- *Corresponding Author:
- A. K. Hassan
57, Abdul Hakeem El-Refahy Street, Apartment 23, Nasr City, Cairo, Egypt
E-mail: kadry_ibrahim173@yahoo.com
| Date of Submission | 10 Sep 2015 |
| Date of Revision | 09 Jun 2016 |
| Date of Acceptance | 17 Jun 2016 |
| Indian J Pharm Sci, 2016; 78(3): 395-401 |
Abstract
Betnovate®, Daktacort®, Daktarin®, Hemoclar®, Kenacomb® and Lotriderm® are O/W creams stabilized by nonionic surfactant/s and distributed in the Egyptian market without stability problems. In this work, the above creams were used as stability reference standard creams to extrapolate numeral values for the proposed parameters of the new accelerated stability testing protocol. The temperature–conductivity relations that represent all the above tested creams have strong direct linear relationships. Determination coefficient R2 values of these relations were ≥0.909 and their yield values represented as temperatures were ≥21º. The stability-indicating phase inversion temperatures of these creams were more than 80º. These common characteristic results represent the basis parameters of the new accelerating stability testing protocol as well as for the determination of the optimum storage temperature of the emulsion under examination. The applicability and validity of this protocol is confirmed by comparing the parameters results obtained from newly formulated O/W 2% miconazole nitrate cream to that obtained from the and also by subjecting all the tested O/W creams including the newly formulated one to accelerating stability testing studies according to the recent approved Issues of ICH Guidelines Q1A (R2). These accelerating stability studies include the measurements of the homogeneity, average weight and leakage test, drug content, pH, viscosity and microbiological analysis of the emulsion. Matching of the compared results and the approval of the stability testing studies confirms the applicability and validity of the new protocol. The new protocol determines the optimum storage temperature condition required for each cream under examination through the determination of the yield value represented as temperature of its temperature–conductivity relation.
Keywords
New accelerating stability testing protocol, R2, Yield value represented as temperature, Stabilityindicating phase inversion temperature, Storage temperature determination
Introduction
In the recent times, there are many problems facing the achieving and performing the accelerating stability testing studies of pharmaceutical and cosmetic O/W creams as well as the determination of the optimum storage temperatures. Tedious procedures, the cost and most important is the time required for applying the accelerating stability testing period which affect the distribution of the drug in the market and consequently the patient seriously.
The objective of this work is to reduce the cost greatly and to save time by applying a new accelerating stability testing protocol for evaluating the stability of O/W creams stabilized by nonionic surfactants in addition to the determination of the optimum storage temperature. Three main reasons pushing us for applying and adopting the new protocol either in parallel with or instead of the current applied one in which the product is tested at temperature 40±2° and relative humidity 75±5% for six months [1]. The first is that, as with all accelerated test procedures, the primary requirement is that the stress applied should speed up, but not alter, the mechanism of deterioration operating at ambient temperatures. The work of Enever, showed that the viscous liquid crystalline phase that disappears above approximately 35° has a considerable stabilizing influence on the emulsions [2]. So it is of error and inappropriate to test any emulsion with accelerated test procedure performed at 40°.
The second is that, the protocol determines the optimum storage temperature required for the stability of O/W cream through the determination of the yield value represented as temperature (YVT) of its temperatureconductivity relation as well as the temperature to which the product will be cooled to obtain a fine dispersion during the preparation process [3]. The third main reason is that the new protocol takes only few days to judge stability.
It is important to denote that the new accelerating stability testing protocol is based on three main factors; the first one is that, the most stable O/W emulsion is determined via the determination of the maximum R² value by applying of simple linear regression (least squares method) to the temperatureconductivity obtained data up to 80° [4]. This means that the temperature-conductivity relation of O/W cream under investigation should have a strong direct linear relationship. So it is very important to determine R² to judge the stability.
The second is the determination of the YVT. This will be explained as the liquid and solid heterogeneous dispersions such as colloidal solutions, emulsions, liquid suspensions, ointments and similar products are following the non-Newtonian equation of flow. The non-Newtonian plastic flow curves do not pass through the origin but rather intersect the shear stress axis (or will if the straight part of the curve is extrapolated to the axis) at a particular point referred to as the yield value. A Bingham body does not begin to flow until a shearing stress, corresponding to the yield value is exceeded. At stresses below the yield value, the substance acts as an elastic material. In effect, a plastic system resembles a Newtonian system at shear stresses above the yield value [5]. It will be mentioned later that the optimum storage temperature required for the stability of the cream is equal to the YVT of its temperature-conductivity relation, so it is important to determine the YVT to judge the stability.
Phase inversion temperature (PIT) represents the third main factor affecting the stability of emulsions. It was discussed in many researches. Shinoda and Saito, concluded that for satisfactory shelf life of emulsions stabilized by nonionic surfactants, the PIT should be 20-70° above the storage temperature. They concluded that the instability of the emulsion is very sensitive to the PIT, so that the selection of a suitable emulsifier by the PIT data is much more accurate and reliable, provided the temperature difference between the storage temperature and the optimum PIT is known which is covered in this work [3]. The emulsification at a higher temperature especially near the PIT and cooling to storage temperature of the emulsion are effective in obtaining fine and uniform dispersions [3].
Parkinson and Sherman concluded that, there is a general relationship between PIT and emulsion stability with the PIT increasing as the rate of globule coalescence decrease. This suggests that it may be possible to use PIT determinations as a simple and rapid method for evaluating emulsion stability [6]. The parameters of the proposed new protocol were based mainly on the above mentioned three factors.
Materials and Methods
The following instruments and reagents were used in the study. RZR1 stirring paddle (Heidolph Instruments Gmbh and Co. KG, Germany); A Jenway model 4510 conductivity/TDS meter (UK); A Jenway model 3510 pH meter (UK); Binder Incubators (Germany); Osworld Stability Chamber (India); Thermo scientific HPLC (USA); Brookfield Digital viscometer (USA); Thermometer 150 (76 mm 1 mm, N2 filled GH, Zeal, Ltd, England); glass-ware grade A (Ilmabor TGI, Germany); Burette (0.1/DIN/AS 50 ml, Germany; MS-H-Pro digital hotplate magnetic stirrer (USA); PGW153e 150.0 g, PGW253e 250.0 g, PGW453e, 750.0 g, d=0.001 g ADAM balances (UK). All equipment was calibrated, approved and ready for use.
Stability reference standard creams (SRSCs)
Betnovate®, Daktacort®, Daktarin®, Hemoclar®, Kenacomb® and Lotriderm® O/W creams were purchased from the Egyptian market. All these O/W creams were stabilized by nonionic surfactant/s as mentioned in their pamphlets and monographs. Their nonionic surfactants are macrogol cetostearyl ether and cetostearyl alcohol, PEG and glycol stearate, macrogol 6-32 stearate and glycol stearate, cetostearomacrogol nonionic, tween 60 and polyoxyethylene fatty alcohol ether and cetosearyl alcohol, respectively.
Stability of SRSCs and their approval
The stability of creams and consequently their distribution in the Egyptian market were approved from three trusted/reliable organizations; the mother international companies, the companies in which these creams are manufactured under license in Egypt and the Ministry of Health in Egypt. These creams were distributed in the country of origin for 10 years before their distribution in the Egyptian market according to the rules of the Ministry of Health in Egypt. They were manufactured in Egypt either in multinational pharmaceutical companies or under the authority of pharmaceutical companies in Egypt. The products names, their batch numbers, production dates, expiration dates, names of nonionic surfactants used, names of the mother international companies and the companies in which these creams are manufactured under license in Egypt were recorded in Table 1.
| Product name | Batch number | Prod. Date | Exp. Date | Nonionic surfactant/s | Mother Company/ Manufactured by |
|---|---|---|---|---|---|
| Betnovate cream | N100797 | Jan-13 | Jan-15 | Macrogolcetostearyl ether/cetostearyl alcohol | GlaxoWellcome, UK/ GlaxoSmithKline, S.A.E., Egypt |
| Daktacort cream | DGE2151 | Jul-13 | Jun-16 | PEG and glycol stearate | Janssen Pharmaceutica, Belgium/Minapharm, Egypt |
| Daktarin cream | DBE0526 | Feb-13 | Jan-15 | Macrogol 6-32 stearate and glycol stearate | Janssen Pharmaceutica, Belgium/Minapharm Egypt. |
| Hemoclar cream | 3EG094 | Jun-13 | May-16 | Cetostearomacrogol nonionic | Sanofi-Aventis, France/ Sanofi-Aventis, S.A.E., Egypt |
| Kenacomb cream | N101485 | May-13 | May-15 | Tween 60 (Polysorbate 60)/polyoxyethylene fatty alcohol ether | GlaxoSmithKline, UK/ Smithkline, Beecham, Egypt |
| Lotriderm cream | 130239 | Jan-13 | Jan-16 | Cetosearyl alcohol | Schering Plough Corporation, USA/Medical Union Pharmaceutical, Egypt |
| Miconazole nitrate cream | 1 | Jun-14 | Jun-16 | Tween 80 / span 80 | Grand Pharma, Egypt |
The products names, their batch numbers, production dates, expiration dates, names of nonionic surfactant/s, the mother companies of these creams and the companies in which these creams are manufactured under license in Egypt. All the products are O/W creams
Table 1: data of the purchased o/w creams.
Preparation of the tested SRSC samples
150 g of each purchased O/W cream were weighed and stirred with RZR1 stirring paddle at speed of 664 rpm for 5 min as a fixed time to assure a complete mixing. Different emulsions were made in triplicate. All parameters were measured after 24 h.
Preparation of the newly formulated O/W 2% miconazole nitrate cream [4]
The following chemicals were used in formulation, miconazole nitrate (Jiangsu Nhwa Pharmaceutical Co. Ltd. China), paraffin oil (Apar Industries Co. Ltd. India), soft paraffin (Jell Pharmaceuticals Pvt Co. Ltd. India) and beeswax (Cisme Co. Italy), Tween 80 and Span 80 (Kolb Co. Switzerland), propyl paraben base (Salycylates and Chemicals Co. Ltd. India) and methyl paraben base (Wuhu Huahai Biology Engineering Co. Ltd. China), sorbitol (Roquette Lestrem Co. France) and water for injection (Grand Pharma for Pharmaceutical Industries Co. Egypt). Emulsion composition was recorded in Table 2.
| Emulsion composition | Gm/100 gm |
|---|---|
| Miconazole nitrate | 2.0 |
| Liquid paraffin | 17.0 |
| Soft paraffin | 12.0 |
| Bees wax | 5.0 |
| Tween 80 | 3.5 |
| Span 80 | 3.5 |
| Propyl paraben | 0.02 |
| Sorbitol | 5.0 |
| Methyl paraben | 0.15 |
| Water for injection | 51.83 |
Water for injection 51.83 The composition of the newly formulated O/W 2% miconazole nitrate cream (9.236 And 9.65 are HLB values of the system and that of the surfactants blend respectively).
Table 2: composition of the newly formulated o/w 2% miconazole nitrate cream.
The emulsions were prepared by the sudden phase inversion method. The water phase was heated to 80±2° and added portion wise to the oily phase containing both emulsifiers at 80±2° within 30 s, while stirring with RZR1 stirring paddle at speed of 664 rpm. Miconazole nitrate was added at 40°±2, while stirring with RZR1 stirring paddle at speed of 664 rpm. The emulsion was mixed up to room temperature 25±2° [3,4]. The emulsion was made in triplicate. All parameters were measured after 24 h.
Yield value (YV), optimum storage temperature, conductivity and PIT range determination
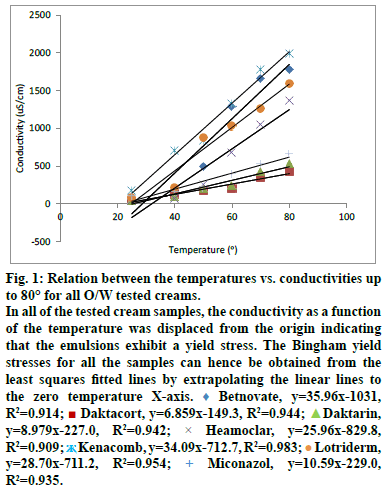
The cream under examination is sheared in the space between the outer wall of a magnetic stirrer (rotating) and the inner wall of a beaker (stationary) into which the stirrer works. 60 ml of the tested cream was continuously agitated at 100 rpm with small propeller stirrer. The force shearing stress of the magnetic stirring agitation and the rate of shear at 100 rpm speed were fixed and allowed to reach equilibrium. The torque resulting from the viscous drag of the cream under examination was affected by increasing the temperature at a steady rate up to 80° using MSH- Pro digital hotplate magnetic stirrer. The specific conductivity of each tested cream was measured directly, as it is, without dilution at room temperature 25±2°, 40°, 50°, 60°, 70° and 80°. In this way, a rheogram can be constructed by plotting temperature versus conductivity by increasing temperature up to 80°. In all of the tested cream samples, the temperature was displaced from the origin indicating that the emulsions exhibit a yield stress. The Bingham yield stresses for all the samples can hence be obtained from the least squares fitted lines by extrapolating the linear lines to the zero temperature X-axis [7].
As known, the material does not begin to flow until a shearing stress, corresponding to the yield value is exceeded. In the present work, YVT and consequently the material does not begin to flow until a shearing stress accompanied with temperature, corresponding to the yield value is exceeded. Since the flow of the material will affect its stability, so for good stability the tested cream should be stored (if possible) in a temperature equals to the yield value which represented as temperature. Accordingly the optimum storage temperature should be equal to the YVT which represents a new approach indicating the importance of the yield value as factor affecting studying of emulsions stability. The points of intersection of the yield values may revealed using figure 1 and can be obtained when needed from the least squares fitted lines by extrapolating the linear lines to the zero temperature X-axis [7].
Figure 1: Relation between the temperatures vs. conductivities up
to 80° for all O/W tested creams.
In all of the tested cream samples, the conductivity as a function
of the temperature was displaced from the origin indicating
that the emulsions exhibit a yield stress. The Bingham yield
stresses for all the samples can hence be obtained from the
least squares fitted lines by extrapolating the linear lines to
the zero temperature X-axis.  Betnovate, y=35.96x-1031,
R2=0.914;
Betnovate, y=35.96x-1031,
R2=0.914;  Daktacort, y=6.859x-149.3, R2=0.944;
Daktacort, y=6.859x-149.3, R2=0.944;  Daktarin,
y=8.979x-227.0, R2=0.942; × Heamoclar, y=25.96x-829.8,
R2=0.909;
Daktarin,
y=8.979x-227.0, R2=0.942; × Heamoclar, y=25.96x-829.8,
R2=0.909;  Kenacomb, y=34.09x-712.7, R2=0.983;
Kenacomb, y=34.09x-712.7, R2=0.983;  Lotriderm,
y=28.70x-711.2, R2=0.954; + Miconazol, y=10.59x-229.0,
R2=0.935.
Lotriderm,
y=28.70x-711.2, R2=0.954; + Miconazol, y=10.59x-229.0,
R2=0.935.
PIT range was detected as a fall of the specific conductivity between any two successive temperature values or they may be detected when two successive conductivity values are nearly equal (steady state of conductivity values). The results quoted are the means of three determinations [4].
Accelerating stability testing for all tested creams
Stability studies were carried out to the purchased O/W SRSCs as well as to the newly formulated O/W 2% miconazole nitrate topical cream according to the recent approved Issues of ICH Guidelines Q1A (R2), in which the product is tested at temperature 40±2° and relative humidity 75±5% for six months [1]. These accelerating stability studies include the measurements of the homogeneity, average weight and leakage test, drug content in the emulsion (identification and assay of the active constituents), pH, viscosity and microbiological analysis of the emulsion. These measurements were performed in addition to the temperature-conductivity relations that represent all the above tested creams. The results obtained from the stability studies indicate that all the tested O/W creams attained their physical, chemical and microbiological attributes and no significant change occurred during the study period.
Results and Discussion
The conductivities of each O/W emulsion set of the purchased O/W SRSCs were measured at room temperature 25±2°, 40°, 50°, 60°, 70°, 80° and a relation between the temperature vs. conductivity up to 80° was plotted by applying the simple linear regression (least squares method) statistical analysis [4]. Temperatures and average of responses of conductivities were recorded in Table 3. Temperature-conductivity measured values were used in the application of the statistical analysis, the rheological measurements as well as the determination of the stability-indicating phase inversion temperatures (SIPIT) range measurements.
| Temperature | Betnovate | Daktacort | Daktarin | Heamoclar | Kenacomb | Lotriderm | Miconazole |
|---|---|---|---|---|---|---|---|
| 25º±2 | 96 | 45 | 47.7 | 44.8 | 171 | 82 | 108.3 |
| 40º | 175 | 131 | 101 | 68.1 | 701 | 216 | 160 |
| 50º | 490 | 179 | 207 | 258 | 833 | 878 | 218 |
| 60º | 1293 | 202 | 247 | 675 | 1332 | 1031 | 394 |
| 70º | 1664 | 345 | 423 | 1048 | 1778.5 | 1265 | 525 |
| 80º | 1785 | 431 | 530 | 1366 | 1987.9 | 1590 | 663 |
Average responses of conductivities in µS/cm at 25±2, 40,50 ,60, 70 and 80º for each O/W tested creams including the newly formulated O/W 2% miconazole nitrate cream .
Table 3: average responses of conductivities.
The least squares regression line equation calculates the slope b (the change in Y for unit change in X), the intercept a (the predicted value of Y when X=0) and R² (known as the coefficient of determination). As known that, in the case of models with an intercept, R² can be interpreted as the proportion of the variation in Y that is accounted for by the predictor variable X after adjusting Y by its mean. It runs from 0 to 1, with 1 indicating perfect prediction of Y from X [4,8].
In all of the tested cream samples, the conductivity as a function of the temperature was displaced from the origin indicating that the emulsions exhibit a yield stress. The Bingham yield stresses (represented as temperatures YVTs) for all the samples can hence be determined/obtained from the least squares fitted lines by extrapolating the linear lines to the zero temperature X-axis. The points of intersection of the yield values may revealed using figure 1 and can be obtained when needed from the least squares fitted lines by extrapolating the linear lines to the zero temperature X-axis [7]. Absence of phase separation, as well as, the absence of the abrupt change in conductivity up on heating up to 80° indicates that the SIPITs of all the tested cream samples are more than 80°.
The results of the statistical analysis, YVT and the SIPIT determination indicated that all the tested creams have common characteristic parameters. These parameters represent the basis of the new accelerating testing protocol required for evaluating the stability of the O/W emulsions stabilized by nonionic surfactant/s, that is to say, the temperature–conductivity relations that represent all the tested creams have strong direct linear relationships. R2 values of these relations were ≥0.909 and their YVTs were ≥21°, in addition to, the SIPITs of these creams were more than 80°.
As mentioned before, since the optimum storage temperature is actually equal to the YVT, so for good stability the tested cream should be stored in a temperature equals to the YVT of its relation. For that, the optimum storage temperatures required for the stability of Betnovate®, Daktacort®, Daktarin®, Hemoclar®, Kenacomb® and Lotriderm® O/W creams are in the range of (24-28°), (20-24°), (24-28°), 32°, (20-24°) and (24-28°), respectively. This represents a new approach indicating the importance of the yield value as factor affecting studying of the emulsions stability and consequently the suitability of the linear regression analysis-Bingham model as a statistical method of analysis of the obtained data was discussed in previous paper [4]. Data were revealed in fig. 1. R2, YVT and PIT results were obtained from fig. 1 and were recorded in Table 4.
| SRSCs and newly formulatedmiconazole cream | R² value | YVTs | SIPITs |
|---|---|---|---|
| Betnovatecream | 0.914 | 28-32º | >80º |
| Daktacortcream | 0.944 | 20-24º | >80º |
| Daktarincream | 0.942 | 24-28º | >80º |
| Heamoclarcream | 0.909 | 32.0º | >80º |
| Kenacombcream | 0.983 | 20-24º | >80º |
| Lotridermcream | 0.954 | 24-28º | >80º |
| Miconazole nitrate cream | 0.935 | 20-24º | >80º |
SRSC: Stability reference standard creams), YVT: Yield values represented as temperaturesand SIPIT: Stability-indicating phase inversion temperatures
Table 4: results of all the tested o/w creams.
The applicability and validity of the new proposed protocol was confirmed by matching the parameters results obtained from the newly formulated O/W 2% miconazole nitrate cream with that obtained from the above mentioned purchased SRSCs. It was confirmed also by subjecting all the tested O/W creams including the newly formulated one to accelerating stability testing studies. These studies were carried out according to the recent approved Issues of ICH Guidelines Q1A (R2), in which the product is tested at temperature 40±2° and relative humidity 75±5% for six months. These accelerating stability studies include the measurements of the homogeneity, average weight and leakage test, drug content in the emulsion (identification and assay of the active constituents), pH, viscosity and microbiological analysis of the emulsion. These measurements were performed in addition to the temperature-conductivity relations that represent all the above tested creams. Temperatures and average of responses of conductivities of the newly formulated 2% miconazole nitrate cream were recorded in Table 3.
The results of the statistical analysis, YVT and the SIPIT determination indicated that the relation of the newly formulated O/W 2% miconazole nitrate cream has strong direct linear relationship between temperature and conductivity. R2 value of this relation is 0.935. YVT of the relation is more than 21° and the SIPIT of this cream is more than 80°. The results obtained from the stability studies indicate that the tested O/W miconazole nitrate cream attained its physical, chemical and microbiological attributes and no significant change occurred during the study period. Matching of the compared results and the approval of the stability testing studies confirms the applicability and validity of the new protocol. Since the optimum storage temperature is actually equal to the YVT, so the optimum storage temperatures required for the stability of the newly formulated cream is mainly 22° (20-24°). Data were revealed in fig. 1. R2, YVT and SIPIT Results were obtained from fig. 1 and were recorded in Table 4.
The new protocol determines the optimum storage temperature condition required for each cream under examination through the determination of the YVT of its temperature-conductivity relation which represents a new approach indicating the importance of the yield value as factor affecting studying of the emulsions stability and consequently the suitability of the linear regression analysis-Bingham model [4] as a statistical method of the analysis of the temperature-conductivity obtained data.
The present work proves the applicability and validity of adopting a new accelerating stability testing protocol for evaluating the accelerated stability of the O/W emulsions stabilized by nonionic surfactant/s. It may be applied either accompanied with or instead of the current applied protocol of the recent approved Issues of ICH Guidelines Q1A (R2).
Criteria of the new protocol provided that, the temperature-conductivity relation of O/W cream under investigation should have strong direct linear relationship. R2 value of this relation should be ≥0.909 and its YVT should be ≥21°, in addition to, the SIPIT of this cream should be more than 80°. The new protocol determines the optimum storage temperature of the cream under examination/investigation through the determination of the YVT which accordingly enables us and the manufacturing companies to designate the optimum storage temperature required for the stability of that cream at different climates zones as well as the temperature to which the product will be cooled to obtain a fine dispersion during the preparation process of the production batch. Shinoda and Saito concluded that, the emulsification at a higher temperature especially near the PIT and cooling to storage temperature of the emulsion are effective in obtaining fine and uniform dispersions [3].
The new protocol provides us an alternative accelerated test procedure as the current applied one is performed at 40° and this temperature will alter rather than speed up the mechanism of deterioration operating at the ambient temperatures. This temperature is not acceptable and inappropriate because the viscous liquid crystalline phase that disappears above approximately 35° has a considerable stabilizing influence on the emulsions.
The new protocol takes only few days to judge the stability, so it reduces cost greatly and save time that affect the distribution of the drug in the market and consequently the patient seriously.
Acknowledgements
The author thanks Grand Pharma for Pharmaceutical Industries Company, Egypt.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Stability testing of new drug substances and products Q1A (R2). International Conference on Harmonization of Technical Requirements for Registration Of Pharmaceuticals For Human Use. 2003.
- Enever RP. Correlation of phase inversion temperature with kinetics of globule coalescence for emulsions stabilized by polyoxyethylene alkyl ether. J Pharm Sci 1976;65:517-20.
- Shinoda K, Saito H. The stability of O/W type emulsions as functions of temperature and the HLB of emulsifiers: The emulsification by PIT-method. J Colloid Sci 1969;30:258-63.
- Hassan AK. Effective surfactants blend concentration determination for O/W emulsion stabilization by two nonionic surfactants by simple linear regression. Indian J Pharm Sci 2015;77:461-9.
- Sinko PJ, Singh Y, editors. Martin’s Physical pharmacy and pharmaceutical sciences. Baltimore: Lippincott Williams and Wilkins, Wolters Kluwer business; 2011.
- Parkinson CJ, Sberman P. Phase inversion temperature as an accelerated method for evaluating emulsion stability. J Colloid Interface Sci 1972; 41:328-30.
- Rashaida AA. Flow of a non-Newtonian Bingham plastic fluid over a rotating disk. Saskatchewan: Department of Mechanical Engineering, University of Saskatchewan; 2005.
- Chatterjee S, Hadi AS. Regression Analysis by Example. 4th ed. New Jersey: John Wiley and Sons Inc; 2006.