- *Corresponding Author:
- T. Vetrichelvan
Department of Pharmaceutical Analysis, Adhiparasakthi College of Pharmacy, Melmaruvathur - 603 319, India.
E-mail: vetrisel@yahoo.com
| Date of Submission | 10 June 2005 |
| Date of Revision | 23 August 2006 |
| Date of Acceptance | 15 April 2007 |
| Indian J Pharm Sci,69 (2) : 307-309 |
Abstract
Two simple and sensitive spectrophotometric methods (A and B) for the determination of racecadotril in bulk drugs and pharmaceutical formulations are described. In method A, methanol was used as solvent and shows absorption maximum at 231 nm. In method B, the solvent used was acetonitrile:water in the ratio of 1:3 and shows absorption maximum at 232 nm. The Beer's law range for method A is 25-100 mg/ml and 20-80 mg/ml for method B. When capsules dosage forms were analyzed, the results obtained by the proposed methods are in good agreement with the labeled amounts and the results were validated statistically.
Introduction
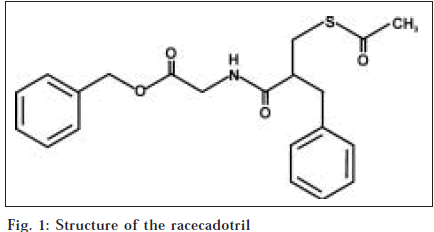
Racecadotril is an effective and safe drug for acute diarrhea in adults and children. Chemically racecadotril is N-[(R,S)-3-acetylmercapto-2-benzylpropanoyl]-glycine benzyl ester [1-3] (fig. 1). The Drug Controller General of India approved it as an antidiarrheal in October 2001 [4]. It is not yet official in any Pharmacopoeia. A survey of literature revealed that a few HPLC [5] methods were reported for the estimation of racecadotril in biological fluids. In the present report, the paper describes two simple, economical and sensitive spectrophotometric methods for the determination of racecadotril in bulk samples and solid dosage forms. In method A, methanol was used as solvent and in method B, acetonitrile:water (1:3) was employed.
Absorbance measurements were made on a Shimadzu1700 double beam UV/Vis spectrophotometer. All the solvents used for analytical studies of racecadotril were of analytical reagent grade. Pharmaceutical grade of racecadotril was kindly gifted by M/s. Micro Labs,Pondicherry, India. The capsules of racecadotril used for the studies were procured from a local Pharmacy. The solubility of racecadotril was determined in a variety of solvents using essentially a method of Schefter and Higuchi [6]. From the solubility studies, methanol and acetonitrile:water (1:3) were selected as solvents for UV spectroscopical studies of racecadotril in bulk drug and capsules dosage form. The λmax was determined in methanol and acetonitrile:water (1:3).
In method A, racecadotril (25 mg) was dissolved in methanol and the total volume was brought to 100 ml with methanol. It was further diluted to obtain 25-100 μg/ml with methanol. The absorbance was measured at 231 nm against methanol as blank. The calibration curve was plotted in the concentration range of 0.025 to 0.1 mg/ml of racecadotril in methanol. In method B, racecadotril (50 mg) was dissolved in acetonitrile:water (1:3) and the total volume was brought to 100 ml. It was further diluted to obtain 20-80 μg/ml with acetonitrile:water (1:3). The absorbance was measured at 232 nm against reagent as blank. The calibration curve was plotted in the concentration range of 0.02-0.08 mg/ml of racecadotril.
In method A, twenty capsules of each formulation S1 and S2 containing racecadotril were powdered, weighed equivalent to 25 mg of racecadotril and dissolved with 20 ml methanol, vigorously shaken for 20 minutes and filtered through Whatmann filter paper No. 41. Repeated the extraction three times, filtered and made up to 100 ml with methanol. The dilutions were made in the same manner as described under bulk drug. The absorbance measurements were made six times for each formulation. The amount of racecadotril was calculated from the respective calibration curve (method A). In method B, Capsules powder equivalent to 50 mg of racecadotril was weighed and extracted with successive quantities of weighed and extracted with successive quantities of paper No. 41 and made up to 100 ml with the same solvent. Subsequent dilutions and absorbance were measured as described under procedure for calibration curve. The amount of racecadotril was calculated from the respective calibration curve (Method B). Recovery experiments were performed by adding six different quantity of drug in previously analyzed sample, but with in the limit of Beer’s Law amount. The percentage of drug recovered was calculated by a mathematical relation followed by Sane et al[7].
The range of linearity for racecadotril was determined in methanol and acetonitrile:water and found to be 25-100 μg/ml and 20-80 μg/ml respectively. Beer’s Law limits [8],molar absorptivity, Sandell’s sensitivity [9], slope and intercept of regression analysis using least square method, precision and accuracy [10] of the analysis are summarized in Table 1. The results of pharmaceutical preparations (capsules) containing racecadotril are shown in Table 2. The outcome of recovery studies revealed the method B is more sensitive and accurate than the method A. As an additional check on the accuracy of the methods, recovery experiments were performed by adding known amount of pure drug to pre- analyzed dosage forms. The percentage of drug recovered (99-102%) was good agreement with the added amount and labeled claim. Recovery experiments indicated the absence of interference from commonly encountered pharmaceutical additives and excipients. The proposed methods were validated statistically [11] and found reproducible results. All statistical data proves validity of the proposed methods, which can be applied in industries for routine analysis of this method to analyze racecadotril in bulk drug and Pharmaceutical preparation. These results indicate that the proposed methods are sensitive, accurate, precise and reproducible.
| Parameters | method A | method B |
|---|---|---|
| λmax (nm) | 231 | 232 |
| Beers law limit (µg/ml) | 25-100 | 20-80 |
| Sandell’s sensitivity a (µg/cm /0.001 A.U) | 0.087412 | 0.077519 |
| Molar extinction coefficient (1 mol–1 cm -1 | 4.411×10 3 | 5.282×10 3 |
| Correlation coefficient (r2 ) | 0.999329 | 0.999623 |
| Regression equation (y=mx+c) | 0.566 | 0.514 |
| Slope (m) | 0.011148 | 0.01299 |
| Intercept© | 0.014737 | 0.007121 |
| Confidence limit with 0.05 level (95%) | ± 0.5800 | ± 0.3965 |
| Confidence limit with 0.01 level (90%) | ± 0.9096 | ± 0.6218 |
aSandell sensitivity (S) = 10-3 /a; S = Number of micrograms of the determined per ml of a solution having a cross section of 1 cm2 and absorbance of 0.001 and a = absorbance of 1 µg/ml solution determined in a cuvette with an optical path length of 1 cm.
Table 1: Optical Characteristics Of Proposed Methods
| Pharmaceutical formulation# | Labeled amount (mg/ capsule) | Amount found in mg* by | Percent recovery | ||
|---|---|---|---|---|---|
| Method A (mg) (mean±SD) | Method B (mg) (mean±SD) | Method A | Method B | ||
| Capsule – S1 | 100 | 100.3±0.1469 | 99.9±0.077 | 99.11 | 100.45 |
| Capsule – S2 | 100 | 100.1±0.2104 | 100.19±0.0194 | 99.08 | 101.65 |
*Mean of six determinations. #The commercial preparations used were, Capsule – S1 is Redotil, and Capsule– S2 is Cadotril.
Table 2: Assay And Recovery Studies Of Racecadotril In Capsule Dosage Form
Acknowledgements
The authors are thankful to Arulthiru Bangaru Adigalar,President, Thirumathi Lakshmi Bangaru Adigalar,Vice-President and Dr.T.Ramesh, Managing Director,- ACMEC- Trust, Melmaruvathur for providing necessary facilities to carry out this work.
References
- Budavari, S., Eds., In; The Merck Index, 13th Edn., Merck Research Laboratories, Division of Merck and Co., Inc., Whitehouse Station, NJ., 2001, 1450.
- Anonymous, CIMS, BIO-GARD (P) Ltd., Bangalore, January 2005, 88, 172.
- Reynolds, J.E.F., Eds., In; Martindale, The Extra Pharmacopoeia, 33rdEdn., The Pharmaceutical Press, London, 2002, 1337.
- Nagpal, J. and Gogia, S., Indian Pediatrics, 2004, 41, 218.
- Zhao, Z. and Liu, SC., Chin. Pharm. J., 2001, 22, 267.
- Schefter, E. and Higuchi, T., J. Pharm. Sci., 1963, 52, 781.
- Sane, R.J., Smita G. J., Mary, F., Aamer R.K. and Premangsus, H., Indian Drugs, 1999, 36, 317.
- Beckett. H.A. and Stenlake B.J., Practical Pharmaceutical Chemistry, 4thEdn., CBS Publishers, New Delhi, 2001, 275.
- Sandell E.B., Colorimetric determination of traces of metals, Inter Science, NewYork, 1950, 29.
- Kamboj, P.C., Pharmaceutical Analysis, 1stEdn.,Vallabh Publications, New Delhi, 2003, 1, 155.
- Gupta, S.C., Fundamentals of Statistics, 4thEdn., Himalaya Publishing House, New Delhi, 1999.