- Corresponding Author:
- Y. Murti
Department of Pharmaceutical Chemistry, Rajiv Academy for Pharmacy, Mathura?281 001, India
E-mail: ymurti@gmail.com
| Date of Submission | 2 April 2010 |
| Date of Revision | 20 June 2011 |
| Date of Acceptance | 24 June 2011 |
| Indian J Pharm Sci, 2011, 73 (3): 333-337 |
Abstract
New quinazolin-4-one derivatives, 6-bromo-2-methyl-3-(substituted phenyl)-(3H)-quinazolin-4-one, were synthesized and evaluated for antimicrobial and antiinflammatory activities. The structures attributed to synthesized compounds 1-8 were supported by the results of elemental analysis as well as by the UV, IR and 1H NMR spectral data. Investigation of antimicrobial activity was performed using cup-plate agar diffusion method against Bacillus subtilis, Staphylococcus aureus and Pseudomonas aeruginosa and Candida albicans, Aspergillus niger and Curvularia lunata. Antiinflammatory activity was evaluated using the carrageenan-induced paw oedema test in rats. The results showed that compounds 2b, 2c, 2d, 2g and 2h exhibited significant antibacterial and antifungal activity comparable to standard drugs and compounds 2b and 2c showed good antiinflammatory activity comparable to ibuprofen.
Keywords
Antibacterial, antifungal, antiinflammatory, quinazolin-4-one, 5-bromoanthranilic acid
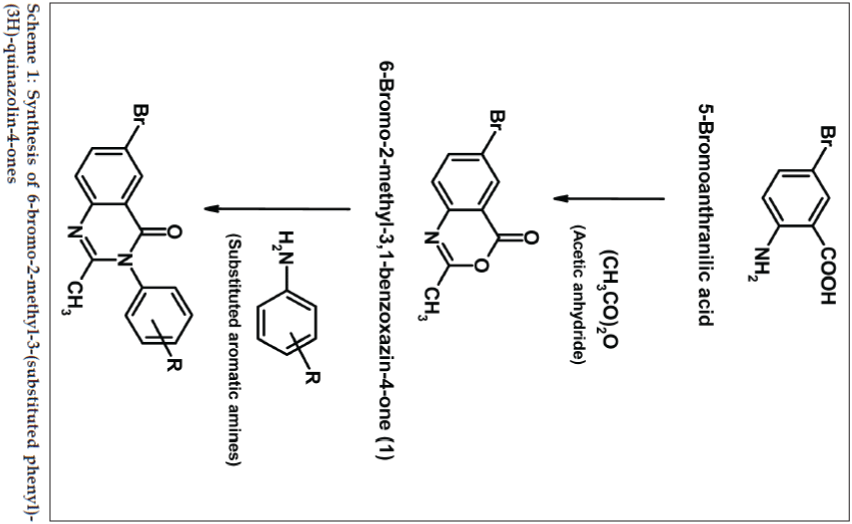
Quinazolin-4-one derivatives exhibited a great therapeutic potential in medicinal chemistry. They have drawn much attention due to their broad range of pharmacological properties, which include vitronectin receptor (αVβ3) antagonist [1], nonpeptide CCK-B antagonist [2], DNA repair enzyme poly (ADP-ribose) polymerase (PARP) inhibitor [3], antibacterial [4], antifungal [4], antiHIV [4], analgesic [5], antiinflammatory [5-7], anticancer [8], antidiuretic [9], sedative-hypnotic [7] and anticonvulsant activities [7,10,11]. Keeping in view the diverse therapeutic activities of quinazolin-4-ones and as part of our ongoing development of efficient protocols for preparation of bioactive heterocycles, the present study describes a simple and novel synthesis of the title compounds in the hope of getting potent biodynamic agents and evaluates their potential for antimicrobial and antiinflammatory activities. 6-Bromo-2-methyl-3,1-benzoxazinone (1) were prepared by the condensation of 5-bromoanthranilic acid with acetic anhydride. This compound (1) was treated with different substituted anilines under the reflux condition gives compounds 6-bromo-2-methyl-3-(substituted phenyl)-(3H)- quinazolin-4-ones (2a-h). The synthetic scheme leading to the formation of targeted compounds is depicted in Scheme 1.
The melting points were determined in open capillary tubes on melting point apparatus and were uncorrected. The absorbance maxima (λmax) were determined on a UV/Vis Spectrophotometer Pharma Spec-1700 (Shimadzu) in chloroform. The FTIR were recorded in KBr on Perkin Elmer Spectrum RXI FTIR system, 1H NMR spectra on Bruker Japan (DRX-300) spectrometer using DMSO-d6 as solvent and tetramethylsilane (TMS) as internal standard. All chemical shifts (d) are in ppm. All the chemicals used were of LR and AR grade and were procured from S. D. Fine Chem. Ltd., Mumbai and E. Merck, Delhi. The purity of the compounds was checked by TLC (Merck Silica-60F254) with chloroform:methanol:petroleum ether (8:1:1) using iodine vapours UV light as detecting agents and Rf value is given in Table 1. Elemental analysis (C, H, and N) of the compounds were performed on Heracus 1108 analyzer. All the animals were maintained under standard conditions and had access to pelleted animal feed and water. The study protocols were approved by the institutional animals ethics committee (IAEC/05/08/R1).
| Comp.no. | R | Molecularformula | %Yield | M.p.(°) | Rf value* | Elemental analysis found (calcd) % calculated (found) | ||
|---|---|---|---|---|---|---|---|---|
| Cc | H | N | ||||||
| 2a | H | C16H16N2O | 72.33 | 189 | 0.87 | 76.16 (76.13) | 6.39 (6.37) | 11.10(11.08) |
| 2b | o-methoxy | C17H18N2O | 67.48 | 138 | 0.83 | 72.32 (72.30) | 6.34 (6.29) | 9.92(9.89) |
| 2c | p-methoxy | C17H18N2O | 71.23 | 135 | 0.85 | 72.32 (72.31) | 6.34 (6.30) | 9.92(9.90) |
| 2d | 3,4-dichloro | C16H14N2OCl2 | 81.21 | 146 | 0.72 | 59.83 (59.81) | 4.39 (4.37) | 8.72(8.70) |
| 2e | o-nitro | C16H15N3O3 | 63.45 | 160 | 0.67 | 66.64 (66.62) | 5.09 (5.07) | 14.13 (14.1) |
| 2f | p-nitro | C16H15N3O3 | 74.27 | 165 | 0.69 | 66.64 (66.63) | 5.09 (5.07) | 14.13 (14.1) |
| 2g | o-bromo | C16H15N2OBr | 61.35 | 146 | 0.82 | 58.02 (57.99) | 4.56 (4.53) | 8.46(8.45) |
| 2h | p-bromo | C16H15N2OBr | 65.43 | 144 | 0.80 | 58.02 (58.00) | 4.56 (4.54) | 8.46(8.44) |
Table 1: Physical characterization data of synthesized compounds (2A-H)
5-Bromoanthranilic acid (1.08 g, 0.005 mol) in 0.01 mol of acetic anhydride was refluxed under anhydrous condition for 5 h. The excess of acetic anhydride was then distilled off under reduced pressure and cooled to room temperature. The corresponding 6-bromo-2- methylbenzoxazin-4-one (1) so obtained was filtered and dried under vacuum; the solid thus obtained was recrystallized from absolute ethanol.
Equimolar quantities of 6-bromo-2-methylbenzoxazin- 4-one (1) (0.01 mol) and substituted anilines (0.01 mol) in glacial acetic acid were refluxed for 5-6 h. The progress of the reaction was monitored using TLC (Merck Silica-60F254). The reaction mixture was cooled and poured on to crushed ice with continuous stirring. The resulting solid was washed with distilled water, filtered, dried in vacuum and recrystallized from absolute ethanol and analyzed. Adopting the above procedure nine different 6-bromo-2-methyl- 3-(substituted phenyl)-(3H)-quinazolin-4-ones (2a-h) were synthesized and their physical and analytical data are presented in Table 1. Spectral analysis of the synthesized compounds were determined and summarized below.
Compound 2a; UV (CHCl3, λmax, nm): 312.0; FTIR (KBr, cm-1): 3010 (aromatic C- H), 2952 (methyl C-H), 1710 (C= O), 1615 (C=N), 1595 (ring C=C), 1212 (C- N), 560 (C- Br); 1H NMR (DMSO-d6, d ppm): 2.13 ppm (s, 3H, -CH3), 6.58-8.60 (m, 8H, aromatic). Compound 2b; UV (CHCl3, λmax, nm): 269.5; FTIR (KBr, cm-1): 3023 (aromatic C- H), 2956 (methyl C-H), 1720 (C= O), 1620 (C=N), 1584 (ring C=C), 1218 (C- N), 687 (C- Br); 1H NMR (DMSO-d6, d ppm): 2.20 ppm (s, 3H, -CH3), 3.37 (s, 3H, methyl group of -OCH3), 6.60-8.50 (m, 7H, aromatic). Compound 2c; UV (CHCl3, λmax, nm): 275.0; FTIR (KBr, cm-1): 3030 (aromatic C- H), 2945 (methyl C-H), 1698 (C= O), 1590 (C=N), 1561 (ring C=C), 1210 (C- N), 679 (C- Br); 1H NMR (DMSO-d6, d ppm): 2.60 ppm (s, 3H, -CH3), 3.45 (s, 3H, methyl group of -OCH3), 6.32-7.77 (m, 7H, aromatic).
Compound 2d; UV (CHCl3, λmax, nm): 331.0; FTIR (KBr, cm-1): 3035 (aromatic C- H), 2951 (methyl C-H), 1700 (C= O), 1625 (C=N), 1595 (ring C=C), 1209 (C- N), 690 (C- Br); 1H NMR (DMSO-d6, d ppm): 3.10 ppm (s, 3H, -CH3), 6.72-8.57 (m, 6H, aromatic). Compound 2e; UV (CHCl3, λmax, nm): 293.5; FTIR (KBr, cm-1): 3032 (aromatic C- H), 2950 (methyl C-H), 1726 (C= O), 1610 (C=N), 1590 (ring C=C), 1206 (C- N), 545 (C- Br); 1H NMR (DMSO-d6, d ppm): 2.45 (s, 3H, -CH3), 7.35-8.40 (m, 7H, aromatic). Compound 2f; UV (CHCl3, λmax, nm): 292.0; FTIR (KBr, cm-1): 3020 (aromatic C- H), 2945 (methyl C-H), 1720 (C= O), 1600 (C=N), 1588 (ring C=C), 1200 (C- N), 650 (C- Br); 1H NMR (DMSO-d6, d ppm): 2.25 (s, 3H, -CH3), 6.12-7.24, (m, 7H, aromatic). Compound 2g; UV (CHCl3, λmax, nm): 310.5; FTIR (KBr, cm-1): 3015 (aromatic C- H), 2955 (methyl C-H), 1705 (C= O), 1616 (C=N), 1596 (ring C=C), 1218 (C- N), 667 (C- Br); 1H NMR (DMSO-d6, d ppm): 2.23 (s, 3H, -CH3), 7.28-8.40 (m, 7H, aromatic). Compound 2h; UV (CHCl3, λmax, nm): 309.0; FTIR (KBr, cm-1): 3028 (aromatic C- H), 2965 (methyl C-H), 1718 (C= O), 1620 (C=N stretch), 1580 (ring C=C stretch), 1210 (C- N), 610 (C- Br); 1H NMR (DMSO-d6, d ppm): 2.35 (s, 3H, -CH3), 6.10-7.40 (m, 7H, aromatic).
All the newly synthesized quinazolin-4-one derivatives (2a-h) were screened for antimicrobial activity by cup-plate agar diffusion method [12] using nutrient agar media against Bacillus subtilis, Staphylococcus aureus and Pseudomonas aeruginosa at 25, 50 and 100 μg/ml concentrations, respectively. All the synthesized compounds were dissolved separately to prepare a stock solution of 1.0 mg/ ml using DMF. About 20 ml of the growth medium was transferred into petri plates and inoculated with 1.5 ml of microbial culture. Filter paper (Whatmann No. 1) sterile disks of 5.0 mm diameter. Each petri plate was divided into 5 equal portions along the diameter to place one disc. Three discs of test sample were placed on three portions together with one disc with reference drug ciprofloxacin (50 μg/ ml) and a disc impregnated with the solvent (DMF) as negative control. Test samples were measured and the average diameters of zones of inhibition (mm) were measured and the average diameters for the test samples were calculated in triplicate sets. The diameters obtained for the test sample were compared with that produced by the standard drug ciprofloxacin by measuring zone of inhibition in mm, by using ciprofloxacin (50 μg/ml) as the standard. Similarly, the antifungal screening of the title compounds (2a-h) was carried out against Candida albicans, Aspergillus niger and Curvularia lunata by using fluconazole (50 μg/ml) as the standard drug for antifungal activity.
The results of antimicrobial activity (Table 2) revealed that all the test compounds showed moderate activity against tested microorganisms. Compounds 2b and 2c exhibited significant antibacterial activity and compounds 2d, 2g and 2h showed good antifungal activity against fungal strains comparable to standard drugs.
| Compd. No. | Zone of inhibition (mm) at 50 µg/ml | ||||||
|---|---|---|---|---|---|---|---|
| Antibacterial activity | Antifungal activity | ||||||
| B. subtilis | S. aureus | P. aeruginosa | C. albicans | A. niger | C. lunata | ||
| 2a | 12.34 | 12.42 | 13.33 | 11.87 | 12.52 | 11.98 | |
| ±0.43 | ±0.65 | ±0.54 | ±0.38 | ±0.48 | ±0.73 | ||
| 2b | 19.12 | 20.98 | 20.36 | 17.33 | 16.58 | 15.98 | |
| ±0.73 | ±0.76 | ±0.43 | ±0.54 | ±0.36 | ±0.18 | ||
| 2c | 18.15 | 21.78 | 19.16 | 17.76 | 17.46 | 16.95 | |
| ±0.48 | ±0.54 | ±0.10 | ±0.43 | ±0.15 | ±0.23 | ||
| 2d | 14.68 | 16.35 | 17.96 | 27.16 | 24.12 | 26.10 | |
| ±0.73 | ±0.58 | ±0.18 | ±0.10 | ±0.28 | ±0.48 | ||
| 2e | 14.02 | 15.26 | 14.65 | 15.96 | 18.68 | 16.65 | |
| ±0.34 | ±0.45 | ±0.23 | ±0.18 | ±0.78 | ±0.43 | ||
| 2f | 15.12 | 16.86 | 16.24 | 15.75 | 17.96 | 16.00 | |
| ±0.18 | ±0.73 | ±0.54 | ±0.23 | ±0.56 | ±0.10 | ||
| 2g | 16.12 | 16.98 | 15.76 | 25.84 | 23.07 | 26.30 | |
| ±0.73 | ±0.76 | ±0.43 | ±0.54 | ±0.64 | ±0.18 | ||
| 2h | 15.15 | 16.78 | 14.86 | 26.73 | 24.77 | 25.96 | |
| ±0.48 | ±0.54 | ±0.10 | ±0.18 | ±0.58 | ±0.23 | ||
| Ciprofloxacin | 20.42 | 23.02 | 21.96 | - | - | - | |
| ±0.38 | ±0.26 | ±0.28 | |||||
| Fluconazole | - | - | - | 28.34 | 25.23 | 27.40 | |
| ±0.76 | ±0.26 | ±0.58 | |||||
| Blank | - | - | - | - | - | - | |
Table 2: Antimicrobial activity of 6-bromo-2-methyl-3-(substituted phenyl)-(3h)-quinazolin-4-ones (2a-h)
All the newly synthesized quinazolin-4-one derivatives (2a-h) were also screened for antiinflammatory activity by using the carrageenaninduced paw oedema method [13] on Wistar rats of either sex weighing 200-300 mg. The rats were randomly divided in to three groups; each consisted of a minimum of 6 animals. The suspension of the newly synthesized compounds, uniformly dispersed in 2% Tween-80 were administered to test animals orally. The control groups received the same experimental handling as test groups in place of test compounds equivalent doses of vehicle alone were administered. Food and water were withdrawn during the test. Ibuprofen was used as the standard anti-inflammatory drug.
Acute oedema in the hind paws of rat was induced by the planter injection of freshly prepared, w/v carrageenan in distilled water (0.1 ml of freshly prepared, w/v carrageenan in distilled water). The foot volume was measured before and 3 h after carrageenan injection by the micropipette method described by Buttle et al. The mean increase in the paw volume in each group was calculated according to the following formula: Percent antiinflammatory activity= [1- Vt/Vc]×100, where Vt and Vc are the oedema volumes in the drug treated and the control groups.
The results of antiinflammatory data (Table 3) indicate that compounds 2b and 2c were found to possess the most potent antiinflammatory activity (39.45 and 40.10, respectively) at 50 mg/kg in comparison with reference drug, ibuprofen, which showed 42.21% of inhibition of oedema at same dose. Furthermore, the test compound 2b, 2c and ibuprofen were subjected to screen at three graded doses of 25, 50 and 100 mg/ kg for antiinflammatory activity. These compounds showed good anti-inflammatory activity at all the three doses than the reference drug.
| Compd. No. | Antiinflammatory activity | |
|---|---|---|
| Oral dose (mg/kg) | % Inhibition of oedema | |
| 2a | 50 | 31.28 a |
| 2b | 25 | 21.76 a |
| 50 | 39.45 a | |
| 100 | 60.23 a | |
| 2c | 25 | 22.12 a |
| 50 | 40.10 a | |
| 100 | 63.35 a | |
| 2d | 50 | 37.37 a |
| 2e | 50 | 36.98 a |
| 2f | 50 | 37.18 a |
| 2g | 50 | 34.41 a |
| 2h | 50 | 33.59 a |
| Ibuprofen | 25 | 22.57 a |
| 50 | 42.21 a | |
| 100 | 65.15 a | |
| a P < 0.05 | ||
Table 3: Antiinflammatory activity of 6-bromo-2-methyl-3-(substituted phenyl)-(3h)-quinazolin-4-ones (2a-h)
The characteristic features of the synthetic route is that 6-bromo-2-methylbenzoxazin-4-one was treated with different anilines to give 6-bromo-2- methyl-3-(substituted phenyl)-(3H)-quinazolin-4-ones. The selection of substituted anilines was based on presence of electron withdrawing and electron releasing groups.
The newly synthesized compounds were evaluated for antimicrobial and antiinflammatory activity. The results of antimicrobial anti antiinflammatory studies are given in Table 2 and 3 respectively. Among all the compounds of the present series, compounds 2b and 2c exhibited highest degree of inhibition against the tested bacterial strains and compound 2d, 2g and 2h exhibited highest degree of inhibition against the tested fungal strains. The data of antimicrobial activity revealed that introduction of methoxy group (-OCH3) increases antibacterial activity while introduction of halo groups (-Cl and -Br) increases antifungal activity against tested organisms respectively.
Among the compounds tested for antiinflammatory activity, the compound 2a having phenyl group as a substituent showed the least percentage inhibition of oedema i.e. 31.28%, while the compounds 2b and 2c, substituted with o-methoxyphenyl group and p-methoxyphenyl group exhibited the maximum antiinflammatory activity, 39.45% and 40.10%, respectively, comparable to ibuprofen.
From the result it was concluded that most of the synthesized compounds were biologically active. It may be concluded that further study of these compounds can lead to the development of a potent antimicrobial and antiinflammatory agents.
Acknowledgements
The authors are thankful to Central Institute for Research on Goats, Farah, Mathura, for providing microbial strains for antimicrobial screening. The authors are also thankful to the Head, Regional Sophisticated Instrumentation Centre (RSIC), Central Drug Research Institute (CDRI), Lucknow, India for providing elemental and spectral data.
References
- Lawson EC, Kinney WA, Costanzo MJ, Hoekstra WJ, Kauffman JA, Luci DK, et al. Structure-function study of quinazolinone-based vitronectin receptor (aVß3) antagonists: Computer-assisted analysis of ligand-receptor interactions. Lett Drug Desi Disc 2004;1:14-8.
- Padia JK, Field M, Hinton J, Meecham K, Pablo J, Pinnock R, et al. Novel nonpeptide CCK-B antagonists: Design and development of quinazolinone derivatives as potent, selective, and orally active CCKB antagonists. J Med Chem 1998;41:1042-9.
- Kulcsár G, Kálai T, Osz E, Sár CP, Jeko J, Sümegi B, et al. Synthesis and study of new 4-quinazolinone inhibitors of the DNA repair enzyme poly(ADP-ribose) polymerase (PARP). ARKIVOC 2003;5:121-31.
- Alagarsamy V, Murugesan S, Dhanabal K, Murugan M, Clercq E De.AntiHIV, antibacterial and antifungal activities of some novel 2-methyl-3-(substituted methylamino)-(3H)-quinazolin-4-ones. Indian J Pharm Sci 2007;69:304-7.
- Alagarsamy V, Muthukumar V, Pavalarani N, Vasanthanathan P, Revathi R. Synthesis, Analgesic and Anti-inflammatory Activities of Some Novel 2,3-Disubstituted Quinazolin-4(3H)-ones.Biol Pharm Bull 2003;26:557-61.
- Kenichi O, Yoshihisa Y, Toyonari O, Toru I, Yoshio I. Synthesis and anti-inflammatory activity of 4(1H)-quinazolinone derivatives. J Med Chem 1985;28:568-76.
- Abdel-Alim M, Abdel-Alim, Abdel-Nasser A, El-Shorbagi, Mahmoud A, El-Gendy,et al.Quinazolinone derivatives of biological interest V. Novel 4(3H)-quinazolinones with sedative-hypnotic, anticonvulsant and anti-inflammatory activities. Collect Czech ChemCommun 1993;58:1963-8.
- Xia Y, Yang ZY, Hour MJ, Kuo SC, Xia P, Bastow KF, et al. Antitumor agents. Part 204: Synthesis and biological evaluation of substituted 2-aryl quinazolinones. Bioorg Med ChemLett 2001;11: 1193-6.
- Lyle FR. U.S. Patent 5,973,257, 1985. ChemAbstr 1985;65:2870.
- Buchanan JG, Sable HZ. In Selective Organic Transformations 2nd ed. New York: Wiley-Interscience; 1972, p. 1-95.
- Wolfe JF, Rathman TL, Sleevi MC, Campbell JA, Greenwood TD. Synthesis and anticonvulsant activity of some new 2-substituted 3-aryl-4(3H)-quinazolinones. J Med Chem 1990;33:161-6.
- Gillespie SH. Medical Microbiology-Illustrated. 1st ed. New York: Butterworth Heinemann; 1994. p. 1.
- Winter CA, Risely EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. ProcSocExpBiol 1962;111:544.