- *Corresponding Author:
- L. Shi
Department of Endocrinology, General Hospital of Northern Theater Command, Shenyang, Liaoning 110016, China
E-mail: 13309889463@163.com
| Date of Received | 24 July 2021 |
| Date of Revision | 04 October 2022 |
| Date of Acceptance | 17 March 2023 |
| Indian J Pharm Sci 2023;85(2):419-425 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect of naringin on the fibrosis of transforming growth factor-beta 1 induced human embryonic lung fibroblasts MRC-5 and its potential mechanism. MRC-5 cells were treated with different concentrations of naringin, 5 ng/ml transforming growth factor-beta 1 and 2 μmol/l nuclear factor kappa B activator phorbol myristate acetate. The experiment was divided into blank group, transforming growth factor-beta 1 group, transforming growth factor-beta 1+naringin group and transforming growth factor-beta 1+naringin+phorbol myristate acetate group. 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide, flow cytometry and scratch experiments were used to analyze cell viability, cell cycle distribution and migration ability. Western blot was used to examine protein levels. With the increase of naringin concentration, MRC-5 cell viability was gradually decreased. Compared with blank group, MRC-5 cell viability, cell cycle, migration rate, alpha-smooth muscle actin, collagen I alpha 1, fibronectin, phosphorylatednuclear factor kappa B p65 and phosphorylated-nuclear factor-kappa B inhibitor alpha expression in transforming growth factor-beta 1 group were increased. Naringin inhibited transforming growth factorbeta 1 induced MRC-5 cell proliferation, migration and fibrosis through inactivating nuclear factor kappa B pathway. Besides, nuclear factor kappa B activator phorbol myristate acetate could reverse naringinmediated the inhibition on transforming growth factor-beta 1 induced MRC-5 cell fibrosis compared with transforming growth factor-beta 1+naringin group. Naringin reduced transforming growth factor-beta 1-induced MRC-5 cell fibrosis by inhibiting nuclear factor kappa B pathway.
Keywords
Naringin, nuclear factor kappa B pathway, pulmonary fibrosis, arthritis, streptomycin
Idiopathic Pulmonary Fibrosis (IPF) is a disease characterized by the inflammatory cell infiltration, activated fibroblasts proliferation, Extracellular Matrix (ECM) deposition, lung tissue structural change and lung dysfunction[1,2]. Due to limited treatment options, IPF has a very high incidence and mortality[3,4]. At present, only a few anti-fibrosis drugs are available for clinical use[5]. Most drugs have adverse reactions and tolerance limitations, resulting in unsatisfactory therapeutic effects for IPF patients[6]. Therefore, the development of new drugs for IPF is critical.
Transforming Growth Factor-Beta 1 (TGF-β1) is a key factor in inducing pulmonary fibrosis, which activates lung fibroblasts to regulate its proliferation and migration[7,8]. Currently, TGF-β1 induced human embryonic lung fibroblasts MRC-5 can be used to construct IPF cell models[9,10]. This provides convenience for us to carry out related research on IPF.
Naringin is a flavonoid extracted from natural plants and has special pharmacological properties, such as anti-arthritis, anti-cancer and antioxidant activities[11,12]. Recently, many studies have shown that pulmonary fibrosis occurrence is related to the activity of Nuclear Factor Kappa B (NF-κB) signal transduction pathway[13,14]. Studies have reported that naringin can play anti-inflammatory and antioxidant roles via suppressing NF-κB pathway[15]. Recently, naringin has been found to reduce renal fibrosis, which has similar pathological characteristics to pulmonary fibrosis[16]. In addition, naringin had been confirmed to have protective effect on pulmonary fibrosis in rats[17]. However, whether naringin inhibits pulmonary fibrosis via mediating NF-κB pathway remains unclear. Therefore, we explored naringin roles in TGF-β1 treated MRC-5 cell fibrosis and the corresponding molecular mechanism.
Materials and Methods
Cell culture and grouping:
MRC-5 cells (Chinese Academy of Sciences Cell Bank, Shanghai, China) were cultured in Dulbecco's Modified Eagle Medium (DMEM) (Gibco, Grand Island, New York, United States of America (USA)) containing 10 % Fetal Bovine Serum (FBS) (Gibco) and 1 % penicillin/streptomycin (Gibco) at 37° with 5 % Carbon dioxide (CO2). Naringin (Yuanye Biotech, Shanghai, China) and NF-κB activator Phorbol Myristate Acetate (PMA) (InvivoGen, Hong Kong, China) were dissolved in Dimethyl Sulfoxide (DMSO) and TGF-β1 (PeproTech, Rockford, Illinois, USA) was dissolved in doubledistilled Water (ddH2O). Final concentration was prepared by diluting the reserve solution in DMEM. In all experiments, the final concentration of naringin was adjusted to 1.25, 2.5, 5, 10, 20 and 40 μmol/l for MRC-5 cell treatment for 48 h, respectively. MRC-5 cells treated with carrier solvent (<0.1 % DMSO) were labeled as blank group, with 5 ng/ml TGF-β1 were labeled as TGF-β1 group, with 5 ng/ml TGF-β1 and 5 μmol/l naringin were labeled as TGF- β1+naringin group, with 5 ng/ml TGF-β1, 5 μmol/l naringin and 2 μmol/l PMA were classified as TGF- β1+naringin+PMA group.
3-(4,5-Dimethylthiazol-2-yl)-2,5 Diphenyl Tetrazolium Bromide (MTT) assay:
Cells cultured in 96-well plates were incubated with MTT solution (Sigma-Aldrich, St. Louis, Missouri, USA) and treated with DMSO to dissolve the crystals. The absorbance was examined by micro plate reader to analyze cell viability.
Flow cytometry:
Cells in each group were treated for 48 h and the supernatant in 6-well plates was discarded to prepare single-cell suspension. Cell suspension was fixed with 75 % ethanol and then incubated with Ribonuclease A (RNase A). Cell cycle distribution was analyzed using flow cytometry after Propidium Iodide (PI) (KeyGen BioTech, Jiangsu, China) staining.
Scratch assay:
Cells were inoculated into 12-well plates until fully fused. The cell monolayer was passed through each well with the tip of a 10 μl pipetting gun and then washed with Phosphate Buffer Saline (PBS). The scratch width was recorded under the microscope (0 h). Then, cells were hatched with serum-free DMEM for 24 h and images were collected. Cell relative migration rate was calculated.
Western Blot (WB) analysis:
MRC-5 cells in each group were treated with Radioimmunoprecipitation Assay (RIPA) lysis buffer (Beyotime, Shanghai, China) to isolate total protein. Protein was quantified by Bicinchoninic Acid (BCA) kit (Beyotime) and transferred to Polyvinylidene Difluoride (PVDF) membranes after isolated by 12 % Sodium Dodecyl Sulphate- Polyacrylamide Gel Electrophoresis (SDS-PAGE) gel. Membrane was incubated with primary antibody (alpha-Smooth Muscle Actin (α-SMA), Collagen I Alpha 1 (COL1A1), Fibronectin (FN), NF-κB p65, p-NF-κB p65, IκBα, and p-IκBα) and Horseradish Peroxidase (HRP)-labeled Immunoglobulin G (IgG) antibody (Abcam, Cambridge, Massachusetts, USA). The signal was detected using an Enhanced Chemiluminescent (ECL) kit (Beyotime) and band strength was analyzed using Image Lab™ software. Protein levels were normalized by Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH).
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 23.0 software was used and measurement data were expressed as x̄ ±s. Analysis of Variance (ANOVA) was used to compare the differences between groups and Student–Newman–Keuls-q (SNK-q) test was used to compare pair-to-group differences. p<0.05 meant the difference was statistically significant.
Results and Discussion
The effect of naringin on MRC-5 cell viability was measured by MTT assay. Compared with 0 μmol/l group, MRC-5 cell viability was reduced in 10, 20 and 40 μmol/l groups, suggesting that naringin had no obvious toxic effect on MRC-5 cells when the concentration was lower than 20 μmol/l (Table 1). Therefore, 5 μmol/l naringin was selected for intervention in subsequent experiments.
| Naringin (μmol/l) | Cell viability (%) |
|---|---|
| 0 | 100.00±7.86 |
| 1.25 | 98.22±8.02 |
| 2.5 | 95.28±7.49 |
| 5 | 89.75±7.86 |
| 10 | 80.28±7.34* |
| 20 | 68.94±6.42* |
| 40 | 51.18±5.31* |
| F | 55.281 |
| p | 0.000 |
Note: Compared to 0 μmol/l group, *p<0.05
Table 1: Effects of Naringin at Different Concentrations on MRC-5 Cell Viability (x̄±s, n=9)
Besides, we assessed the effect of naringin on TGF- β1-induced MRC-5 cell viability and the results were shown as Table 2. MRC-5 cell viability was higher in TGF-β1 group than in blank group and was lower in TGF-β1+naringin group than in TGF-β1 group. MRC-5 cell viability in TGF-β1+naringin+PMA group was remarkably enhanced compared with TGF-β1+naringin group.
| Group | Cell viability (%) |
|---|---|
| Blank | 100.00±5.39 |
| TGF-β1 | 137.25±7.07* |
| TGF-β1+naringin | 91.38±5.16& |
| TGF-β1+naringin+PMA | 129.84±6.81# |
| F | 118.202 |
| p | 0.000 |
Note: Compared to blank group, *p<0.05; compared to TGF-β1 group, &p<0.05 and compared to TGF-β1+naringin group, #p<0.05
Table 2: Effects of Naringin on MRC-5 Cell Viability Under TGF-β1 Treatment (x̄±s, n=9)
Flow cytometry was used to detect the effect of naringin on cell cycle distribution and the results were shown in Table 3. The results indicated that compared with blank group, cell ratio was significantly reduced in Gap/Growth 1 (G0/G1) phase, while was enhanced in Synthesis (S) phase and Growth 2 (G2)/Mitotic (M) phase in TGF-β1 group. Cell ratio in G0/G1 phase was increased, while was decreased in S phase and G2/M phase in TGF-β1+naringin group compared with TGF-β1 group. In addition, cell ratio in G0/G1 phase was decreased, while was increased in S phase and G2/M phase in TGF-β1+naringin+PMA group compared with TGF-β1+naringin group.
| Group | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|
| Blank | 62.28±6.03 | 26.07±2.12 | 11.65±1.07 |
| TGF-β1 | 45.68±4.41* | 31.77±2.68* | 22.55±2.15* |
| TGF-β1+naringin | 58.77±5.72& | 26.98±2.11& | 14.25±1.56& |
| TGF-β1+naringin+PMA | 48.04±4.82# | 30.87±2.59# | 21.09±2.09# |
| F | 21.011 | 12.512 | 76.468 |
| p | 0.013 | 0.000 | 0.000 |
Note: Compared to blank group, *p<0.05; compared to TGF-β1 group, &p<0.05 and compared to TGF-β1+naringin group, #p<0.05
Table 3: Effects of Naringin on the Cell Cycle Distribution of MRC-5 Cells Under TGF-β1 Treatment (x̄±s, n=9)
Then, we evaluated the effect of naringin on cell migration ability using scratch assay and the results were listed as fig. 1 and Table 4. The migration ability of MRC-5 cells in TGF-β1 group was increased compared with blank group. Moreover, the migration ability of MRC-5 cells in TGF-β1+naringin group was lower than in TGF-β1 group. Besides, the migration ability of MRC-5 cells in TGF-β1+naringin+PMA group was remarkably enhanced compared to TGF- β1+naringin group.
| Group | Relative migration rate (%) |
|---|---|
| Blank | 20.25±2.07 |
| TGF-β1 | 39.81±3.88* |
| TGF-β1+naringin | 24.36±2.19& |
| TGF-β1+naringin+PMA | 36.47±3.53# |
| F | 86.819 |
| p | 0.000 |
Note: Compared to blank group, *p<0.05; compared to TGF-β1 group, &p<0.05 and compared to TGF-β1+naringin group, #p<0.05
Table 4: Effect of Naringin on TGF-β1 Induced MRC-5 Cell Migration (x̄±s, n=9)
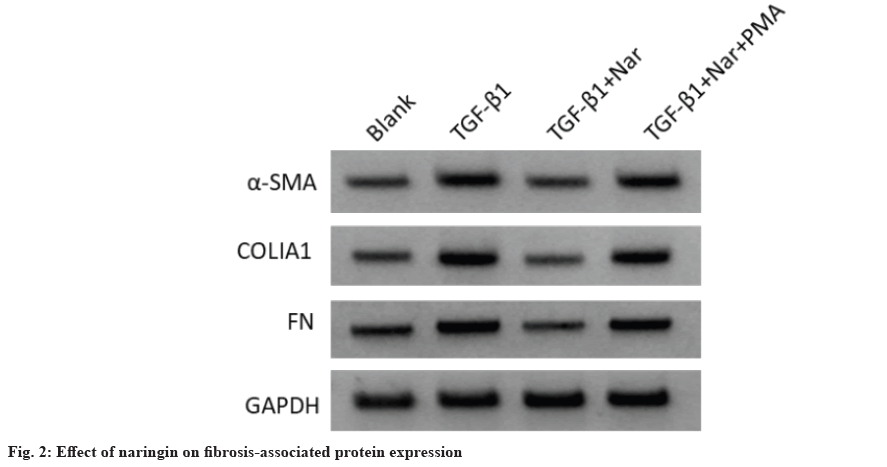
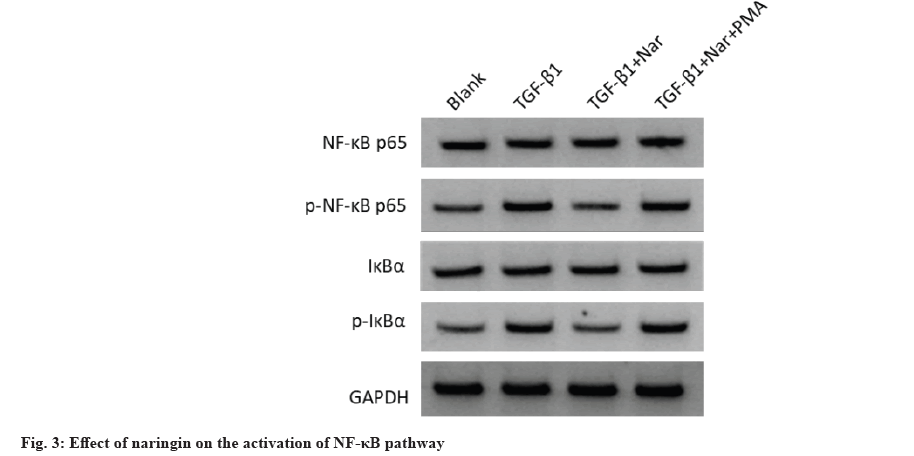
In addition, WB analysis was used to assess the effect of naringin on fibrosis-associated protein expression (fig. 2 and Table 5). The α-SMA, COLIA1 and FN levels were increased in TGF-β1 group compared to the blank group, while decreased in TGF- β1+naringin group compared to the TGF-β1 group. Also, these levels in TGF-β1+naringin+PMA group were remarkably higher than in TGF-β1+naringin group. Additionally, we measured NF-κB pathwayrelated protein levels to detect the effect of naringin on the activation of NF-κB pathway (fig. 3 and Table 6). The results showed that p-NF-κB p65 and p-IκBα levels were enhanced in TGF-β1 group compared with the blank group, while decreased in TGF-β1+naringin group compared with the TGF-β1 group. Also, p-NF-κB p65 and p-IκBα levels in TGF-β1+naringin+PMA group were increased compared with TGF-β1+naringin group. There was no significant difference in NF-κB p65 and IκBα expression among all groups.
| Group | α-SMA | COLIA1 | FN |
|---|---|---|---|
| Blank | 0.62±0.06 | 0.21±0.02 | 0.36±0.03 |
| TGF-β1 | 0.93±0.09* | 0.51±0.05* | 0.62±0.06* |
| TGF-β1+naringin | 0.66±0.07& | 0.25±0.03& | 0.39±0.04& |
| TGF-β1+naringin+PMA | 0.89±0.08# | 0.47±0.04# | 0.58±0.05# |
| F | 38.87 | 153.778 | 72.384 |
| p | 0.000 | 0.000 | 0.000 |
Note: Compared to blank group, *p<0.05; compared to TGF-β1 group, &p<0.05 and compared to TGF-β1+naringin group, #p<0.05
Table 5: Effect of Naringin on the Levels of Fibrosis-Associated Protein in TGF-β1 Induced MRC-5 Cells (x̄±s, n=9)
| Group | NF-κB p65 | p-NF-κB p65 | IκBα | p-IκBα |
|---|---|---|---|---|
| Blank | 0.87±0.08 | 0.57±0.05 | 0.61±0.06 | 0.46±0.04 |
| TGF-β1 | 0.84±0.07 | 0.96±0.09* | 0.62±0.06 | 0.83±0.08* |
| TGF-β1+naringin | 0.86±0.08 | 0.65±0.06& | 0.62±0.05 | 0.49±0.05& |
| TGF-β1+naringin+PMA | 0.89±0.09 | 0.93±0.09# | 0.60±0.05 | 0.79±0.07# |
| F | 0.605 | 62.354 | 0.271 | 88.422 |
| p | 0.617 | 0.000 | 0.846 | 0.000 |
Note: Compared to blank group, *p<0.05; compared to TGF-β1 group, &p<0.05 and compared to TGF-β1+naringin group, #p<0.05
Table 6: Effect of Naringin on the Levels of NF-κB Related Protein in TGF-β1 Induced MRC-5 Cells (x̄±s, n=9)
IPF is a chronic disease occurring in human lungs, which poses a great threat to the life of patients[18]. Naringin is a bioflavonoid commonly found especially in citrus fruits, which has a variety of properties. Previous studies have shown that naringin has antifibrotic properties in many fibrosis-related diseases, including pulmonary fibrosis, renal fibrosis and hepatic fibrosis[16,19,20]. TGF-β1 is a vital factor in pulmonary fibrosis[21,22]. Here, TGF-β1 induced MRC-5 cells were used to establish IPF cell model. Our data showed that naringin could inhibit MRC-5 cell proliferation. Meanwhile, naringin could also suppress the proliferation and cell cycle process of TGF-β1 induced MRC-5 cells. Additionally, scratch assay data showed that naringin attenuated TGF-β1 induced migration in MRC-5 cells.
TGF-β1 can stimulate mesenchymal cells to produce a large amount of ECM, leading to fibrosis development[23]. Meanwhile, TGF-β1 stimulates fibroblasts toward FN and enhances fibroblastmediated ECM contraction, resulting in an in vitro model of pulmonary fibrosis. FN released from lung fibroblasts depends on chemotaxis and collagen gel contraction of lung fibroblasts[24]. WB analysis showed that TGF-β1 increased α-SMA and FN levels in MRC-5 cells, while naringin effectively decreased α-SMA and FN levels. Study has highlighted that collagen is the main component of synthesized ECM in pulmonary fibrosis[25]. Building on previous studies, we assessed whether naringin inhibited COLIA1 production in TGF-β1-stimulated MRC- 5 cells and found that naringin inhibited COLIA1 protein level. The above data indicate that naringin may play an antifibrotic role by mediating TGF-β1 induced ECM synthesis in fibroblasts.
Recently, TGF-β1 has been found to activate downstream Suppressor of Mothers against Decapentaplegic (SMAD) pathways, including NF- κB and Phosphatidylinositol 3 Kinase (PI3K)/Protein Kinase B (AKT)[26,27]. NF-κB pathway is a vital intracellular signaling pathway for cell growth and protein synthesis[28]. Abnormal activation of NF-κB pathway is also critical in fibrosis-related diseases[29]. Naringin could reduce the Matrix Metalloproteinase (MMP) catabolism and inflammation by reducing NF-κB and p53 pathway expression[30]. However, it has not been reported whether naringin mediates pulmonary fibrosis via the NF-κB pathway. Our study confirmed that TGF-β1 could lead to the activation of NF-κB pathway and increasing of p-NF-κB p65 and p-IκBα protein expression in MRC-5 cells. Naringin treatment inhibited NF-κB pathway and reduced fibrosis marker levels, speculating that naringin could inhibit NF-κB pathway to reduce fibrosis. To verify this hypothesis, we conducted experiments with PMA, a specific activator of NF-κB pathway, which further confirmed the hypothesis. These results reveal that NF-κB pathway may play a key role in naringin induced pulmonary fibrosis inhibition.
In conclusion, our study demonstrated that naringin restrained TGF-β1 induced MRC-5 cell proliferation, migration and fibrosis via inhibiting NF-κB pathway. The results of this experiment indicate that naringin may exert antifibrotic effects and can be developed as a new therapeutic drug for IPF.
Author’s contributions:
Yuan Kong and Fei Sun have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Hewlett JC, Kropski JA, Blackwell TS. Idiopathic pulmonary fibrosis: Epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol 2018;71:112-27.
[Crossref] [Google Scholar] [PubMed]
- Glass DS, Grossfeld D, Renna HA, Agarwala P, Spiegler P, Kasselman LJ, et al. Idiopathic pulmonary fibrosis: Molecular mechanisms and potential treatment approaches. Respir Investig 2020;58(5):320-35.
[Crossref] [Google Scholar] [PubMed]
- Petnak T, Lertjitbanjong P, Thongprayoon C, Moua T. Impact of antifibrotic therapy on mortality and acute exacerbation in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Chest 2021;160(5):1751-63.
[Crossref] [Google Scholar] [PubMed]
- Barratt SL, Creamer A, Hayton C, Chaudhuri N. Idiopathic pulmonary fibrosis (IPF): An overview. J Clin Med 2018;7(8):201.
[Crossref] [Google Scholar] [PubMed]
- Maher TM, Strek ME. Antifibrotic therapy for idiopathic pulmonary fibrosis: Time to treat. Respir Res 2019;20(1):205.
[Crossref] [Google Scholar] [PubMed]
- Saito S, Alkhatib A, Kolls JK, Kondoh Y, Lasky JA. Pharmacotherapy and adjunctive treatment for idiopathic pulmonary fibrosis (IPF). J Thorac Dis 201;11(14):S1740-54.
[Crossref] [Google Scholar] [PubMed]
- Ye Z, Hu Y. TGF-β1: Gentlemanly orchestrator in idiopathic pulmonary fibrosis. Int J Mol Med 2021;48(1):132.
[Crossref] [Google Scholar] [PubMed]
- Boutanquoi PM, Burgy O, Beltramo G, Bellaye PS, Dondaine L, Marcion G, et al. TRIM33 prevents pulmonary fibrosis by impairing TGF-β1 signalling. Eur Respir J 2020;55(6):1901346.
[Crossref] [Google Scholar] [PubMed]
- Gong H, Zheng C, Lyu X, Dong L, Tan S, Zhang X. Inhibition of Sirt2 alleviates fibroblasts activation and pulmonary fibrosis via Smad2/3 pathway. Front Pharmacol 2021;12:756131.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Zhang W, Zhang R, Xia Y. Knockdown of FBLN2 suppresses TGF-β1-induced MRC-5 cell migration and fibrosis by down regulating VTN. Tissue Cell 2023;81:102005.
[Crossref] [Google Scholar] [PubMed]
- Aihaiti Y, Song Cai Y, Tuerhong X, Ni Yang Y, Ma Y, Shi Zheng H, et al. Therapeutic effects of naringin in rheumatoid arthritis: Network pharmacology and experimental validation. Front Pharmacol 2021;12:672054.
[Crossref] [Google Scholar] [PubMed]
- Zhao H, Liu M, Liu H, Suo R, Lu C. Naringin protects endothelial cells from apoptosis and inflammation by regulating the hippo-yap pathway. Biosci Rep 2020;40(3):BSR20193431.
[Crossref] [Google Scholar] [PubMed]
- Zhu M, An Y, Zhang X, Wang Z, Duan H. Experimental pulmonary fibrosis was suppressed by microRNA-506 through NF-kappa-mediated apoptosis and inflammation. Cell Tissue Res 2019;378(2):255-65.
[Crossref] [Google Scholar] [PubMed]
- Liu B, Rong Y, Sun D, Li W, Chen H, Cao B, et al. Costunolide inhibits pulmonary fibrosis via regulating NF-kB and TGF-β1/Smad2/Nrf2-NOX4 signaling pathways. Biochem Biophys Res Commun 2019;510(2):329-33.
[Crossref] [Google Scholar] [PubMed]
- Syed AA, Reza MI, Shafiq M, Kumariya S, Singh P, Husain A, et al. Naringin ameliorates type 2 diabetes mellitus-induced steatohepatitis by inhibiting RAGE/NF-κB mediated mitochondrial apoptosis. Life Sci 2020;257:118118.
[Crossref] [Google Scholar] [PubMed]
- Wang R, Wu G, Dai T, Lang Y, Chi Z, Yang S, et al. Naringin attenuates renal interstitial fibrosis by regulating the TGF-β/Smad signaling pathway and inflammation. Exp Ther Med 2021;21(1):66.
[Crossref] [Google Scholar] [PubMed]
- Turgut NH, Kara H, Elagoz S, Deveci K, Gungor H, Arslanbas E. The protective effect of naringin against bleomycin-induced pulmonary fibrosis in Wistar rats. Pulm Med 2016;2016:7601393.
[Crossref] [Google Scholar] [PubMed]
- Yu M, Liu Y, Xu D, Zhang R, Lan L, Xu H. Prediction of the development of pulmonary fibrosis using serial thin-section CT and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J Radiol 2020;21(6):746-55.
[Crossref] [Google Scholar] [PubMed]
- Zhou C, Lai Y, Huang P, Xie L, Lin H, Zhou Z, et al. Naringin attenuates alcoholic liver injury by reducing lipid accumulation and oxidative stress. Life Sci 2019;216:305-12.
[Crossref] [Google Scholar] [PubMed]
- Wei Y, Sun L, Liu C, Li L. Naringin regulates endoplasmic reticulum stress and mitophagy through the ATF3/PINK1 signaling axis to alleviate pulmonary fibrosis. Naunyn Schmiedeberg's Arch Pharmacol 2023:1-5.
[Crossref] [Google Scholar] [PubMed]
- Jin J, Togo S, Kadoya K, Tulafu M, Namba Y, Iwai M, et al. Pirfenidone attenuates lung fibrotic fibroblast responses to transforming growth factor-β1. Respir Res 2019;20(1):119.
[Crossref] [Google Scholar] [PubMed]
- Kim MS, Baek AR, Lee JH, Jang AS, Kim DJ, Chin SS, et al. IL-37 attenuates lung fibrosis by inducing autophagy and regulating TGF-β1 production in mice. J Immunol 2019;203(8):2265-75.
[Crossref] [Google Scholar] [PubMed]
- Li S, Liu J, Tan J, Li L, Kaltreider MJ, Zhao J, et al. Inhibition of Raf1 ameliorates bleomycin-induced pulmonary fibrosis through attenuation of TGF-β1 signaling. Am J Physiol Lung Cell Mol Physiol 2018;315(2):L241-7.
[Crossref] [Google Scholar] [PubMed]
- Hu X, Xu Q, Wan H, Hu Y, Xing S, Yang H, et al. PI3K-Akt-mTOR/PFKFB3 pathway mediated lung fibroblast aerobic glycolysis and collagen synthesis in lipopolysaccharide-induced pulmonary fibrosis. Lab Invest 2020;100(6):801-11.
- Deng Z, Fear MW, Choi YS, Wood FM, Allahham A, Mutsaers SE, et al. The extracellular matrix and mechanotransduction in pulmonary fibrosis. Int J Biochem Cell Biol 2020;126:105802.
[Crossref] [Google Scholar] [PubMed]
- You W, Hong Y, He H, Huang X, Tao W, Liang X, et al. TGF-β mediates aortic smooth muscle cell senescence in Marfan syndrome. Aging 2019;11(11):3574-84.
[Crossref] [Google Scholar] [PubMed]
- Zhang Z, Zhang X, Zhao D, Liu B, Wang B, Yu W, et al. TGF-β1 promotes the osteoinduction of human osteoblasts via the PI3K/AKT/mTOR/S6K1 signalling pathway. Mol Med Rep 2019;19(5):3505-18.
[Crossref] [Google Scholar] [PubMed]
- Kunnumakkara A, Shabnam B, Girisa S, Harsha C, Banik K, Devi TB, et al. Inflammation, NF-κB and chronic diseases: How are they linked? Crit Rev Immunol 2020;40(1):1-39.
[Crossref] [Google Scholar] [PubMed]
- Peng L, Wen L, Shi QF, Gao F, Huang B, Meng J, et al. Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-κB/NLRP3-mediated epithelial–mesenchymal transition and inflammation. Cell Death Dis 2020;11(11):978.
[Crossref] [Google Scholar] [PubMed]
- Gao G, Chang F, Zhang T, Huang X, Yu C, Hu Z, et al. Naringin protects against interleukin 1β (IL-1β)-induced human nucleus pulposus cells degeneration via downregulation nuclear factor kappa B (NF-κB) pathway and p53 expression. Med Sci Monit 2019;25:9963-72.
[Crossref] [Google Scholar] [PubMed]