- *Corresponding Author:
- Raghu ram rao A
Department of Pharmaceutical Chemistry, V. L. College of Pharmacy, Raichur - 584 101, India
E-mail: raghumed@hotmail.com
| Date of Received : | 21 January 2006 |
| Date of Revised : | 23 July 2007 |
| Date of Accepted : | 25 December 2007 |
| Indian J. Pharm. Sci., 2007, 69 (6): 853-856 |
Abstract
The synthesis of eight new 3-[(N,N-dialkylamino)allkyl]-2(1H)-thioquinazolin-4(3H)-ones (3a-d) and their oxo analogues (4a-d) has been described. H 1 -Antihistaminic activities have been evaluated using in vitro and in vivo standard animal models in comparison to chlorpheniramine maleate as reference drug. Among the test compounds, 3d was found to be the most potent with an IC 50 value of 1.23 µM (in vitro) offering 70.24% protection in vivo .
The clinical and pharmacological versatility of H1- receptor antagonists need not be overemphasized [1-7]. A number of ligands with varied structural features have been found to be useful. Previously we reported some quinazoline derivatives with potent antihistaminic activities [8,9]. We have reported the synthesis of some [(N,N-dialkylamino)allkyl]-2-phenyl-quinazolin- 4(3H)-ones, which showed moderate affinity towards H1-receptors [10-12]. With an aim of expanding correlation between their structural features and biological potency, we attempted the synthesis and biological evaluation of 3-[(N,N-dialkylamino)alkyl]-2(1H)- thioquinazolin-4(3H)-ones and their oxo analogues. The present communication deals with their chemistry and pharmacological evaluation.
Melting points were determined in open capillary tubes on a Thermonik precision melting point cum Boiling Point Apparatus, Model C-PMB-2, Mumbai and were uncorrected. Purity of the compounds was verified by running TLC, using precoated plates (E. Merck Kieselgel 60 F254). The IR Spectra were recorded using KBr pellets on a Perkin Elmer 337 spectrophotometer (υmax: cm-1) 1H-NMR spectra on a Varian EM-390 90 MHz spectrometer using TMS as internal standard (chemical shifts in δ ppm) and mass spectra (EI-MS) at 70 eV on VG Micromass 7070 H mass spectrometer. Elemental analyses were carried out using Heraus Carlo Erba 1180 CHN analyzer.
Histamine dihydrochloride (used as an agonist) was procured from Himedia, Mumbai. Chlorpheniramine maleate IP (Avil®) obtained from Sanofi Aventis, Mumbai was used for the recovery of the test animals after histamine aerosol administration.
General procedure used for the synthesis of (N,Ndialkylamino) alkyl-2-amino benzamides (2a-d) involved addition to 0.01 mol (1.63 g) of isatoic anhydride (1) in 25 ml of dioxane, four different dialkylamino alkyl amines, [for 2a N,N-dimethyl propylamine (2 g, 0.015 mol), for 2b, 2-(diethylamino)ethylamine (2.32 g, 0.015 mol), for 2c, 3-(diethylamino)-1- propylamine (2.6 g, 0.015 mol) and for 2d, N,Ndibutyl propylamine (3.72 g, 0.015 mol)] in 5 ml dioxane separately drop-wise with constant stirring at 5-100C for 30 min as depicted in Scheme 1. The mixture was continuously stirred for two more hours and kept overnight at 5-100. The excess of solvent was distilled off and crushed ice (100 g) was added onto the residue obtained and pH was adjusted to 5.0 using dilute acetic acid (10%). The product was extracted with ethyl acetate and evaporated to dryness. The resinous material thus obtained was triturated with petroleum ether to yield the crude product, which was used in next step without further purification.
The general procedure employed for the synthesis of 3-[(N,N-dialkylamino)allkyl]-1,2,3,4-tetra hydroquinazolin-2(1H)-thio-4(3H)-one (3a-d) is as follows; To compound 2 (0.01 mol: 2.26 g for 3a, 2.42 g for 3b, 2.56 g for 3c and 3.12 g for 3d), alcoholic potassium hydroxide (20%, 10 ml), carbon disulphide (15 ml) were added and heated under reflux for 12 h as depicted in Scheme 1. Excess of carbon disulphide was removed and to the residue, crushed ice (100 g) was added, filtered and washed with cold water, dried and recrystallized in an appropriate solvent(s).
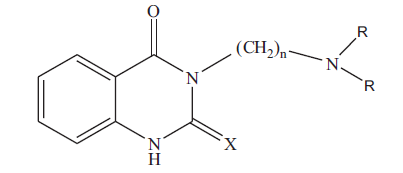
3-[(N,N-dimethylamino)propyl]-quinazolin-2(1H)-thio- 4(3H)-one (3a): colorless crystals; Rf 0.53 (chloroform: methanol, 4.8: 0.2); IR (KBr): 3281 (NH). 1636(C=0), 1277(C=S) cm-1; 1H NMR (CDCI3): δ 2.00 (m, 2H, CH2-CH2-CH2-), 2.28 (s, 6H, N(CH3)2), 2.45 (t, 2H,- CH2-CH2-CH2-), 4.5(t, 2H,-CH2-CH2-CH2), 7.1-7.3 (m,3H, aromatic-H), 10.6 (s, 1H, NH) ppm; MS (m/z, %): 263 (M+,14). Similarly 3b, 3c and 3d were characterized based on their physical, analytical and spectral data. The physical data of 3a-d is given in Table 1.
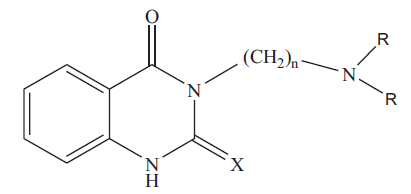 |
||||||||
| Compound no. | R | N | X | Molecular formula | mpa (°C) | Yield (%) | Elemental analyses C,H,N | Rfb |
|---|---|---|---|---|---|---|---|---|
| 3a | CH3 | 3 | S | C13H17N3OS | 180 | 85 | 59.30:6.45:15.95 | 0.53 |
| (59.32:6.46:15.97) | ||||||||
| 3b | C2H5 | 2 | S | C14H19N3OS | 148 | 92 | 60.62:6.82:15.12 | 0.49 |
| (60.65:6.86:15.16) | ||||||||
| 3c | C2H5 | 3 | S | C15H21N3OS | 150 | 90 | 61.80:7.20:14.40 | 0.44 |
| (61.86:7.22:14.43) | ||||||||
| 3d | C4H9 | 3 | S | C19H29N3OS | 160 | 91 | 66.69:8.34:11.98 | 0.38 |
| (66.71:8.36:12.10) | ||||||||
| 4a | CH3 | 3 | O | C13H17N3O2 | 153 | 94 | (63.12:6.85:16.98) | 0.57 |
| 63.15:6.88:17.00 | ||||||||
| 4b | C2H5 | 2 | O | C14H19N3O2 | 155 | 86 | 64.35:7.25:16.05 | 0.55 |
| (64.37:7.28:16.09) | ||||||||
| 4c | C2H5 | 3 | O | C15H21N3O2 | 149 | 89 | 65.44:7.61:15.25 | 0.49 |
| (65.45:7.64.27) | ||||||||
| 4d | C4H9 | 3 | O | C19H29N3O2 | 140 | 82 | 68.85:8.74:12.66 | 0.41 |
| (68.88:8.76:12.69) | ||||||||
aCompounds 3a-d were recrystallized from chloroform and 4a-d from methanol. bEluent used in tlc was chloroform:methanol (96:4).
Table 1: Physical and Analytical Data of Compounds 3a-D and 4a-D
General procedure employed for the synthesis of 3- [N,N-dialkylamino)alkyl]-1,2,3,4-tetrahydroquinazolin- 2,4(1H,3H)-dione (4a-d) is as follows. To compound 3 (0.01 mol: 2.63 g for 4a, 2.77g for 4b, 2.91 g for 4c and 3.47 g for 4d), 15 ml of alcoholic potassium hydroxide (20%) was added and the reaction mixture was heated under reflux for 2 h with continuous addition of 1 g of potassium permanganate in small quantities at a time. The reaction mixture was filtered while hot and kept aside for cooling. The solid product thus obtained was recrystallized from methanol.
3-[(N,N-dimethylamino)propyl]-quinazolin-2,4(1H,3H)- dione (4a): colorless crystals, Rf 0.41 (chloroform: methanol, 4.8:0.2), IR (KBr):3281 (NH), 1642(C=O) cm-1, 1H NMR (CDCl3) : δ 1.9 (m,2H,-CH2-CH2- CH2-), 2.1(s, 6H,-N(CH3)2), 2.3(t,2H,-CH2-CH2-CH2-), 4.2(t,2H,-CH2-CH2-CH2-), 7.1-7.3 (m, 3H, aromatic - H), 10.5 (s, 1H, NH) ppm, MS (m/z, %): 247(M+,11). Similarly 4b, 4c and 4d were characterized based on their physical, analytical and spectral data. The physical data of 4a-d is given in Table 1.
Guinea pigs of either sex, weighing 250-400 g were obtained from National Institute of Nutrition, Hyderabad, (India) and housed in wire mesh cages in a restricted access room with constant condition (23±20C, 12 h light/dark) for 3-4 days for acclimatization were used for the experiments. The animals were fed with standard laboratory pellets (Hindustan Lever Ltd. Mumbai) and purified water ad libitum. Prior to the experiment, animals were fasted overnight (i.e. they were deprived of food but maintained on purified water). All the animal experiments have been carried out according to the internationally valid guidelines and after the approval of Institutional Animal Ethical Committee (IAEC). The details of experimental procedures have been published from these laboratories earlier [11, 12].
In order to verify and ascertain reproducibility, three sets of experiments were carried out and the average IC50 value was found out (Table 2), chlorpheniramine maleate (CPM) was used as standard antihistaminic agent. Thirty-two healthy adult Hartley guinea pigs of either sex were divided into 16 groups of 2 animals each weighing around 400 g, previously fasted for overnight were kept in the histamine chamber. The animal was exposed to aerosol (0.2% aqueous solution of histamine acid phosphate in a Vaponephrin Pocket Nebulizer) until they collapse. Those that collapse within 2 min were revived with fresh air and used for this test. After 12 h, the animals were given an oral dose of test compound suspended in 1% acacia aqueous solution. After 1 h, the guinea pigs were exposed to the aerosol challenge. Those animals that withstand the antigenic challenge, i.e. collapse within 6 min, were deemed to be protected Percentage protection has been measured by calculating the time for onset of convulsions (Table 2).
 |
|||
| Compound no. | in vitro #IC50 ± SD µM | in vitro | |
|---|---|---|---|
| Mean ± SD (onset of convulsions) | % Protection | ||
| 3a | 1.52 ± 0.57 | 906 ± 9.57 | 68.65 |
| 3b | 1.61 ± 0.42 | 900 ± 6.43 | 68.19 |
| 3c | 1.33 ± 0.69 | 915 ± 5.38 | 69.33 |
| 3d | 1.23 ± 0.54 | 927 ± 8.44 | 70.24 |
| 4a | 1.51 ± 0.82 | 892 ± 8.15 | 67.59 |
| 4b | 1.70 ± 0.36 | 890 ± 5.50 | 67.44 |
| 4c | 1.41 ± 0.73 | 895 ± 9.14 | 67.82 |
| 4d | 1.32 ± 0.46 | 900 ± 9.57 | 68.19 |
| Control | -- | 88 ± 4.78 | -- |
| Standards | 1.0 ± 0.52 | 1225 ± 6.30 | 92.82 |
sChloropheneramine maleate was used as standard drug. #IC 50 denotes inhibitory concentration for 50% inhibition.
Table 2: Antihistaminic Activity of Compounds 3a-D and 4a-D.
Synthesis of title compounds (3a-d) and (4a-d) has been carried out as depicted in Scheme 1. The treatment of isatoic anhydride (1) with (N,Ndialkylamino) alkyl amines gave the corresponding benzamides (2a-d), which in turn were reacted with carbon disulphide in presence of potassium hydroxide yielded the title compounds (3a-d) quantitatively.
The suitability of using carbon disulphide for cyclizing the amides (2a-d) has been found to be advantageous over the other cyclizing agents like thiophosgene or phosgene. They are not only poisonous but also hazardous to the environment involving tedious work up procedures. Compounds (3a-d) on treatment with potassium permanganate in acetic acid yielded their corresponding oxo analogues (4a-d).
All of the synthesized compounds were characterized by their physical, analytical and spectral data (Table 1). The IR spectra of compounds (3a-d) have shown the presence of strong absorption peaks around 1640 cm-1 characteristic of carbonyl group. The absence of signals around 1150 cm-1 confirmed the total conversion of thio to oxo group in 4. The 1H NMR spectra exhibited the methylene protons at 2-4.5 δ ppm (Experimental). The mass spectral data indicated stable molecular ion peaks for all compounds (Experimental) with fragmentation pattern characteristic of their structure.
The in vitro H1- antihistaminic activity was carried out using isolated guinea pig ileum method [10,11] and the details of the procedure appeared in Bahekar and Rao12. From the graphical presentation of the logarithmic dose of the test compounds and their corresponding blockade, the IC50 (the dose corresponding to 50% inhibiton) of the agonist response was determined (Table 2). Chlorpheniramine maleate (CPM) was used as standard antihistaminic agent. It was interesting to observe that all the test compounds have shown inhibition against the histaminic contractions.
Although some failed to block the response to the agonistic challenge totally, a majority of them could cause 100% blockade at higher dose levels. However none of the compounds either produced an irreversible blockade or total indifference to the agonistic influence. Amongst them compound 3d was found to be the most potent offering IC50 of 1.23±0.54 µM.
For in vivo activity histamine chamber method13 was used. The compounds with three methylene groups (n=3) in the side chain were found to be more active than those compounds with two (n=2), in general. The increased chain length may be playing a key role in this respect. The substitution pattern on amino group does not seem to affect the biological activity significantly. Compounds 3d was found to exhibit good protection against histamine challenge in guinea pigs i.e. protection for 927s against control 88s.
Taking the clues from these SAR observations, with the help of molecular modeling studies, the synthesis and evaluation programme for potentially more active compounds is being worked out. Further investigations on the possible CNS effects and anticholinergic activity of the compounds, is in progress and the results will form the basis for our future publications.
Acknowledgements
The authors wish to thank the Principals of University College of Pharmaceutical Sciences, Kakatiya University, Warangal and V. L. College of Pharmacy, Raichur for providing laboratory facilities. The help rendered by RSIC, Lucknow is gratefully acknowledged.
References
- Maarouf AR, El-Bendary ER, Goda FE. Synthesis and evaluation ofsome novel quinazolinone derivatives as diuretic agents. Arch Pharm (Weinheim) 2004;337:527.
- Archana. Srivastava VK, Kumar A. Synthesis of newer thiadiazolyl and thiazolidinonyl quinazolin-4(3H)-ones as potential anticonvulsant agents. Eur J Med Chem 2002;37:873.
- Hey JH, Del Peado M, Sherwood J, Kreutner W, Egan RW. Comparative analysis of the cardiotoxicity proclivities of second generation antihistaminics in an experimental model predictive of adverse clinical ECG effect. Arzneim-Forsch./Drug Res 1996;46:153-8.
- Abdel-Alim AM, EI-Shorbagi AA, EI-Shareif HAH, EI-Gendy MA, Amin MA. Synthesis and biological activities of 6-bromo-2,3-disubstituted-4(3H)-quinazolinones. Indian J Chem 1994;33B:260-5.
- Wolfe JF, Rathman TL, Sleevi MC, Campbell JA, Greenwood TD. Synthesis and anticonvulsant activity of some new 2-substituted-3-aryl-4(3H)-quinazolinones. J Med Chem 1990;33:161-6.
- Alagarsamy V, Yadav MR, Giridhar R. Synthesis and pharmacological investigation of novel 1-alkyl-4-(4-substituted aryl/heteroaryl)-1,2,4-triazolo [4,3- a] quinazolin-5 (4H)-ones as a new class of H1-antihistaminic agents. Arzneimittelforsch Drug Res 2006;56:834-41
- Alagarsamy V, Giridhar R, Yadav MR. Synthesis and pharmacological investigation of novel 1-substituted-4-phenyl-1,2,4-triazolo[4,3-a]quinazolin-5(4H)-ones as a new class of H1-antihistaminic agents. Bioorg Med ChemLett 2005;15:1877-80.
- Rao AR, Reddy VM. Synthesis and H1-antihistaminic activity of beta-alkoxyethyl and beta-(N,N-dialkylamino) ethyl-(3-aryl-3,4-dihydro-4-oxoquinazolin-2-yl) methyl ethers. Pharmazie 1992;47:794-6.
- Rao AR, Reddy VM. New esters of heteryl acetic and mercaptoacetic acids. Synthesis and H1-antihistaminic evaluation. Arzneimittelforsch Drug Res 1993;43:663-7.
- Raju VS, Raju MB, Bahekar RH, Rajan KS, Rao AR. New antihistaminic agents –5 Synthesis and H1-antihistaminic evaluation of 3-(N,N-dialkylamino) alkyl derivatives of 2-phenyl-3,4-dihydroquinazolin-4(3H)-ones. Indian Drugs 1999;36:759-61.
- Singh SD, Raju MB, Bahekar RH, Rajan KS, Rao AR. New antihistaminic agents –5 Synthesis and H1-antihistaminic evaluation of 3-(N, N-dialkylamino) alkyl derivatives of 6-halo-2-phenyl-3, 4-dihydroquinazolin-4(3H)-ones. Indian J Chem 2001;40B:813-6.
- Bahekar RH, Rao AR. Bronchodilation and structure-activity relationship studies on new 6-substituted benzimidazo[1,2-c]quinazolines. Arzneimittelforsch Drug Res 2000;50:712-6
- Ghosh MN. In: Fundamentals of Experimental Pharmacology. 2nd ed. Kolkatta: Scientific Book Agency; 1984. p. 60-2.





