- Corresponding Author:
- R. K. Khar
Department of Pharmaceutics, Faculty of Pharmacy, Jamia Hamdard, New Delhi - 110 062, India
E-mail: roopkhar@hotmail.com
| Date of Submission | 29 September 2010 |
| Date of Acceptance | 10 October 2010 |
| Indian J. Pharm. Sci., 2010, 72 (6): 675-688 |
Abstract
Nucleic acid-based therapeutics have gained a lot of interest for the treatment of diverse ophthalmic pathologies. The first to enter in clinic has been an oligonucleotide, Vitravene® for the treatment of cytomegalovirus infection. More recently, research on aptamers for the treatment of age related macular degeneration has led to the development of Macugen® . Despite intense potential, effective ocular delivery of nucleic acids is a major challenge since therapeutic targets for nucleic acid-based drugs are mainly located in the posterior eye segment, requiring repeated invasive administration. Of late, nanotechnology-based nano-vectors have been developed in order to overcome the drawbacks of viral and other non-viral vectors. The diversity of nano-vectors allows for ease of use, flexibility in application, low-cost of production, higher transfection efficiency and enhanced genomic safety. Using nano-vector strategies, nucleic acids can be delivered either encapsulated or complexed with cationic lipids, polymers or peptides forming sustained release systems, which can be tailored according to the ocular tissue being targeted. The present review focuses on developments and advances in various nano-vectors for the ocular delivery of nucleic acid-based therapeutics, the barriers that such delivery systems face and methods to overcome them.
Keywords
Dendrimers, liposomes, nucleic acid, nano-vectors, nanoparticles, ocular delivery
Nucleic acids such as oligonucleotide (ODN), small interfering RNA (siRNA) and aptamer play an increasingly vital role in our arsenal of therapeutic agents[1,2]. Recent advances in biotechnology and molecular biology have enabled not only a significant increase in the number of nucleic acid-based drugs but also to produce these macromolecules in large quantities. The application of nucleic acids in the treatment of a variety of ailments has been well documented, ocular disorders not being an exception[3]. Indeed, nucleic acid-based therapeutics have gained a lot of interest for the treatment of blinding disorders like glaucoma[4], retinitis pigmentosa[5], macular degeneration[6], neovascularization[7] and many other ocular disorders such as keratitis, corneal haze, corneal dystrophies and allograft rejection[8].
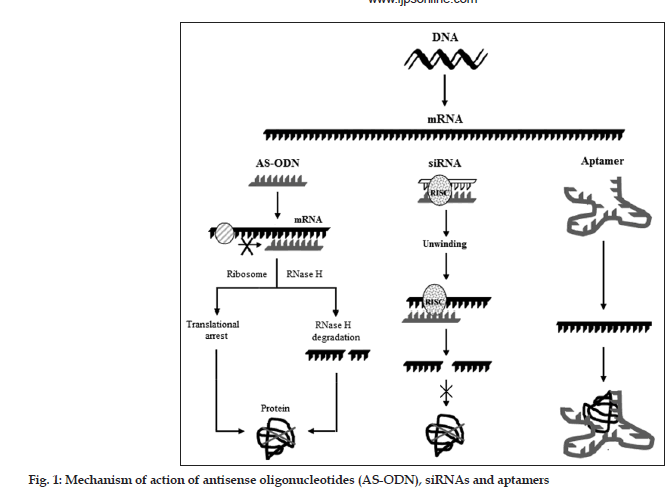
Among the promising nucleic acid-based therapeutic approaches, the first one is antisense oligonucleotide (AS-ODN)[1]. AS-ODN stops the production of undesirable proteins by binding to a target RNA sequence owing to its complementary design. A more recent approach for targeting mRNA is the use of double-stranded nucleic acids made up of 21–23 nucleotides called as small interfering RNA (siRNA). siRNAs are able, intracellularly, to assemble into a multiprotein complex, termed RNA-induced silencing complex (RISC). The RISC contains a helicase activity that unwinds the two strands of siRNA molecules and allows the antisense strand to bind to the targeted RNA molecule[9]. Further, RISC also exhibits endonuclease activity which hydrolyzes the target mRNA homologous at the site where the antisense strand is bound. Recently, a single RNA palindromic sequence of 50–70 oligonucleotides that can hybridize with itself to form a stem-loop “hairpin” structure was also developed[2]. Once in the cytoplasm the structure can be cleaved into 21-nucleotide siRNA duplexes. Another potential nucleic acid approach to treat ocular diseases is the use of aptamers. Aptamers are DNA or RNA molecules, selected from random pools, based on their ability to bind to nucleic acids, proteins, small organic compounds, and even entire organisms[1,2].
Nucleic acid delivery holds special promise for the treatment of ocular diseases. As with any other modality of administration, a major challenge in ocular nucleic acid therapy has become the effective delivery of nucleic acid to the target cell. The design of macromolecules for the treatment of ocular disorders is a unique task that is limited by the functional physiology as well as by the location of the eye[1,10]. Eye is relatively an isolated organ that has a number of avascular components such as cornea, lens and trabecular meshwork. Topical therapy as an eyedrop formulation is by far the most effective for a number of disease states and diagnostic procedures. However, due to a number of protective mechanisms (blinking reflex, tear turn over, lacrimation) and systems (eyelids, cornea, sclera, conjunctiva), the majority of the topical dose will be delivered, not to the ocular tissues but to the general circulation via the nasolacrimal duct and nasal/gastric mucosa[3]. Therein lies another problem with most of the nucleic acids utilized for ophthalmic diseases. Many of these agents are typically lead compounds that were originally developed for oral/parenteral therapy, and therefore, can be expected to elicit profound side effects when absorbed systemically. In addition to the potential adverse effects, there are also instances where the local effects of these agents themselves are severely limiting. Thus, in order to maximize the therapeutic index of ophthalmic nucleic acid agents it is necessary to design strategies or carrier systems to deliver nucleic acids to a specific site within the eye[11]. Designing carrier system for ocular delivery of AS-ODNs, siRNA or aptamer is a challenging task because of the high solubility, high molecular weight and size, presence of surface charge on the therapeutic agent and intrinsic complexities associated with the structure of ocular tissues[12].
Historically, viral vectors have been the preferred systems for the transfer of nucleic acids into tissues of interest. Despite high expression efficiency of viral vectors, poor transduction efficiency, inherent immunogenicity and toxicity limit their use in human eyes. Non-viral physical methods can overcome the immunogenicity and toxicity problems but their expression efficiency is relatively low[10,13]. In recent years, the use of nanotechnological approaches has received increasing attention for achieving the delivery of nucleic acids into the eye. They offer the advantages of a possible exit strategy, the modulation of treatment in function of the clinical evolution, and greater genomic safety. The focus of the present review is to provide an overview on the principles of AS-ODN, siRNA and aptamer therapy with an emphasis on the delivery of these drugs to the eye through use of nanotechnological approaches such as liposomes, nanoparticles, DNA nanoparticles, dendrimers and nanoemulsions.
Nucleic Acids In Ocular Therapy
Antisense oligonucleotides
AS-ODNs are synthetic molecules composed of 13 to about 25 nucleotides, which are complementary to mRNA strands in a region of a coding sequence designed as sense strand[1]. They bind to specific intracellular mRNA strands and stop translation of the mRNA, and hence synthesis of protein expressed by the targeted gene. In the most commonly described translational arrest mechanism, AS-ODN binds to the single strand mRNA by Watson–Crick base pairing forming a double-helix hybrid, thereby sterically blocking the translation of this transcript into a protein (fig. 1). The hybridized mRNA also prevents the binding of factors that initiate or modulate the translation and may block the movement of ribosomes along the mRNA. The formation of a stable hybrid depends on the binding affinity and sequence-specificity of the AS-ODN. Another widely described mechanism was a catalytic one and was based on RNase-H mediated cleavage of the target mRNA. RNase-H is a ribonuclease that recognizes RNA–DNA duplexes and selectively cleaves the RNA strand. After the RNA molecule is cleaved, the AS-ODN dissociates from the duplex and binds to a second target mRNA molecule. Vitravene® discovered by ISIS® and marketed by Novartis Ophthalmics® for the treatment of cytomegalovirus (CMV) retinitis, was the first AS-ODN approved by the FDA in the year 1998. Many other AS-ODNs for ocular administration are under trials[14-16].
Small interfering RNA (siRNA)
Into a variety of hosts, the long double stranded RNA (dsRNA) induces post transcriptional silencing of all homologous host genes and are metabolized to siRNA by the action of an endogenous enzyme, ribonuclease[1]. The siRNA molecules then assemble to RISC. The RISC exhibits helicase activity that unwinds the two strands of siRNA molecules and allows the antisense strand to bind to the targeted RNA molecule (fig. 1). RISC also demonstrates endonuclease activity by virtue of which it hydrolyzes the target mRNA homologous at the site where the antisense strand is bound[11]. A chemically modified siRNA targeting vascular endothelial growth factor (VEGF) receptor was shown to inhibit neovascularisation in several validated preclinical models[17]. Because of their antisense mechanism of action they are suitable alternatives to AS-ODNs for ocular therapeutics. Further, the higher stability of double-stranded siRNAs compared to AS-ODNs and their ability to inhibit gene expression in mammalian cells makes them highly efficient macromolecules[18,19]. Another modification to siRNA is short hairpin RNA (shRNA). shRNA are DNA-directed expression cassettes encoding a single RNA palindromic sequence of 50–70 oligonucleotides that can hybridize with itself to form a stem-loop hairpin structure. Once in the cytoplasm the structure can be cleaved to 21-nucleotide siRNA duplexes. shRNA are easily amplified to obtain large quantities and potentially offer a prolonged gene suppression[20].
Aptamers
Aptamers are RNA or DNA molecules selected in vitro from enormous populations of random sequences that recognize specific ligands. They bind specifically to functional domains of the target protein, due to their unique 3-D structures, thereby modulating the biological function of the molecule (fig. 1)[21,22]. Pegaptanib sodium, an aptamer, has recently been approved by FDA to treat age-related macular degeneration (AMD) of the eye[21-24]. The pegaptanib sodium was discovered by Eyetech Pharmaceuticals, Inc., and marketed by Pfizer Inc. under the trade name Macugen®. Aptamers themselves are small (5–25 kDa), prone to hydrolytic breakdown by nucleases and are rapidly eliminated from the body by renal clearance[23]. Aptamers with 2-O-methyl nucleotides, 2-fluoro pyrimidine, and a 3-end cap modification significantly enhances the in vivo stability of aptamers. Further modifications, using polyethylene glycol or cholesterol as anchor groups have been shown to improve the pharmacokinetic parameters and bioavailability of the aptamers[24].
Ocular Nucleic Acid Delivery
Although ocular delivery of nucleic acids holds a lot of promise, the major impediment in practical application comes from the low concentrations of nucleic acids reaching the site of action. This is because the ocular barriers required for the maintenance and protection of ocular functions tend to block the entry of macromolecular therapeutics[10]. The selection of the route of administration depends primarily on the target tissue, being traditionally the topical and subconjunctival for targeting anterior segments. The topical instillation is the easiest and least invasive method, but is virtually ineffective for the delivery of genetic material to the intraocular space and posterior segment, which in most cases are the target sites for nucleic acids. Owing to their high molecular weight and negative charge, nucleic acids do not penetrate the corneal surface and remain confined to the superficial epithelial layer, and thus are more suitable as a delivery method for corneal and conjunctival conditions[3,10].
The ocular distribution of ODNs after topical ocular application has been studied. In a study using rat eye, instilled eye drops of ODNs remained localized on the surface and were observed on the corneal epithelium[25]. In another study using rabbit eye, the ODNs were detected in the corneal epithelium, conjunctiva, and sclera, with negligible levels in the iris[26]. The challenge of achieving effective concentrations of nucleic acids in the intraocular regions has led to the prevalence of invasive administration. Subconjunctival delivery is a little invasive and may lead to drug penetration into the anterior and posterior segments of the eye, but with significant systemic absorption[27]. A single subconjunctival injection of an anti-TGF-β ODN in a rabbit model of glaucoma filtration surgery allowed for prolonged bleb survival. Subconjunctival injection of a VEGF siRNA mix allowed potential targeting of corneal cells leading to a significant inhibition of corneal neovascularisation or HSV-1 infection in animal models[17].
Delivery of nucleic acids through intravenous administration is associated with poor pharmacokinetics arising out of blood-aqueous barrier in the anterior part of the eye and blood-retinal barrier in the posterior part of the eye[28]. Sub retinal and intravitreal injections are generally considered to be the most effective ways of delivering materials to the back of the eye[29]. Subretinal injection may be optimal for delivery to outer retina, specifically photoreceptors and RPE, whereas intravitreal injection has been shown to be more effective for delivery to retinal ganglion cells and inner retinal interneurons[30]. Subretinal injection is being employed in the current ongoing gene therapy trials for RPE65-associated LCA[31-33]. Direct injection into the sub retinal space, although technically challenging, allows for increased contact time of the injected nucleic acid and the posterior retinal layers, but the area of contact is restricted only to the injection site. Moreover, there are chances of induction of lesions in RPE cells, limiting its suitability in clinical settings. Intravitreal injection is more clinically acceptable and several approved ARMD drugs are already delivered via intravitreal injection. By contrast, nucleic acids have very low intravitreal half-lives thereby necessitating frequent administrations to achieve a continuous presence in the retina, which might lead to the risk of endophthalmitis, lens damage and retinal detachment[30]. Administration of drugs via the sclera could be an alternative for the delivery of nucleic acids. Several studies have highlighted the potential of transscleral delivery of various macromolecules (≤70 kDa). Recent data demonstrated the successful delivery of an aptamer across the sclera by diffusion. Drug diffusion or transport through the sclera is interesting because of its large and accessible surface area, high degree of hydration, low number of cells and the fact that drug permeability does not decline significantly with age. Shuler et al., have determined the transscleral and intrascleral permeability of a fluorescein-labelled ODN (24 bases) after intrascleral injection. The studies revealed that ODN diffusion through the sclera to the posterior segment is possible. To allow nucleic acids to gain access to the target site, they should be delivered by a nanosized carrier which permits subcellular cytoplasmic delivery. Finally the delivery system needs to remain for a prolonged time in the vitreous avoiding repeated administration[34]. Nevertheless, choosing a mode of administration would depend on the target cell type. Basic knowledge needs to be increased on how different nucleic acids penetrate into the eye and ocular tissues, distribute in the vitreous humor, penetrate into the retinal layers and/or cells, and are eliminated from the eye. This should serve as a basis for designing novel drug delivery systems. The later should be non-or minimally invasive and should allow for a controlled or sustained release of the nucleic acid in the ocular media.
Nano-Vectors For Ocular Nucleic Acid Delivery
The ideal vector should be taken up efficiently and extensively in the concerned tissue with minimal ectopic uptake or expression. Levels of gene expression should not induce toxicity but should be high enough to promote phenotypic improvement. Gene expression should start rapidly after treatment delivery and persist throughout the life of the organism especially for chronic disease treatment. The vector should be able to be delivered by a safe non-invasive method, should be well tolerated and should not cause significant inflammation, immune response, toxicity, or other adverse physiological conditions[5,35]. In spite of significant successes with viral vectors in nucleic acid delivery and therapy, several chemical non-viral methods are currently being explored[3]. This is because viral vectors (lentivirus, retrovirus, adenovirus) are associated with risk of adverse immune effects and insertional mutagenesis[3,5]. Considerable progress has also been achieved in non-viral delivery using physical methods (iontophoresis, electroporation, nucleofection, gene gun) with the advantage of low immunogenicity and toxicity[3,5]. However, physical methods are also associated with low expression efficiency which limits their use[11]. In recent years, nanotechnologybased chemical methods have received increasing attention for achieving the delivery of nucleic acids to the eye[5,11]. Nanocarriers have high gene-carrying capacity, low risk of immunogenicity, and relatively low cost. These nanocarriers termed as “nanovectors” can be thought of as completely “synthetic viruses” whose composition is well defined and completely controlled (unlike actual viral vectors). Several nano-vectors have been developed to enhance the ocular delivery of nucleic acids[5,11]. The formation of complex between nucleic acid and nanovector is based on electrostatic interaction between negatively charged nucleic acid at physiological pH and cationic nano-vector. The positive surface charge of the nano-vector facilitates its attachment to the cell surface and subsequent endocytosis. The cationic lipids in the form of liposomes[36] and lipid nanoparticles[37,38], cationic polymers in form of micelles[39], polyplexes[40] and polymeric nanoparticles[41], peptides as compacted DNA nanoparticles[42] and dendrimers[43] have improved the cellular uptake of nucleic acids with variable success in vitro and in vivo. The salient features of these nano-vectors make them attractive for the treatment of chronic ocular conditions such as uveitis, glaucoma, retinal edema, neovascularization as well as intraocular tumors and other conditions such as capsular fibrosis, ocular neovascularization and proliferative vitreoretinopathy. Nano-vectors can be targeted to different tissues in the eye by varying the injection site or could be given topically in the form of eye drops.
Liposomes
Liposomes are the micron- or submicron-sized vesicles composed of one or more concentric lipid bilayers, separated by water or aqueous buffer compartments with a diameter ranging from 80 nm to 10 μm. Liposomes are attractive as ocular delivery vehicles as they are biodegradable, sustain the release of drug and remain localized at the site of administration.
Hydrophilic therapeutics can be encapsulated in the inner aqueous cavity and lipophilic therapeutics can be incorporated in the membrane. Liposomes can act as carriers for a wide variety of drug molecules, proteins, nucleic acids, genes and even plasmids giving them tremendous potential for ophthalmic drug delivery. Another potential advantage of liposomes is their ability to come in intimate contact with the ocular surfaces, thereby increasing the probability of ocular drug absorption[44-46]. Liposomes rely on electrostatic interactions between the anionic DNA and lipid molecules. Complexes obtained are more compact than naked DNA and their positive or neutral global charge enhance intracellular delivery[44]. Cationic lipids, such as dioleoyl trimethylammonium propane (DOTAP), trimethylammonioacetyl- didodecyl-D-glutamate (TMAG), dioleyloxypropyl trimethyl ammonium chloride (DOTMA) and (dimethylaminoethanecarbamoyl) cholesterol are electrostatically favourable for complexing negatively charged DNA[47-49]. In addition, dioleoylphosphatidylethanolamine (DOPE) and dioleylphosphatidylcholine (DOPC) are often used as the neutral lipids because of their pH-sensitive ability to destabilize the lysosomal membranes after cellular entry of the liposome through endocytosis. A highly efficient DNA transfection technique, ‘lipofection’, was introduced by Felgner et al. Commercially available Lipofectin™ consists of a 1:1 weight mixture of positively charged DOTMA and neutral lipid DOPC[37]. Zelphati and Szoka (1996) reported that Lipofectin™ and ODNs complex leads to a marked increase in antisense activity and ODN nuclear uptake. Complexes of ODNs and Lipofectin™ bind to and penetrate cellular membranes, and ODNs are subsequently released into the cytoplasm[47]. Liposomes can also be used to protect drug molecules from the attack of metabolic enzymes present at the tear/corneal epithelium interface. Liposomal encapsulation was shown to cause up to a 15- fold increase in inulin concentration in cornea and conjunctiva. The increased uptake of inulin by conjunctiva and cornea was attributed to the physical adsorption of lipid vesicles onto the epithelial surface of the membrane[30]. Liposomal encapsulation is the most common non-viral method to deliver plasmids and ODNs to the corneal cells and also to the inner retinal layers and the RPE cells[30]. Despite the findings where β-galactosidase expression in retinal ganglion cells and specific gene expression in corneal and conjunctival epithelial cells was observed for up to 1 month after topical instillation of liposomes with lacZ plasmid, the mode and mechanism of liposomes penetration from the ocular surface to the retina remains enigmatic. After intracameral injection of liposomes containing lacZ plasmid to rat eye, β-galactosidase expression was observed in the basal layer of the corneal epithelium, corneal endothelium, ciliary epithelium, ciliary body stroma, iris, and RGCs. The mechanism postulated was that the liposomes injected into the anterior chamber diffuse into the vitreous humor and reach the retina against the normal aqueous humor flow from the posterior chamber to the anterior chamber. Further, transfection of the corneal epithelium is probably due to the penetration and infiltration of liposomes through the full thickness of the cornea finally reaching the basal corneal epithelium[30,50].
Liposomes have also been used for the delivery of antisense ODNs for gene silencing. The antisense effect was seen only with small-sized liposomes with a membrane active component such as DOPE or an added peptide JTS-1 (membrane-active pHdependent peptide) that releases the ODN through membrane fusion[51]. Yoon et al. reported that twice or thrice weekly subconjunctival injection of a plasmid encoding the extracellular region of brain-specific angiogenesis inhibitor 1 (BAI1-ECR) gene fused to GFP in liposomes led to a significant inhibition of corneal neovascularisation[52]. Oligothymidylate ODN injected into the vitreous humor of rabbit eyes led to a 9.3 fold higher ODN concentration in the vitreous humor than that observed when naked ODNs were used. Liposomes have also been used to increase the stability and reduce the toxicity of intravitreally injected nucleic acids[53]. Bochot et al. (2000) used liposomes for intravitreal administration of ODNs for the treatment of ocular viral infections[54]. The authors found that the use of liposomes for intravitreal administration is very promising since liposomes are able to protect ODNs against degradation by nucleases[54]. The DNA properties can further be improved by using haemagglutinating virus of Japan (HVJ) containing the viral high-mobility group 1 protein. The high-mobility group 1 is a nonhistone nuclear protein which enables the intracellular trafficking of the plasmid DNA into the nuclei. It also mediates the fusion of liposomes with the cell membrane. Yet another methodology to enhance liposomal gene transfer is the use of protamine sulfate[54].
To further improve the stability of liposomes in the vitreous, pegylation strategies have been developed. PEG coating allows surface charge masking of the liposomes thus inducing a repulsive effect towards proteins aggregation. PEG-coated liposomes resulted in increased ODNs uptake in retina/choroid. However, mild and transient intraocular inflammation was also associated[54]. Subretinal injection of LacZ plasmid formulated with 10% NeuroPorter led to transgene expression in RPE cells without any observed damage to retinal tissues. However, significant photoreceptors damage was observed when LacZ plasmid formulated with 40% Lipofectamine 2000 was used. Transferrin receptors are widely expressed both in the plasma membrane of cells in the retina and at the blood-retinal barrier. Pegylated immunoliposomes encapsulating the rat 8D3 monoclonal antibody were constructed to allow crossing of the blood-ocular barriers. Gene expression in the inner retina, ciliary body, iris, sebaceous glands of the tarsal plate and corneal epithelium was observed in the presence of glial fibrillary acidic protein (GFAP) promoter. Simian virus SV40 promoter led to diffuse gene expression in the RPE cells[55,56].
Nanoparticles
Another nanotechnology-based strategy for the prolonged ocular delivery of nucleic acids, mainly ODNs and aptamers is the use of nanoparticles. Nano-sized particles are considered to be efficient and particles less than 25 nm have the potential to efficiently pass through the pores in the nuclear membrane, thus overcoming a significant barrier to successful transfection[12]. Based on the previous reports, particles successful for nucleic acid delivery usually have a hydrodynamic diameter of less than 500 nm[3]. Nanoparticles have also been used extensively for ocular applications other than nucleic acid delivery[3,5]. For example, cerium oxide nanoparticles have been used to alleviate oxidative stress in models of light-induced retinal degeneration[38]. Nanoparticles are used to deliver growth factors to cells and are helpful in managing corneal allograft rejection[38]. Usually, nanoparticles consist of a polymer base (polymeric nanoparticles), a lipid base (solid lipid nanoparticles) or a peptide base (peptide nanoparticles) that encapsulate or condense with the DNA of interest.
Polymeric nanoparticles - Among the polymers explored, polyesters (PLA, PGA and PLGA) are most attractive for the fabrication of nucleic acid-loaded nanoparticles for ocular delivery, due to their slow erosion rate, biocompatibility, and biodegradability and also because of their interactions with various ocular receptors[3]. The interest of polymeric nanoparticles to deliver nucleic acids into the retina was a result of in vitro and in vivo studies demonstrating transretinal movement of plasmidloaded PLA and PLGA nanoparticles after intravitreal injection, with a preferential localization in the RPE cells[41]. A preferential red nuclear fluorescent protein (RNFP) plasmid expression within the RPE cell layer was observed, without any toxic effects up to eight days in culture. Polyester nanoparticles have also been used for retinal DNA delivery. These nanosized particles can escape the early endo-lysosomal formation and enter the cytosolic compartment by a mechanism of surface charge reversal, thereby improving their delivery efficiency[41]. In another study, antiviral activity of a phosphorothioate ASODN and a control phosphodiester AS-ODN, either encapsulated or adsorbed in albumin nanoparticles was evaluated in MRC-5 fibroblasts infected with human CMV[57]. Nanoparticles entrapping AS-ODNs showed slightly higher antiviral efficacies compared to nanoparticles having AS-ODNs on their surface. No significant differences was observed between phosphorothioate and phosphodiester AS-ODNs delivered by nanoparticles. The results indicated that nano-encapsulation of AS-ODNs increases their antiviral efficacy by preventing their accessibility to nucleases[57-59]. In an attempt to deduce the biodistribution of albumin nanoparticles, intracellular trafficking of AS-ODNs labelled with FITC was studied[57]. Two weeks after the intravitreal injection of albumin nanoparticles to rats, the histopathological studies confirmed the association of nanoparticles to ocular cells following absence of cellular infiltration. No inflammatory reactions in the retinal tissue and in the surrounding ocular tissues of the eye were seen. However, the retinal epithelium was disrupted to a certain degree by the histological manipulation itself. After two weeks of single intravitreal injection the studies revealed that the nanocarrier appeared to diffuse through the vitreous space. A significant amount of nanoparticles were mainly found in a thin layer overlying the retina and a few of them were in adjacent sites of the blood-aqueous barrier and in the ciliary body[57].
Implantable polymeric microspheres can be directly injected into the site of action providing a sustained release of AS-ODNs for extended periods of time but are unable to increase the intracellular penetration of the released AS-ODN. To overcome this limitation Gomes dos Santos et al. designed a “trojan” delivery system consisting of nano-sized cationic complexes of anti-TGFβ2 phosphorothioate AS-ODN encapsulated within PLGA microspheres[60]. The naked AS-ODNPLGA microspheres had a bleb survival time of 28 days for 50% of the treated eyes, whereas nano-sized complex of AS-ODN-PLGA microspheres significantly prolonged the bleb survival to 42 days for 100% of treated eyes following subconjunctival injection in a rabbit glaucoma trabeculectomy model. The better efficiency of the nanosized complex released from microspheres was probably due to a better intracellular delivery in conjunctival cells[60]. Chitosan (CH) and its oligomer CHO, and hyaluronan (HY) are the natural polymers explored for the production of nanoparticles intended for ocular delivery of nucleic acids[61]. Following topical instillation of plasmid-loaded nanoparticle suspensions (HY:CHO and HY:CH at a mass ratio 1:2) to the rabbit eyes, gene expression was detected at a dose of 25 g/eye and 50 g/eye for HY:CHO and HY:CH nanoparticles, respectively. The expression lasted as long as 7 days for HY:CHO nanoparticles. The enhanced and extended expression of these nanoparticulate systems were attributed to the incorporation of HY in the nanoparticles and nanoparticle interactions[61]. The placebo nanoparticles of fluoresceinamine- HY and CH or CHO were prepared to study the ocular uptake/ penetration mechanism of these nanoparticles. Two hours postinstillation, these nanoparticles were found to penetrate through the corneal epithelium of the rabbit eyes. The nanoparticles were deposited inside the cells rather than in the intercellular spaces, indicating transcellular transportation. HY nanoparticles were retained in the epithelial cells for longer duration than the HY solution, suggesting stronger interactions of HY with the cornea when HY was in a nanoparticulate form, and thus a slower clearance of the nanoparticles from the eye. These results indicate that HY, along with CH, is suitable for the fabrication of nanoparticles for ocular delivery of nucleic acids[61].
Solid lipid nanoparticles (SLNs) - A newer type of lipid-based nucleic acid carrier are SLNs. Compared to traditional liposomes, SLNs are reportedly easier to make and their size is less than 200 nm in diameter. Thus far, the only reports of delivery of SLNs to ocular tissue studied their ability to direct gene expression in the ARPE- 19 cell line[62]. The authors reported that the SLNs particles were taken up via clathrin-mediated endocytosis, but the ARPE-19 cells did not transfect well with less than 2.5% transfection efficiency. Additional research will be needed to prove SLNs as clinically useful vectors[62].
Compacted DNA nanoparticles - Excellent preliminary studies have been undertaken with polyester nanoparticles, but so far they have not been used for delivery of nucleic acid to the mammalian retina. Alternatively, compacted DNA nanoparticles have proved to be a very useful vehicle for nucleic acid delivery and meet the majority of the requirements discussed above for a successful vector[35]. The formation of compacted DNA nanoparticles relies on the principles of DNA condensation. They typically contain a highly negatively charged circular or linear segment of DNA or RNA which is compacted with a polycationic polymer. Polycationic polymers or condensing agents can vary from organic polyamines to inorganic polycations to polypeptides such as polylysine, which is used in some of the therapeutic nanoparticles currently in use[42,63]. The size of the compacted DNA nanoparticles is quite small (10-100 nm), and therefore, these particles are taken up at the cell surface and trafficked to the nucleus within a short period of time. Compacted DNA nanoparticles yield medium to high transfection efficiency; in many cases expression levels are several folds greater than those observed after treatment with naked plasmid DNA. These results are dependent on specifics of the nanoparticle formulation, particle size, or electric charge[63,64].
Cationic polyethylenimine spontaneously forms nanoparticles with DNA and is used for nucleic acid delivery[29]. Polyethylenimine-based nanoparticles exhibited relatively high transfection efficiency in various organs although they are still less efficient than viral vectors due to toxicity concerns[29]. Compacted polyethylene glycol (PEG) nanoparticles of plasmid DNA have been used to efficiently transfect post-mitotic cells in vitro and in vivo[65]. Small size (10-20 nm), allowing easy diffusion through the vitreous humor makes these systems very attractive for nucleic acid applications. Additionally, these nanoparticles are stable under a wide range of temperatures and pH, protect encapsulated DNA or RNA from DNase or RNase degradation and, are capable of containing plasmids up to 20 kb and retain full functional competence following in vivo administration[65]. Studies in humans and mice showed little to no toxicity in the targeted tissues after repetitive administration of the nanoparticles which adds another advantage over some viral vectors. Recently, CK30PEG10K, a 30-mer lysine peptide with an N-terminal cysteine conjugated to 10 kDa PEG has been used for plasmid DNA delivery to different ocular tissues in mice using acetate or trifluoroacetate as the lysine amine counterion[35]. These nanoparticles can compact any type of nucleic acid and each particle contains only one molecule of DNA owing to their very small size (8-20 nm in diameter)[35]. Further, by varying the polylysine counterion at the time of compaction, various shapes of nanoparticles can be achieved[35]. Intravitreal injections of compacted DNA nanoparticles caused strong gene expression in the inner retina and in ocular tissues near the vitreous humor, whereas subretinal injection resulted in substantial transfection of the photoreceptor layer and RPE[35]. Moreover, delivery of these compacted DNA nanoparticles did not show any toxicity or decrease in retinal function[35]. Cell penetrating peptides that show high translocation across the cell membrane can also be fine-tuned for the delivery of nucleic acids to the ocular tissues especially retina. These molecules show rapid cellular entry in a temperature dependent manner and get localized to RPE, photoreceptor and ganglion cells[66]. These can compact DNA into nanocarriers and achieve transgene expression in human embryonic retinoblasts. However, the varied chemical nature of the cell penetrating peptides makes peptide design difficult and optimal delivery using peptide still remains a challenge[3].
Dendrimers
Dendrimers are three dimensional, hyperbranched, monodisperse molecules with defined molecular weights and host-guest entrapment properties. Characteristically, the dendrimers are globe- or ellipsoid-shaped, with three distinct components: (1) a central core, (2) repeated branches, and (3) surface functional groups. The structure of these polymers consists of repeated branching around the central core that results in a nearly-perfect threedimensional geometrical pattern. The central core is a molecule with at least two reactive functional groups. The repeated branches are organized in a series of radically concentric layers called generations[67]. At higher generations dendrimers resemble spheres with countless cavities within their branches which make it possible to encapsulate macromolecular therapeutic agents into the macromolecule interior. The surface functional groups, which determine dendrimer’s physical properties, are located on the surface of dendrimer molecules. The large number of functional groups (such as carboxyl and amine) on the surface of dendrimers may be expected to have potential application in enhancing the solubility of poorly water-soluble drugs by electrostatic interaction or covalent conjugation[67]. Dendrimers synthesized stepby- step, have well organized structures and precisely controllable nano-scale scaffolding and nanocontainer properties, which in some sense mimic the properties of macromolecules such as nucleic acids. Dendrimers used in ocular delivery are usually less than 100 nm in diameter with multiple functional groups on their surface, rendering them ideal carriers for macromolecular therapeutics[67].
Dendrimers made up of amine-containing cationic polymers such as poly-L-lysine[68], polyamidoamine[28] and polyethylenimine dendrimers are capable of condensing DNA, and also interact with the negatively charged cell surface molecules to facilitate cellular uptake. Poly-L-lysine is the most common polymer and has been investigated in vitro for ocular delivery of plasmid DNA to RPE cells. Gene transfer into RPE using the commercially available starburst polyamidoamine dendrimer Superfect has also been successful[28]. An in vivo study demonstrated targeted delivery of shRNA and a reporter red fluorescent protein to retinal ganglion cells after intravitreal injections of polyethylenimine dendrimers[29]. The shRNA-loaded polyethylenimine dendrimers reduce the melanopsin expression in the transfected area to an undetectable level[29]. Dendrimers can also be conjugated with ligands to achieve cell-specific DNA delivery through receptor-mediated uptake[68,69]. Marano et al. used synthetic lipid–lysine dendrimers with a tail of lipidic aminocarboxylic acids and a poly-lysine head in an attempt to improve the delivery of the ODN-1 (anti-VEGF) into the nuclei of cultured D407 retinal pigment epithelium cells[68,69]. These lipid–lysine dendrimers facilitate transmembrane transportation, act as lipid solubilizers and protect the labile DNA from nuclease digestion. It was found that dendrimers with a higher number of positive charges possess greater transfection efficacies. ELISA test showed that all the dendrimers were able to penetrate the nuclear membrane of cultured cells and deliver ODN-1 to the target site on the dsDNA strand, as indicated by the significant inhibition (P<0.05) of VEGF expression. In vivo studies also indicated that the presence of lipid-lysine dendrimer could prolong the delivery of ODN-1. The dendrimer-ODN conjugate was well tolerated, with excellent biodistribution and no observable increase in inflammation-associated antigens. The transfection efficiency of the dendrimers increases with their charge and the most effective structural combination was three branched chains of intermediate length with 8 positive charges. These results altogether demonstrated that lipid-lysine dendrimers could be used for gene delivery and maintain longterm modulation of gene expression in vivo, thus are valuable tools for gene therapy[69,70]. Shaunak et al. have synthesized water-soluble conjugates of carboxylated PAMAM dendrimer (3.5G) and D(+)-glucosamine and D(+)-glucosamine-6-sulfate[71]. These conjugates, when injected subconjunctivally in a rabbit model of scar tissue formation after glaucoma filtration surgery, increased the longterm success of the surgery from 30% to 80% and inhibited the pro-inflammatory and pro-angiogenic responses in the tested animals. Rabbits treated with conjugated dendrimers also showed minimal scar tissue formation compared to placebo-treated animals. Furthermore, no clinical or biochemical toxicity, or microbial infection was found in animals during the experimental period[71]. Cell-adhesion peptides were also attached to dendrimers which were later used to link with collagen scaffolds. All peptides have shown promoted effect on stratification of corneal epithelial cells. Biocompatible conjugates of dendrimers with collagen scaffolds also promoted the adhesion and proliferation of human corneal epithelial cells. These dendrimer-cross-linked collagen gels can be used to develop scaffolds for artificial corneal and many other tissue engineering applications[71].
Polyplexes
Polyplexes are complexes of nucleic acids such as plasmid DNA and cationic polymers with numerous amine, lysine or arginine groups protonated at physiological pH. If only a fraction of the groups are protonated at physiological pH, as for polyethylenimines and dendrimers, the polymers are able to buffer endosomes and enable the release of endocytosed DNA into the cytosol. Polypexes are nanosized (diameter 8 nm), have large vector capacity, are stable in nuclease-rich environments, and have relatively high transfection efficiency for both dividing and nondividing cells. Conjunctival matrix fibrosis and inflammation induced in a mouse model were inhibited after subconjunctival injection of siRNAs targeting the type-2 TGFβ receptor formulated with a polymeric lipid transfecting agent (TransIT-TKO)[17].
A ternary complex composed of a core of YFPencoding plasmid DNA/cationic polymer polyplex enveloped in an anionic dendrimer with phtalocyanine as photosensitizer was formulated[72,73]. Following internalization by endocytosis, the photosensitizer is released from gene carriers by laser irradiation and plasmid was delivered. Illumination induced a confined and localized transient gene expression within the conjunctival epithelial cells in the illuminated area only[73]. Intravitreal injection of polyoxyethylenepolyspermine (PEO-PSP) block copolymers containing fluorescein isothiocyanate-labeled anti-fibronectin antisense ODNs led to reduced fibronectin mRNA levels and fibronectin protein expression 2 and 6 days post-treatment[74]. Systemic administration of siRNAs formulated with polymer TargeTran, led to a significant inhibition of corneal neovascularization induced by CpG or HSV-1 infection[17].
Polymeric micelles
Eye drops were developed using poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) (PEO–PPO–PEO) polymeric micelles loaded with LacZ gene plasmids encoding for β-gal[39]. The expression of this gene was driven by different promoters, i.e., cytomegalovirus early gene (pCMVLacZ), keratin 12 gene (pK12-LacZ), and keratocan gene (pKera 3.2-LacZ). After topical instillations of the polymeric micelle eye drops containing epithelium-specific pK12-LacZ to mice and rabbits, increased β-gal activity was observed in both mouse and rabbit corneas[39]. The delivery mechanisms of this nanocarrier were believed to be paracellular transport and endocytosis[39]. With the eye drops of polymeric micelles containing pKera 3.2-LacZ, stroma-specific LacZ expression was found in the stroma only after the pretreatment with EDTA, a tight junction opener, before topical instillation[39]. This suggests that the epithelial barrier accounted for the lack of gene delivery into the stroma. The results also demonstrated retarded delivery of pKera 3.2-LacZ after pretreatment with RGD peptide (arginine– glycine–aspartic acid), an endocytotic inhibitor[39]. Gene delivery to the stroma after perturbation of tight junctions in the epithelial layer by EDTA and retarded gene delivery after the pretreatment of endocytotic inhibitor evidenced endocytosis of polymeric micelles[39]. A comprehensive list of nanovectors investigated for ocular delivery of nucleic acid-based therapeutics is summarized in Table 1.
Obstacles To Nano-Vector Nucleic Acid Delivery
Eye anatomy- Although nano-vectors for ocular delivery of nucleic acids hold a great promise, the major impediment in practical application comes from the low concentration of formulations at the target site. This is because the ocular barriers required for the protection and maintenance of ocular functions tends to block the entry of nano-vectors. Obstacles to ocular nucleic acid delivery with different routes of administration are described in fig. 2.
Biodistribution- The other major obstacle is to locate and visualize suboptical nano-vectors in large areas of tissues. Further, the biodistribution of nano-vector and their persistence in tissues and organs is still not well known.
Manufacturing- Manufacturing of nano-vectors requires not only the clean-room area but also makes extreme demands on the manufacturing of biological components and their complexation to the nanoparticles. Safe bionanomanufacturing is still a largely unexplored area.
Cell-by-cell nucleic acid delivery- Although nanovectors provide controlled and targeted drug delivery however, delivery of a precise amount of nucleic acid to individual cells in vivo is an extremely difficult task. One way of addressing this problem is in-situ production of therapeutic genes under the control of molecular biosensors. The molecular biosensors can regulate the amount of drug per cell according to the need as detected in a feedback loop with an upstream molecular biosensor.
| Vector | Nucleic acid | Delivery route | Cell line/ animalspecies | Reference |
|---|---|---|---|---|
| Liposome | ||||
| DOTMA/DOPE/ Cholesterol | Luciferase | Intravitreal | Rabbit | 36 |
| Phospholipid/Cholesterol/PEG-DSPE | ODN | Intravitreal | Rabbit | 53 |
| Pegylated immunoliposome | β-galactosidase with GFAP | Intravenous | Mouse | 55 |
| DOTMA/DOPE/protamine sulphate | Secreated alkaline phosphatase | Transfection | ARPE-19 | 75 |
| DOTAP/DOPE/PEG | Luciferase | Transfection | D407 | 76 |
| Artificial viral envelope liposome | β-galactosidase, ODN | Intravitreal | Rat | 77 |
| Polymeric Nanoparticle | ||||
| PLGA | GFP, RNFP | Transfection | ARPE-19 | 41 |
| GFP, RNFP | Intravitreal | Rat | 41 | |
| Anti-VEGF RNA aptamer | Trans-scleral | Rabbit | 78 | |
| Albumin | Flt23K | Intrastromal | Mice | 57 |
| Chitosan/Hyaluronic acid | pEGFP, β galactosidase | Transfection | HCE, IOBA-NHC | 61 |
| Oligomeric chitosan | pDNA | Transfection | IOBA-NHC | 79 |
| PBCA | pEGFP-N1 Plasmid DNA | Transfection | HepG2 cells | 80 |
| Solid Lipid Nanoparticles | ||||
| Precirol® ATO5/ DOTAP | pCMS-EGFP plasmid | Transfection | ARPE-19, HEK 293 | 62 |
| Compacted DNA Nanoparticle | ||||
| Polyethylenimine | shRNA targeting melanopsin | Intravitreal | Mouse | 29 |
| Anti-TGFb-2 ODN | Transfection | Rodent primary retinal | 60 | |
| CK30PEG10K | GFP | Intravitreal, subretinal | Mouse | 35 |
| PEG | Plasmid DNA | Transfection | Post-mitotic cells | 65 |
| GGG(ARKKAAKA)4 | siRNA, GFP | Transfection | HER 911 | 66 |
| Dendrimers | ||||
| Polyamidoamine | GFP | Transfection | Human primary RPE cells | 28 |
| PLL, PEG-PLL, PLL | Luciferase | Transfection | D407 | 68 |
| Lipid-lysine | Anti-VEGF ODN | Intravitreal | Rat | 69 |
| Carboxylated PAMAM/ glucosamine | - | Subconjunctival | Rabbit | 71 |
| Polyplexes | ||||
| TargeTran | siRNA | Subconjunctival, | Mouse, Mice | 17 |
| Intravenous | ||||
| Cationic polyplex/ anionic dendrimer with phtalocyanine | YFP encoding plasmid DNA | Transfection | Conjunctival epithelial cells | 73 |
| Polyoxyethylene-polyspermine | Anti-fibronectin ASODNs | Intravitreal | 74 | |
| Polymeric Micelles | ||||
| PEO-PPO-PEO | plasmid DNA lacZ gene | Topical instillation | Mice, Rabbits | 39 |
Table 1: Nano-Vectors Used For Ocular Delivery Of Nucleic Acids.
Biological consequence - The biggest advantage of nano-vectors is that one can at least minimize unintended biological consequences by using highly targeted nano drug delivery systems. Targeting as well as the fact that 1 to 2 orders of magnitude smaller amounts of therapeutic agents are delivered in vivo, goes a great deal of the way towards reducing possible unintended consequences and adverse effects. Nonetheless, it is difficult to predict the toxicological response to a given nano-vector in most cases. In general, the toxicity of nano-vector reflects their chemistry[3].
Conclusion
The examples of nanotechnology in ophthalmology cited in this perspective demonstrate that nanotechnology will play an important role in both early-and late-stage intervention in the management of blinding diseases. Interaction of cationic polymers/ lipids with negatively charged nucleic acids results in condensation of the material into particles of several hundred nanometers, protection of nucleic acids from enzymes, and mediation of cellular entry. The ability to target nano-vectors to different cell types and/or regions of the cell makes them customizable for diverse applications. While recent trends in the literature show the undoubted advantage of nanovectors for ocular delivery of nucleic acid-based therapeutic agents, most of the vectors chosen for delivery are not specially designed to overcome anatomical barriers. Since barriers for ocular nucleic acid delivery are unique, novel strategies that can circumvent those barriers need to be explored. These efforts will help in modulating the chemistry of the carrier and in developing vectors fine-tuned for ocular delivery. The remaining concerns facing the use of these vectors deal with efficient targeting of the vast array of disease genes, and curing genetic diseases diagnosed after the onset of degeneration. Further, nanoscale-engineered cell substrata such as nanowires and carbon nanotubes could be designed for use as future nano-vectors for the management of different disease states in ophthalmology.
References
- Fattal E, Bochot A. Ocular delivery of nucleic acids: Antisense oligonucleotides, aptamers and siRNA. Adv Drug Deliv Rev 2006;58:1203-23.
- Klausner EA, Peer D, Chapman RL, Multack RF, Andurkar SV. Corneal gene therapy. J Control Release 2007;124:107-33.
- Naik R, Mukhopadhyay A, Ganguli M. Gene delivery to the retina: Focus on non-viral approaches. Drug Dis Today 2009;14:306-15.
- Liu X, Rasmussen CA, Gabelt BT, Brandt CR, Kaufman PL. Gene Therapy Targeting Glaucoma: Where Are We Surg Ophthalmol 2009;54:472-86.
- Conley SM, Naash MI. Nanoparticles for retinal gene therapy. Prog Retin Eye Res 2010;29:376-97.
- Colella P, Cotugno1 G, Auricchio A. Ocular gene therapy: Current progress and future prospects. Trends Mol Med 2008;15:23-31.
- Reich SJ, Bennett J. Gene therapy for ocular neovascularization: A cure in sight. Curr Opin Genet Dev 2003,13:317-22.
- Hao J, Li SK, Kaob WW, Liu CY. Gene delivery to cornea. Brain Res Bulletin 2010;81:256-61.
- Murata M, Takanami T, Shimizu S, Kubota Y, Horiuchi S, Habano W, et al. Inhibition of ocular angiogenesis by diced small interfering RNAs (siRNAs) specific to vascular endothelial growth factor (VEGF). Curr Eye Res 2006;31:171-80.
- Bloquel C, Bourges JL, Touchard E, Berdugo M, BenEzra D, Behar- Cohen F. Non-viral ocular gene therapy: Potential ocular therapeutic avenues. Adv Drug Deliv Rev 2006;58:1224-42.
- Cai X, Conley S, Naash M. Nanoparticle applications in ocular gene therapy. Vision Res 2008;48:319-24.
- Gaudana R, Jwala J, Boddu SH, Mitra AK. Recent Perspectives in Ocular Drug Delivery. Pharm Res 2009;26:1197-216.
- Borra´s T. Recent developments in ocular gene therapy. Exp Eye Res 2003;76:643-52.
- Stein CA, Cheng YC. Antisense oligonucleotides as therapeutic agents-is the bullet really magical Science 1993;261:1004-12.
- Leonetti JP, Degols G, Clarenc JP, Mechti N, Lebleu B. Cell delivery and mechanisms of action of antisense oligonucleotides. Prog Nucleic Acid Res Mol Biol 1993;44:143-66
- Agrawal S. Importance of nucleotide sequence and chemical modifications of antisense oligonucleotides. Biochim Biophys Acta 1999;1489:53-68.
- Kim B, Tang Q, Biswas PS, Xu J, Schiffelers RM, Xie FY, et al. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: Therapeutic strategy for herpetic stromal keratitis. Am J Pathol 2004;165:2177-85.
- Robinson GS, Pierce EA, Rook SL, Foley E, Webb R, Smith LE. Oligodeoxynucleotides inhibit retinal neovascularization in amurinemodel of proliferative retinopathy. Proc Natl Acad Sci USA 1996;93:4851-56.
- Garrett KL, Shen WY, Rakoczy PE. In vivo use of oligonucleotides to inhibit choroidal neovascularisation in the eye. J Gene Med 2001;3: 373-83.
- Poddevin B, Meguenni S, Elias I, Vasseur M, Blumenfeld M. Improved anti-herpes simplex virus type 1 activity of a phosphodiester antisense oligonucleotide containing a 3′-terminal hairpin-like structure. Antisense Res Dev 1994;4:147-54.
- Proske D, Blank M, Buhmann R, Resch A. Aptamers-basic research, drug development, and clinical applications. Appl Microbiol Biotechnol 2005;69:367-74.
- Nimjee SM, Rusconi CP, Sullenger BA. Aptamers: An emerging classof therapeutics. Annu Rev Med 2005;56:555-83.
- Ng EW, Shima DT, Calias P, Cunningham Jr ET, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov 2006;5:123-32.
- Burmeister PE, Lewis SD, Silva RF, Preiss JR, Horwitz LR, Pendergrast PS, et al. Direct in vitro selection of a 2′-O-methyl aptamer to VEGF. Chem Biol 2005;12:25-3.
- Berdugo M, Valamanesh F, Andrieu C, Klein C, Benezra D, Courtois Y, et al. Delivery of antisense oligonucleotide to the cornea by iontophoresis, Antisense Nucleic Acid Drug Dev 2003;13:107-14.
- Bochot A, Mashhour B, Puisieux F, Couvreur P, Fattal E. Comparison of the ocular distribution of a model oligonucleotide after topical instillation in rabbits of conventional and new dosage forms. J Drug Target 1998;6:309-13.
- Cordeiro MF, Mead A, Ali RR, Alexander RA, Murray S, Chen C, et al. Novel antisense oligonucleotides targeting TGFβ inhibit in vivo scarring and improve surgical outcome. Gene Ther 2003;10:59-71.
- Chaum E, Hatton MP, Stein G. Polyplex-mediated gene transfer into human retinal pigment epithelial cells in vitro. J Cell Biochem 1999;76:153-60.
- Liao HW, Yau KW. In vivo gene delivery in the retina using polyethylenimine. BioTechniques 2007;42:285-8.
- Masuda I, Matsuo T, Yasuda T, Matsuo N. Gene transfer with liposomes to the intraocular tissues by different routes of administration. Invest Ophthalmol Visual Sci 1996;37:1914-20.
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med 2008;358:2231-9.
- Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA 2008;105:15112-7.
- Bressler SB. Introduction: Understanding the role of angiogenesis and antiangiogenic agents in age-related macular degeneration. Ophthalmology 2009;116:S1-S7.
- Shuler Jr RK, Dioguardi PK, Henjy C, Nickerson JM, Cruysberg LP, Edelhauser HF. Scleral permeability of a small, single-stranded oligonucleotide. J Ocular Pharmacol Ther 2004;20:159-68.
- Farjo R, Skaggs J, Quiambao AB, Cooper MJ, Naash MI. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS One 2006;1:e38.
- Kawakami S, Harada A, Sakanaka K, Nishida K, Nakamura J, Sakaeda T, et al. In vivo gene transfection via intravitreal injection of cationic liposome/plasmid DNA complexes in rabbits. Int J Pharm 2004;278:255-62.
- Philip L, Felgner T, Thomas R, Gadek, Marilyn H, Roman R, et al. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA 1987;84:7413-7.
- Chen Y, Moiseyev G, Takahashi Y, Ma JX. RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65-/- mice. Invest Ophthalmol Visual Sci 2006;47:1177-84.
- Liaw J, Chang SF, Hsiao FC. In vivo gene delivery into ocular tissues by eye drops of poly(ethylene oxide)-poly (propylene oxide)- poly(ethylene oxide) (PEO-PPO-PEO) polymeric micelles. Gene Ther 2001;8:999-1004.
- Ruponen M, Rönkkö S, Honkakoski P, Pelkonen J, Tammi M, Urtti Extracellular glycosaminoglycans modify cellular trafficking of lipoplexes and polyplexes. J Biol Chem 2001;276:33875-80.
- Bejjani RA, BenEzra D, Cohen H, Rieger J, Andrieu C, Jeanny JC, et al. Nanoparticles for gene delivery to retinal pigment epithelial cells. Mol Vis 2005;11:124-32.
- Ziady AG, Gedeon CR, Muhammad O, Stillwell V, Oette SM, Fink TL, et al. Minimal toxicity of stabilized compacted DNA nanoparticles in the murine lung. Mol Ther 2003;8:948-56.
- Vandammeand TF, Brobeck L. Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J Control Release 2005;102:23-38.
- Singh K, Mezei M. Liposomal ophthalmic drug delivery system. I.Triamcinolone acetonide. Int J Pharm 1983;16:339-44.
- Singh K, Mezei M. Liposomal ophthalmic drug delivery system. II. Dihydrostreptomycin sulfate. Int J Pharm 1984;19:263-9.
- Taniguchi K, Itakura K, Yamazawa K, Morisaki S, Hayashi Y, Yamada. Efficacy of a liposome preparation of anti-inflammatory steroid as an ocular drug delivery system. J Pharmacobiodyn 1988;11:39-46.
- Zelphati O, Szoka FC Jr. Mechanism of oligonucleotide release from cationic liposomes. Proc Natl Acad Sci USA 1996;93:11493-8.
- Liu F, Yang J, Huang L, Liu D. New cationic lipid formulations forgene transfer. Pharm Res 1996;13:1856-60.
- Salvati A, Ciani L, Ristori S, Martini G, Masi A, Arcangeli A. Physicochemical characterization and transfection efficacy of cationic liposomes containing the pEGFP plasmid. Biophys Chem 2006;121: 21-9.
- Matsuo T, Masuda I, Yasuda T, Matsuo N. Gene transfer to the retina of rat by liposome eye drops. Biochem Biophys Res Commun 1996;219:947-50.
- Neuner-Jehle M, Berghe LV, Bonnel S, Uteza Y, Benmeziane F, Rouillot JS, et al. Ocular cell transfection with the human basic fibroblast growth factor gene delays photoreceptor cell degeneration in RCS rats. Hum Gene Ther 2000;11:1875-90.
- Yoon KC, Ahn KY, Lee JH, Chun BJ, Park SW, Seo MS, et al. Lipid-mediated delivery of brainspecific angiogenesis inhibitor 1 gene reduces corneal neovascularization in an in vivo rabbit model. Gene Ther 2005;12:617-24.
- Bochot A, Fattal E, Boutet V, Deverre JR, Jeanny JC, Chacun H, et al. Intravitreal delivery of oligonucleotides by sterically stabilized liposomes. Invest Ophthalmol Visual Sci 2002;43:253-9.
- Bochot A, Couvreur P, Fattal E. Intravitreal administration of antisense oligonucleotides: Potential of liposomal delivery. Prog Retin Eye Res 2000;19:131-47.
- Zhu C, Zhang Y, Pardridge WM. Widespread expression of an exogenous gene in the eye after intravenous administration. Invest Ophthalmol Vis Sci 2002;43:3075-80.
- Zhang Y, Schlachetzki F, Li JY, Boado RJ, Pardridge WM. Organ- specific gene expression in the rhesus monkey eye following intravenous non-viral gene transfer. Mol Vis 2003;9:465-72.
- Jani PD, Singh N, Jenkins C, Raghava S, Mo Y, Amin S, et al. Nanoparticles sustain expression of Flt intraceptors in the cornea and inhibit injury-induced corneal angiogenesis. Invest Ophthalmol Vis Sci 2007;48:2030-6.
- Abul-Hassan K, Walmsley R, Boulton M. Optimization of non-viral gene transfer to human primary retinal pigment epithelial cells. Curr Eye Res 2000;20:361-6.
- Jääskeläinen I, Peltola S, Honkakoski P, Mönkkönen J, Urtti A. A lipid carrier with a membrane active component and a small complex size are required for efficient cellular delivery of anti-sense phosphorothioate oligonucleotides. Eur J Pharm Sci 2000;10:187-93.
- Gomes dos Santos AL, Bochot A, Doyle A, Tsapis N, Siepmann J, Siepmann F, et al. Sustained release of nanosized complexes of polyethylenimine and anti-TGF-beta 2 oligonucleotide improves the outcome of glaucoma surgery. J Control Release 2006;112:369-81.
- Fuente M, Seijo B, Maria JA. Novel Hyaluronic Acid-Chitosan Nanoparticles for Ocular Gene Therapy. Invest Ophthalmol Vis Sci 2008;49:2016-24.
- del Pozo-Rodr´guez A, Delgado D, Solin´s MA, Gasco´n AR, Pedraz JL. Solid lipid nanoparticles for retinal gene therapy: Transfection and intracellular trafficking in RPE cells. Int J Pharm 2008;380:177-83.
- Sun W, Ziady AG. Real-time imaging of gene delivery and expression with DNA nanoparticle technologies. Methods Mol Biol 2009;544: 525-46.
- Liu G, Li D, Pasumarthy MK, Kowalczyk TH, Gedeon CR, Hyatt SL, et al. Nanoparticles of compacted DNA transfect postmitotic cells. J Biol Chem 2003;278:32578-86.
- Parker RS, Cashman SM, Singh RK. A poly(ethylene) glycolylated peptide for ocular delivery compacts DNA into nanoparticles for gene delivery to post-mitotic tissues in vivo. J Gene Med 2010;12:86-96.
- Johnson LN, Cashman SM, Kumar-Singh R. Cell-penetrating peptide for enhanced delivery of nucleic acids and drugs to ocular tissues including retina and cornea. Mol Ther 2008;16:107-14.
- Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, et al. A new class of polymers: Starburst-dendritic macromolecules. Polym J 1985;17:117-32.
- Männistö M, Vanderkerken S, Toncheva V, Elomaa M, Ruponen M, Schacht E, et al. Structure-activity relationships of poly(L-lysines): Effects of pegylation and molecular shape on physicochemical and biological properties in gene delivery. J Control Release 2002;83: 169-82.
- Marano RJ, Wimmer N, Kearns PS, Thomas BG, Toth I, Brankov M, et al. Inhibition of in vitro VEGF expression and choroidal neovascularization by synthetic dendrimer peptide mediated delivery of a sense oligonucleotide. Exp Eye Res 2004;79:525-35.
- Marano RJ, Toth I, Wimmer N, Brankov M, Rakoczy PE. Dendrimer delivery of an anti-VEGF oligonucleotide into the eye: A long-term study into inhibition of laser-induced CNV, distribution, uptake and toxicity. Gene ther 2005;12:1544-50.
- Shaunak S, Thomas S, Gianasi E, Godwin A, Jones E, Teo I, et al. Polyvalent dendrimer glucosamine conjugates prevent scar tissue formation. Nat Biotechnol 2004;22:977-84.
- Thakor D, Spigelman I, Tabata Y, Nishimura I. Subcutaneous peripheral injection of cationized gelatin/DNA polyplexes as a platform for non- viral gene transfer to sensory neurons. Mol Ther 2007;15:2124-31.
- Nishiyama N, Iriyama A, Jang WD, Miyata K, Itaka K, Inoue Y, et al. Light-induced gene transfer from packaged DNA enveloped in a dendrimeric photosensitizer. Nat Mater 2005;4:934-41.
- Roy S, Zhang K, Roth T, Vinogradov S, Kao RS, Kabanov A. Reduction of fibronectin expression by intravitreal administration of antisense oligonucleotides. Nat Biotechnol 1999;17:476-9.
- Mannermaa E, Rönkkö S, Ruponen M, Reinisalo M, Urtti A. Long-lasting secretion of transgene product from differentiated and filter-grown retinal pigment epithelial cells after nonviral gene transfer. Curr Eye Res 2005;30:345-53.
- Peeters L, Sanders NN, Jones A, Demeester J, De Smedt SC. Post-pegylated lipoplexes are promising vehicles for gene delivery in RPE cells. J Control Release 2007;121:208-17.
- Otsuji T, Ogata N, Takahashi K, Matsushima M, Uyama M, Kaneda Y. In vivo gene transfer into choroidal neovascularization by the HVJ liposome method. Graefes Arch Clin Exp Ophthalmol 2000;238:191-9.
- Carrasquillo KG, Ricker JA, Rigas IK, Miller JW, Gragoudas ES, Adamis AP. Controlled delivery of the anti-VEGF aptamers EYE001 with poly(lactic-co-glycolic)acid microspheres. Investigative Ophthalmol Visual Sci 2003;44:290-9.
- Klausner EA, Zhang Z, Chapman RL, Multack RF, Volin MV. Ultrapure chitosan oligomers as carriers for corneal gene transfer. Biomaterials 2010;31:1814-20.
- Duan J, Zhang Y, Chen W, Shen C, Liao M, Pan Y, et al. Cationic Polybutyl Cyanoacrylate Nanoparticles for DNA Delivery. J BiomedBiotechnol 2009;2009:149254.