- *Corresponding Author:
- A. H. M. Viswanatha swamy
Department of Pharmacology, KLE University’s College of Pharmacy, Hubli-580 031
E-mail: vmhiremath2004@yahoo.com
| Date of Submission | 21 February 2013 |
| Date of Revision | 25 September 2013 |
| Date of Acceptance | 30 September 2013 |
| Indian J Pharm Sci 2013; 75(6): 657-663 |
Abstract
This study was designed to evaluate the neuroprotective activity of ethanol extract of Pongamia pinnata stem bark in monosodium glutamate-induced neurotoxicity in rats. Neurotoxicity was induced by intraperitoneal injection of monosodium glutamate 2 g per kg body weight daily for 7 days. Ethanol extract of Pongamia pinnata stem bark (200 and 400 mg/kg) was administered orally after 1 h of monosodium glutamate treatment. Dextromethorphan (30 mg/kg, p.o.) was used as standard drug for the comparison. The degree of protection was determined by various behavioural, locomotor, muscle grip activity, lipid peroxidation and measurement of antioxidant status of glutathione, catalase and superoxide dismutase. Estimation of calcium, sodium and potassium ions in brain tissue and gamma aminobutyric acid level in serum was carried out. The histopathological study of brain tissue was also carried out. Treatment with Pongamia pinnata significantly improved monosodium glutamate-induced alteration in behavioural and locomotor activity and muscle strength. Significant decrease in lipid peroxidation and increase in glutathione, superoxide dismutase and catalase was observed in Pongamia pinnata treated group. Further, Pongamia pinnata also significantly reduced the monosodium glutamate-induced excitotoxicity by decreasing the level of Ca +2 and Na + with concomitant increase in the level of K + . Serum gamma aminobutyric acid level was also increased in Pongamia pinnata treated animals. Further, the histopathological evidence supports the neuroprotective activity of Pongamia pinnata. In conclusion, the present study suggests that the ethanol extract of stem bark of Pongamia pinnata possesses significant neuroprotective activity in albino rats.
Keywords
Pongamia pinnata, neuroprotective, monosodium glutamate, dextromethorphan
The neurodegenerative diseases are in focus due to their increasing health and socioeconomical implications. Alzheimer’s disease (AD), Parkinson’s disease (PD) and motor neuron diseases are more likely to occur with increasing age either sporadically or as a familial disorder [1]. Monosodium glutamate (MSG) is the sodium salt of the glutamic acid, a nonessential amino acid. The MSG is widely used as a flavouring agent in food industry as well as at homes and restaurants. It acts through the activation of both ionotropic and metabotropic glutamate receptor (iGluR and mGluR) found in the central nervous system (CNS). Hyperactivation of these receptors has been reported to produce excitotoxicity and neuronal death [2]. It is also known that MSG or sodium salt of glutamate exerts excitotoxicity by over activation of glutamate receptors namely The neurodegenerative diseases are in focus due to their increasing health and socioeconomical implications. Alzheimer’s disease (AD), Parkinson’s disease (PD) and motor neuron diseases are more likely to occur with increasing age either sporadically or as a familial disorder [1]. Monosodium glutamate (MSG) is the sodium salt of the glutamic acid, a nonessential amino acid. The MSG is widely used as a flavouring agent in food industry as well as at homes and restaurants. It acts through the activation of both ionotropic and metabotropic glutamate receptor (iGluR and mGluR) found in the central nervous system (CNS). Hyperactivation of these receptors has been reported to produce excitotoxicity and neuronal death [2]. It is also known that MSG or sodium salt of glutamate exerts excitotoxicity by over activation of glutamate receptors namely N-methyl D-aspartate (NMDA), α-amino-3-hydroxy- 5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors (KARs). Further, MSG is also known to affect dopaminergic neurons. The main toxicity of MSG includes neurotoxicity, disorders of endocrine glands associated with neurological activity, learning difficulties, epileptic seizures, increase in glucose levels and increased incidences of metabolic diseases [3]. One of the ways to counteract these deleterious effects of neurotoxicity produced by excess MSG is to evaluate suitable medicines of herbal origin.
In recent years much attention has been paid to traditional herbal medicines [4]. The Pongamia pinnata (PP) commonly known as Indian beech tree, karanj and pongam oil tree. Traditionally, various parts of the plant are used in the treatment of cold, cough, bronchitis, diarrhoea, dyspepsia, flatulence, beriberi, baldness, jaundice, anorexia, intestinal paralysis, fever glycosuria, urinary disorders and others [5,6]. The PP has been reported for its antibacterial and antifungal [7,8], anthelmintic [9], antidyslipidaemic and antioxidant [10], anticonvulsant [11], antiinflammatory [12], antiviral [13], antiulcer [14], antihyperglycaemic [15], antihyperammonemic[16] and antidiarrhoeal activity. Phytochemical review reveals the presence of alkaloids, glabrin, glabrosaponin, kaempferol, kanugin, karangin, pinnatin, pongamol, pongapin, saponin, β-sitosterol, tannin and flavonoids such as furanoflavonoids and chalcones [17,18]. It has been reported that the plants containing furanoflavonoids and chalcones exert neuroprotective activity in various neurodegenerative diseases [19,20]. However, there is no scientific evidence for the neuroprotective activity of PP. Hence, the present study has been undertaken to evaluate the neuroprotective activity of PP stem bark extract in MSG-induced neurotoxicity model.
Materials and Methods
Dextromethorphan (Centurion Laboratories Pvt. Ltd., Vadodara, India), MSG (Desmo Exports Limited, Mumbai, India), GABA (Sigma Chemicals, Mumbai, India), Calcium kit (Erba Diagnostics Mannheim GmbH, Solan, HP, India) and Sodium and Potassium kit (Crest Biosystems, Verna, Goa, India) were used in the present study. All the chemicals were of analytical grade. Wistar albino rats (150-200 g) of either sex were used. They were housed in clean polypropylene cages under standard conditions of temperature (25±2°) and 12 h lightdark cycle and fed with standard diet (Gold Mohur, Lipton India Ltd.) and water ad libitum. Experimental protocols were reviewed and approved by the Institutional Animal Ethics Committee. For the preparation of extract, stem bark of PP was collected from the institute’s botanical garden and was identified and authenticated by a botanist. The coarsely powdered PP stem was extracted with ethanol (70-80°) in a soxhlet extractor, concentrated in reduced pressure and stored in a desiccator (percent yield was 6.91).
Experimental protocol
A total of 30 rats were randomly divided into five groups. Each group contained six rats and received the following treatment for 7 days. Group 1 (normal) rats were administered with an aqueous suspension of 1% w/v CMC, (5 ml/kg, p.o.). MSG was prepared in normal saline for i.p. and dextromethorphan was prepared in 1% CMC for oral administration for the use in groups 2 to 5. The doses of plant extract, dextromethorphan and MSG were selected based on previous literature [21]. Group 2 rats were treated with MSG (2 g/kg, i.p.). Group 3 rats were administered with an ethanol extract of PP (200 mg/kg, p.o.) after 1 h of treatment with MSG (2 g/kg, i.p.). Group 4 rats were administered with an ethanol extract of PP (400 mg/kg, p.o.) after 1 h of treatment with MSG (2 g/kg, i.p.). Group 5 rats were administered with dextromethorphan (30 mg/kg. p.o.) after 1 h of treatment with MSG (2 g/kg, i.p.).
The general behavioural changes, if any, were observed for 30 min daily after the administration of MSG. On 8th day, the rats were evaluated for locomotor and muscle relaxant activity. Hyperactiveness and aggressiveness exhibited by the rats was recorded. Rats were scored 5 when they exhibited aggression and 0 for behaving normal behaviour. Locomotor activity was evaluated by using actophotometer. In this, animals of all the groups were placed in actophotometer for 10 min and the score was recorded. Muscle relaxant property was evaluated by using Rotarod apparatus. Animals of all the groups were placed on the rotating rods and the time of animal fall from the rotating rods was recorded.
Biochemical estimations
On 9th day, rats were sacrificed and brain was quickly isolated and stored at −20°. Blood samples were centrifuged to get clear serum for estimating GABA level. The brain tissue 10% w/v was homogenised with Tris–HCl buffer (pH 7.4) using handheld homogeniser (Remi Homogeniser, India). Homogenised samples were centrifuged at 5000 rpm for 10 min (Remi Centrifuge, India). Supernatant was used for biochemical estimations. Protein concentration was measured by Lowery et al. using bovine serum albumin as standard.
Reduced glutathione was determined by the method of Ellman [22]. Briefly, 1 ml of homogenate was added to 1 ml of 10% trichloroacetic acid (TCA) and centrifuged. One millilitre of supernatant was treated with 3 ml of phosphate buffer (pH 8.0) and 0.5 ml of Ellmans reagent. The absorbance was observed immediately at 412 nm. The amount of glutathione was calculated using the extension coefficient value of 13,600/M/cm. Lipid peroxidation was estimated in terms of thiobarbituric acid reactive species (TBARS) [23]. Briefly, 0.1 ml of the tissue homogenate was added with 2.0 ml of the TCA–TBA–HCl reagent (15% w/v TCA, 0.375% w/v TBA and 0.25 N HCl). The contents were boiled for 15 min, cooled and centrifuged at 1000 rpm for 10 min. The absorbance of clear supernatant was read at 535 nm and malondialdehyde concentration of the sample was calculated using extinction coefficient of 1.56×105/M/cm. The estimation of superoxide dismutase (SOD) was determined by the method of Kakkar et al. [24]. Briefly, 0.1 ml of the sample was mixed with 1.2 ml of sodium pyrophosphate buffer (pH 8.3, 0.052 M), 0.1 ml of 186 μM of phenazine methasulphate (PMS) and 0.3 ml of 300 μM nitro blue tetrazolium (NBT). The reaction was started by adding 0.2 ml of NADH (750 μM). The mixture was incubated at 30° for 90 s. The reaction was stopped by the addition of 0.1 ml glacial acetic acid. The reaction mixture was stirred vigorously and shaken with 4 ml of n-butanol. The mixture was allowed to stand for 10 min, centrifuged to separate the butanol layer. The colour intensity of the chromogen was measured against n-butanol at 560 nm using spectrophotometer. SOD activity was defined as its concentration required to decrease the rate of reaction by 50% in 1 min under the assay conditions. Catalase (CAT) was assayed colorimetrically as described by Sinha [25]. The reaction mixture contained 1.0 ml phosphate buffer (pH 7.0), 0.1 ml of tissue homogenate (supernatant) and 0.4 ml of 0.2 M H2O2. The reaction was stopped by the addition of 2.0 ml of dichromate-acetic acid reagent. Colour intensity was measured colorimetrically at 620 nm and CAT activity expressed as micromoles of H2O2 consumed/ min/mg protein. The concentration of electrolytes like calcium, sodium and potassium in the brain was measured by using commercial kits by Erba diagnostics (for calcium) and Crest Biosystems (for sodium and potassium). The GABA level in serum was assayed by paper chromatography as mentioned by Mishra et al. with modifications [26]. The serum sample (100 μl) was added to 1.5 ml of absolute alcohol and centrifuged at 3000g for 15 min. Fifty microlitre of the sample was used for paper chromatography using the mobile phase containing n-butanol, glacial acetic acid and water. Optical density of eluted sample was taken on a spectrophotometer at wavelength of 509 nm and compared with standard GABA solution. The GABA concentration in serum was expressed in pmol/ml.
Histopathological studies
The brain tissue was dissected out and fixed in 10% formalin. The paraffin sections were prepared and stained with haematoxylin and eosin and examined using light microscopy.
Statistical analysis
Results are expressed as the mean±SEM. Results were analysed by one-way analysis of variance (ANOVA) followed by Dunnet’s multiple comparison test using Graph Pad PRISM software. P<0.05 is considered as significant.
Results and Discussion
Overactivation of NMDA receptors by the MSG or sodium salt of glutamate exerts excitotoxicity. Over activation of NMDA along with other glutmate/ glycine receptors disturb the calcium homeostasis, which is the key mediator of glutamate-induced excitotoxic neuronal damage. Dextromethorphan, a synthetic opioid agonist functions additionally as an NMDAR antagonist. Hence in the present study, we have used dextromethorphan against MSG-induced neurotoxicity. The accumulation of high intracellular calcium together with increased level of sodium and decreased level of potassium intracellularly triggers a cascade of membrane, cytoplasmic and nuclear events leading to mitochondrial dysfunction and free radical generation resulting in neurotoxicity [27].
In the present study, MSG resulted in behavioural and physiological alterations like precipitation of aggressiveness, decreased locomotor activity and loss of muscle strength (Table 1). The administration of MSG resulted in aggressiveness and hyperactiveness in all animals. This observation may be due loss in the hippocampal region of the brain as suggested by histopathological studies. This is known to result in increased levels of glutamate, which leads to aggressive behaviour. Similar observations were also reported [27]. Treatment with PP as well as standard drug, dextromethorphan did not exhibit any effect on MSG-induced aggressiveness and hyperactiveness, which indicates that the MSG might be producing behavioural changes through receptors other than NMDA receptors.
| Treatment | Score | Score of | Time in seconds |
|---|---|---|---|
| (Aggressiveness/ | locomotor | (To fall from the | |
| Hyperactiveness) | activity | rotating rod) | |
| Normal | 0.00±0.00 | 192.8±12.49 | 110.20±8.10 |
| Control | 5.00±0.00c | 107.5±10.62c | 54.67±5.41c |
| (MSG-2 g/kg) | |||
| MSG+PP | 5.00±0.00 | 146.8±7.48* | 87.83±10.32* |
| (200 mg/kg) | |||
| MSG+PP | 4.16±0.83 | 170.3±7.93* | 105.3±8.77* |
| (400 mg/kg) | |||
| MSG+DXMP | 4.16±0.83 | 145.0±7.55* | 87.50±4.56* |
| (30 mg/kg) |
All values are expressed as mean±SEM, (n=6). *P<0.05 as compared with control and cP<0.05 (MSG v/s normal) and data was analysed by ANOVA followed by dunnet’s multiple comparison test
Table 1: Effect of pp on general behaviour, Locomotor activity and muscle strength in msg-induced neurotoxicity
The MSG significantly decreased locomotor activity compared with the normal animals (Table 1). Treatment with PP at the dose of 200 and 400 mg/kg as well as standard drug, dextromethorphan significantly reversed the MSG induced decrease in locomotor activity score. The MSG is known to impair locomotor activity by causing the damage to the dopaminergic neurons by generating free radicals. Dextromethorphan and PP might be overcoming the deleterious effect of MSG by protecting the damage by free radicals. This is evidenced in the antioxidant effects of the treatment.
Treatment with MSG significantly decreased the time to fall from the rotating rod compared with normal group (Table 1). This effect might be due to over activation of glutmate pathway leading to neurotoxocity and counter effects on the release of GABA. This is reflected in the decreased level of GABA when treated with MSG (Table 2). Treatment with PP at the both doses as well as standard drug dextromethorphan significantly increased the time to fall from the rotating rod compared with MSG treated group (Table 1). Thus, the reversal of MSG-induced loss of muscle grip activity with the treatment of PP may be attributed to its antioxidant activity, which provides protection against neurotoxicity as well as its central GABA activity.
| Treatment | Calcium | Sodium | Potassium | GABA | |||||
|---|---|---|---|---|---|---|---|---|---|
| (nM) | (mM) | (mM) | (pmol/ml) | ||||||
| Normal | 79.53±1.58 | 9.56±0.37 | 79.18±2.26 | 452.6±9.19 | |||||
| Saline+Vehicle | |||||||||
| Control | 138.1±2.78c | 14.53±0.55c | 53.03±3.36c | 253.4±7.23c | |||||
| (MSG 2g/kg) | |||||||||
| MSG+PP | 121.6±2.08* | 12.56±0.44* | 57.42±3.26 | 268.5±11.30 | |||||
| (200 mg/kg) | |||||||||
| MSG+PP | 116.2±3.62* | 11.90±0.30* | 68.45±2.88* | 300.1±10.08* | |||||
| (400 mg/kg) | |||||||||
| MSG+DXMP | 98.45±2.80* | 9.98±0.40* | 69.23±2.44* | 298.1±10.13* | |||||
| (30 mg/kg) |
All values are expressed as mean±SEM, (n=6). *P<0.05 as compared with control and cP<0.05 (MSG v/s Normal) and data was analysed by ANOVA followed by Dunnet’s multiple comparison test.
Table 2: Effect of pp on calcium, Sodium, Potassium and gaba levels in msg-induced neurotoxicity
It is a well-known fact that the reactive oxygen species are involved in the pathogenesis of MSGinduced neurotoxicity. In the present study, increase in brain tissue MDA level along with decrease in levels of GSH, SOD and CAT is considered as an indication of the oxidative stress and neuronal damage. Significant increase in the level of lipid peroxide (LPO) was found in MSG treated animals compared with normal animals. The LPO measured by thiobarbituric reactive substances (TBARS) has been generally accepted as an indicator of oxidative stress. Increase in LPO level results in loss of function and integrity of neuronal membranes, which increases nonspecific permeability to ions leading to disruption of membrane structure. The treatment with dextromethorphan and PP dose dependently decreased the LPO levels compared with MSG treated group (Table 3), which suggests a protective effect of PP against MSG-induced oxidative stress.
| Treatment | GSH | LPO (nmol | SOD (Ux/mg | CAT (Uy/mg |
|---|---|---|---|---|
| (nmol/g of | MDA/g of | protein) | protein) | |
| wet tissue) | wet tissue) | |||
| Normal | 4.12±0.29 | 3.23±0.39 | 28.47±1.62 | 52.43±1.84 |
| Saline+Vehicle | ||||
| Control | 1.40±0.17c | 10.34±0.87c | 12.98±1.49c | 29.62±1.01c |
| (MSG 2g/kg) | ||||
| MSG+PP | 2.14±0.12* | 7.61±0.55* | 20.58±1.35* | 34.58±1.03* |
| (200 mg/kg) | ||||
| MSG+PP | 2.46±0.16* | 6.16±0.63* | 26.61±1.85* | 36.17±1.32* |
| (400 mg/kg) | ||||
| MSG+DXMP | 2.13±0.15* | 4.33±0.61* | 30.8±3.06* | 41.88±1.38* |
| (30 mg/kg) |
All values are expressed as mean±sem, (n=6). *p<0.05 as compared with control and cp<0.05 (msg v/s normal) and data was analysed by anova followed by Dunnet’s multiple comparison test. Ux= one unit of activity was taken as the enzyme reaction, which gave 50% inhibition of nbt reduction in 1 min. Uy= µmole h2o2 consumed/min, Nbt= nitro blue tetrazolium, Gsh= glutathione, Lpo=lipid peroxide, Sod=superoxide dismutase , Cat=catalase
Table 3: Effect of pp on glutathione, Lipid peroxidation catalase and superoxide dismutase level in msg-induced neurotoxicity
Animals treated with MSG significantly decreased the level of GSH compared with normal animals.
The GSH is a major scavenger of free radicals in cytoplasm and an important inhibitor of free radical mediated lipid peroxidation. Excitotoxicityinduced depletion of glutathione in rat brain tissue is due to the breakdown of the glutamate-cystine antiporter system and thereby reducing the level of GSH as observed in the present study in MSG-treated animals. The GSH level was significantly increased in dextromethorphan and PP treated animals at both lower and higher doses compared with MSG treated group (Table 3) indicating the protection of the neurons against MSG-induced neurotoxicity by restoring GSH level.
The SOD and CAT levels were significantly lower in MSG treated group compared with normal group. The SOD protects the cell against free radical injury by converting O2− radical to H2O2 and prevents the formation of OH− radicals through O2− driven Fenton reaction. The H2O2 formed by SOD is removed by the CAT. Hence, if the activity of CAT is not adequate to degrade H2O2, more H2O2 is converted into toxic hydroxyl radicals. In the present study, MSG-induced oxidative stress significantly reduced the SOD and CAT activities. Significant increase in the SOD and CAT levels were found in PP treated groups at the dose of 200 and 400 mg/kg and dextromethorphan compared with MSG treated group (Table 3). Thus, the protective mechanism of PP may be due to its potent antioxidant property.
Excitotoxicity is a result of over activation of glutamate receptors, that is NMDA, AMPA and KARs leading to increased Ca+2 and Na+ levels and decreased K+ level in the cells resulting to cellular swelling and neuronal death[28]. In MSG treated group, the levels of calcium and sodium ions were significantly elevated and that of potassium ions significantly decreased compared with normal group indicating neurotoxicity. The groups treated with PP at the dose of 200 and 400 mg/kg and dextromethorphan significantly decreased the level of Ca+2 and Na+ levels and significantly increased the level of K+ compared with MSG treated group and brought the level of these ions near to normal level (Table 2). The GABA level in MSG treated group was significantly decreased compared with normal group, which may be due to inhibition of GABA synthesis or destruction of GABAergic neurons. The groups treated with PP at the dose of 400 mg/kg and dextromethorphan significantly increased the level of GABA level compared with MSG treated group (Table 2). Further, dextromethorphan was used as a standard drug in MSG-induced neurotoxicity model. The dextromethorphan is an NMDA receptor antagonist and has been reported to possess neuroprotective property [29]. Over activation of NMDA receptors is responsible for the alteration of these electrolyte levels hence, NMDA receptor antagonists may be beneficial in maintaining the levels of these ions. In the present study, both PP and dextromethorphan were found to be effective in maintaining the levels of Ca+2, Na+ and K+ in the brain indicating that the PP and dextromethorphan act similarly.
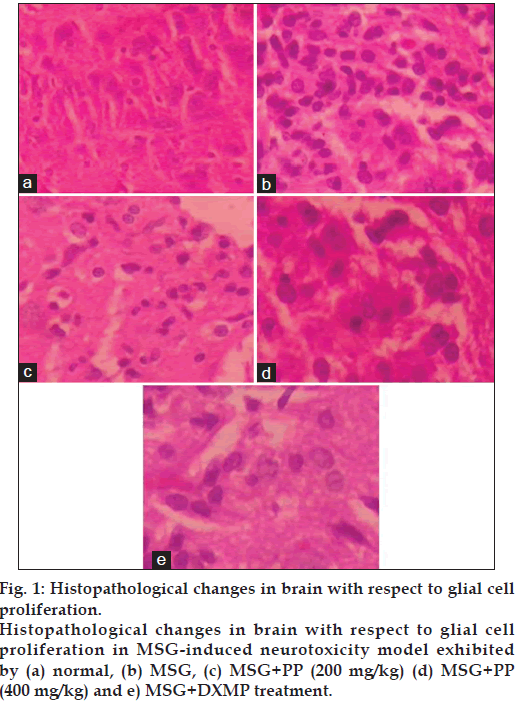
The results of histopathological study showed that there are no morphological changes of the brain in the normal animals. The cerebral cortex was found to be normal, no oedema appeared. No neuronal eosinophilia was found and nucleus was normal. There is no astrocytic or oligodendroglial changes were observed (fig. 1). Hippocampus was found to be normal. In MSG treated group, marked cerebral oedema, neuronal eosinophilia, nuclear pyknosis and neuronal karyorrhexis were observed. Treatment with PP effectively reduced the cerebral oedema. There was no an astrocytic and oligodendroglial change as well as reduced incidence of neuronal eosinophilia, nuclear pyknosis and neuronal karyorrhexis, thereby PP prevented hippocampal damage (fig. 2).
Figure 1: Histopathological changes in brain with respect to glial cell proliferation. Histopathological changes in brain with respect to glial cell proliferation in MSG-induced neurotoxicity model exhibited by (a) normal, (b) MSG, (c) MSG+PP (200 mg/kg) (d) MSG+PP (400 mg/kg) and e) MSG+DXMP treatment.
Taken together, the present study showed the neuroprotective effect of PP, which could be due to the various phytoconstituents such as flavonoids (furanoflavonoids) and chalcones as the plants containing these phytoconstituents have been reported to be beneficial in neurodegenerative diseases. It can be concluded that PP exerts neuroprotective property, which may be due to furanoflavonoids or chalcones possessing its potent antioxidant property and either GABAergic or NMDA receptor antagonising property.
Acknowledgements
The authors thank the Principal, KLE University’s College of Pharmacy, Hubli for providing the necessary facilities for the present research work and Dr. B. D. Huddar, Professor and Head, Department of Botany, HSK Science Institute, Hubli for authenticating the plant.
References
- Mayeux R. Epidemiology of Neurodegeneration. Annu Rev Neurosci 2003;26:81-104.
- Pavlovic V, Pavlovic D, Kocic G, Sokolovic D. Ascorbic acid modulates monosodium glutamate induced cytotoxicity in rat thymus. BratislLekListy 2009;110:205-9.
- Egbuonu AC, Obidoa O, Ezeokonkwo CA, Ezeanyika LU, Ejikeme PM. Hepatotoxic effects of low dose oral administration of monosodium glutamate in male albino rats. Afr J Biotech 2009;8:3031-5.
- Park SJ, Nam KW, Lee HJ, Cho EY, Koo U, Mar W. Neuroprotective effects of an alkaloid-free ethyl acetate extract from the root of SophoraflavescensAit. against focal cerebral ischemia in rats.Phytomedicine 2009;16:1042-105.
- Khare CP. Encyclopedia of Indian Medicinal Plants, Rational Western therapy, Ayurvedic and other traditional usage, Botany. Berlin: Springer Verlag; 2004. p. 378-9.
- Chopade VV, Tankar AN, Pande VV, Takade AR, Gowekar NM, Bhandari SR, et al. Pongamiapinnata: Phytochemical constituents, traditional uses and pharmacological properties: A review. Int J Green Pharm 2008;2:72-5.
- Chandrashekar KS, Prasanna KS. Antimicrobial activity of Pongamiapinnataleaves. Int J Med Res 2010;1:18-20.
- Arote SR, Dahikar SB, Yeole PG. Phytochemical screening and antibacterial properties of leaves of Pongamiapinnata Linn. (Fabaceae) from India. Afr J Biotech 2009;8:6393-6.
- Nirmal SA, Malwadkar G, Laware RB. Anthelmintic activity of Pongamiaglabra. J SciTechnol 2007;29:755-7.
- Bhatia G, Puri A, Maurya R, Yadav PP, Khan MM, Khanna AK, et al. Antidyslipidemic and antioxidant activities of different fractions of
- Pongamiapinnata(lin.) fruits. Med Chem Res 2008;17:281-9.
- Manigauha A, Patel S, Monga J, Ali H. Evaluation of anticonvulsant activity of Pongamiapinnata Linn in experimental animals. Int J Pharm Tech Res 2009;1:1119-21.
- Smitha GN, Asif AK, Mukesh SS, Geetanjali SS. Antiinflammatory activity of Pongamiapinnatastem bark in rats. J Pharm Res 2010;3:828-30.
- Rameshthangam P, Ramasamy P. Antiviral activity of bis (2-methylheptyl) phthalate isolated from Pongamiapinnata leaves against White Spot Syndrome Virus of PenaeusmonodonFabricius. Virus Res 2007;126:38-44.
- Prabha T, Dorababu M, Goel S, Agrawal PK, Singh A, Joshi VK, et al. Effect of methanol extract of Pongamiapinnata Linn seed on Gastro-duodenal ulceration and mucosal offensive and defensive factors in rats. Indian J ExpBiol 2009;47:649-59.
- Badole SL, Bodhankar SL. Antihyperglycemic Activity of PongamiapinnataStem Bark in Diabetic Mice. Pharm Biol 2008;46:900-5.
- Essa MM, Subramanian P. Protective role of PongamiaPinnata leaf extract on tissue antioxidant status and lipid peroxidation in Ammonium chloride induced hyperammonemic rats. ToxicolMech Methods 2006;16:477-83.
- Sangwan S, Rao DV, Sharma RA. A Review on PongamiaPinnata (L.) Pierre: A Great Versatile Leguminous Plant. Nat Sci 2010;8:130-9.
- Badole SL, Bodhankar SL. Investigation of antihyperglycemic activity of aqueous and petroleum ether extract of stem bark of Pongamiapinnataon serum glucose level in diabetic mice. J Ethnopharmacol2009;123:115-20.
- Narender A, Khaliq T, Purib A, Chander R. Antidyslipidemic activity of furano-flavonoids isolated from Indigoferatinctoria. Bioorg Med Chem 2006;16:3411-4.
- Nobre-Junior HV, Oliveira RA, Maia FD, Nogueira MA, Moraes MO, Bandeira MA, et al. Neuroprotective effects of chalcones from Myracrodruonurundeuvaon 6-hydroxydopamine-induced cytotoxicityin rat mesencephalic cells. Neurochem Res 2009;34:1066-75.
- Ramnathan M, Sivakumar S, Anandvijaykumar PR, Saravanababu C, Pandian PR. Neuroprotective evaluation of standardized extract of Centellaasciaticain monosodium glutamate treated rats. Indian J ExpBiol 2007;45:425-31.
- Ellman GL. Tissue sulphydryl groups. Arch BiochemBiophys 1959;82:70-7.
- Niehaus WG, Samuelsson B. Formation of malondialdehyde from phospholipids arachidonate during microsomal lipid peroxidation. Eur J Biochem 1968;6:126-30.
- Kakkar P, Das B, Vishwanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J BiochemBiophys 1984;21:130-2.
- Sinha AK. Colourimetric assay of catalase. Anal Biochem 1972;47:389-94.
- Mishra OP, Singhala D, Upadhyay RS, Prasad R, Atria D. Cerebrospinal fluid zinc, magnesium, copper and gamma-aminobutyric acid levels in febrile seizures. J PediatrNeurol 2007;5:39-44.
- Mallick HN. Understanding safety of glutamate in food and brain. Indian J PhysiolPharmacol 2007;51:216-34.
- Wang Y, Qin Z. Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis 2010;15:1382-402.