/w microemulsion and an amphiphilic cream. Eur J Pharm Biopharm 2007;67:6990-706.
- Bergonzi MC, Hamdouch R, Mazzacuva F, Isacchi B, Bilia AR. Optimization, characterization and in vitro evaluation of curcumin microemulsions. Food Sci Technol 2014;59:148-55.
- Setthacheewakul S, Mahattanadul S, Phadoongsombut N, Pichayakorn W, Wiwattanapatapee R. Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur J Pharm Biopharm 2010;76:475-85.
- Duan Y, Wang J, Yang X, Du H, Xi Y, Zhai G. Curcumin-loaded mixed micelles: preparation, optimization, physicochemical properties and cytotoxicity in vitro. Drug Deliv 2015;22(1):50-7.
- Letchford K, Liggins R, Burt H. Solubilization of hydrophobic drugs by methoxy poly(ethylene glycol)-block-polycaprolactone diblock copolymer micelles: theoretical and experimental data and correlations. J Pharm Sci 2008;97:1179-90.
- Ma Z, Shayeganpour A, Brocks DR, Lavasanifar A, Samuel J. High-performance liquid chromatography analysis of curcumin in rat plasma: application to pharmacokinetics of polymeric micellar formulation of curcumin. Biomed Chromatogr 2007;21:546-52.
- Kusum Devi V, Saisivam S, Maria GR, Deepti PU. Design and Evaluation of Matrix Diffusion Controlled Transdermal Patches of Verapamil Hydrochloride. Drug Dev Ind Pharm 2003;29(5):495-503.
- Limpongsa E, Umprayn K. Preparation and evaluation of diltiazem hydrochloride diffusion-controlled transdermal delivery system. AAPS PharmSciTech 2008;9(2):464-70.
- Patel NA, Patel NJ, Patel RP. Design and evaluation of transdermal drug delivery system for curcumin as an anti-inflammatory drug. Drug Dev Ind Pharm 2009;35(2):234-42.
- Patra S, Roy E, Madhuri R, Sharma PK. The next generation cell-penetrating peptide and carbon dot conjugated nano-liposome for transdermal delivery of curcumin. Biomater Sci 2016;4:418-29.
- Madhavi BB, Masan KS, Madipoju P. Enhanced Transdermal Drug Penetration of Curcumin via Ethosomes. Malays J Pharm Sci 2013;11:49-58.
- Mohanty C, Sahoo SK. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials 2010;31:6597-611.
- Sindhu K, Indra R, Rajaram A, Sreeram KJ, Rajaram R. Investigations on the interaction of gold-curcumin nanoparticles with human peripheral blood lymphocytes. J Biomed Nanotechnol 2011;7:56.
- Bisht S, Mizuma M, Feldmann G, Ottenhof NA, Hong SM, Pramanik D, et al. Systemic administration of polymeric nanoparticle-encapsulated curcumin (NanoCurc) blocks tumor growth and metastases in preclinical models of pancreatic cancer. Mol Cancer Ther 2010;9:2255-64.
- Anuchapreeda S, Fukumori Y, Okonogi S, Ichikawa H, Anuchapreeda S, Fukumori Y, et al. Preparation of lipid nanoemulsions incorporating curcumin for cancer therapy. J Nanotechnol 2012;1-11.
- Lim KJ, Bisht S, Bar EE, Maitra A, Eberhart CG. A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biol Ther 2011;11:464-73.