- *Corresponding Author:
- A. A. Shirwaikar
Department of Pharmaceutics, India
E-mail: arunshirwaikar@yahoo.co.in
| Date of Submission | 19 June 2006 |
| Date of Revision | 27 June 2007 |
| Date of Acceptance | 15 September 2007 |
| Indian J Pharm Sci, 2007, 69 (5): 633-639 |
Abstract
Co-processed particles of microcrystalline cellulose and mannitol were fabricated by spray drying technique to be used as a direct compression excipient in fast dissolving tablet formulation. Microcrystalline cellulose passed through sieve no.80, having a volumetric mean diameter (d 50 ) of 28.35 µm, was used to form composite particles with powdered mannitol which was previously passed through sieve no. 80, in various mixing ratios. The composite particles were evaluated for their powder and compression properties. An increase in the microcrystalline cellulose proportion imparted greater compressibility to the composite particles, but the flowability of these mixtures was decreased. Although microcrystalline cellulose and mannitol have been extensively used in the formulation of fast dissolving tablets, the non-wetting property of the hard compact central core may delay the disintegration time. Optimized co-processed formulation containing mannitol and microcrystalline cellulose in the ratio of 1.25:1 was found to have optimized powder and compressibility characteristics with fast disintegrating property (<15 s). Photomicrographs have shown that the mannitol crystals are fine and uniformly distributed in the microcrystalline matrix in spray dried form compared to physical mixture of the same combination. The fast disintegration may be due to the partial amorphization and formation of submicron particles of mannitol. These results indicated that improved fast dissolving tablets could be prepared by the co-processed mixture of microcrystalline cellulose and mannitol. Finally fast dissolving tablets of glipizide were prepared by blending with other excipients and compressed into tablets. Sensory study on disintegration time and mouth feel attributes ranked the present formulation based on grittiness, chalkiness and overall preference as the best.
Keywords
Rapidly dissolving oral forms, co-processed excipients, spray drying, fl owability, tablet porosity, angle of spatula, wetting time, in vitro and in vivo disintegration time
Oral tablet administration to patients is a significant problem and has become the object of public attention [1]. The problem can be resolved by the creation of rapidly dispersing or dissolving oral forms, which do not require water to aid swallowing. The simplicity and cost effectiveness of the direct compression process have positioned direct compression as an attractive alternative to traditional granulation technologies. Although simple in terms of unit process involved, the direct compression process is highly influenced by powder characteristics such as flowability, compressibility and dilution potential. Most formulations (70-80%) contain excipients at a higher concentration than the active drug [2]. The physico-mechanical properties of excipients that ensure a robust and successful process are good flowability, good compressibility, low or no moisture sensitivity, low lubricant sensitivity, and good machine ability even in high speed tableting machines with reduced dwell times [3]. Excipients with improved functionality can be obtained by developing new chemical excipients, new grades of existing materials and new combination of existing materials [4]. New combinations of existing excipients are an interesting option for improving excipient functionality because all formulations contain multiple excipients. Many possible combinations of existing excipients can be used to achieve the desired set of performance characteristics. Solid substances are characterized by three levels of solid state; the molecular, particle and bulk level. These levels are closely linked to one another, with the changes in one level reflecting in another level. A much broader platform for the manipulation of excipient functionality is provided by co-processing or particle engineering two or more excipients. Co-processing is based on the novel concept of two or more excipients interacting at the sub particle level, the objective of which is to provide a synergy of functionality improvement as well as masking the undesirable properties of individual [5]. Co-processed excipients are prepared by incorporating one excipient into the particle structure of other excipients using processes such as co-drying. A similar principle was applied in developing silicified microcrystalline cellulose which is the most widely used co-processed excipient [6,7]. Coprocessing excipient leads to the formation of excipients granulates with superior properties compared with physical mixtures of components or individual components. Usually a combination of plastic and brittle materials is used for co-processing. This combination prevents storage of too much elastic energy during the compression, which results in a small amount of stress relaxation and a reduced tendency of capping and lamination thereby optimum tableting performance [8]. Hence, co-processing these two kinds of materials produces a synergistic effect in terms of compressibility by selectively overcoming the disadvantages and can help improve functionalities such as compaction performance, flow properties, strain rate sensitivities, lubricant sensitivity or sensitivity to moisture. Because of their high aqueous solubility and sweetness, which imparts a pleasing mouth feel and good taste masking, nearly all formulations for rapidly dissolving tablets contain sugar based materials. Mannitol exhibits low moldability and a high dissolution rate and less sensitivity to humidity. Hard compacts of microcrystalline cellulose disintegrate rapidly due to the rapid passage of water into the compact and the instantaneous rupture of hydrogen bonds. Combinations of mannitol and microcrystalline cellulose are commonly used in fast dissolving/ disintegrating tablets. Higher concentration of microcrystalline cellulose may slow the disintegration of tablet due to physical entrapment of small particles between deformed microcrystalline cellulose which delays wetting and dissolution.
Materials and Methods
The following materials were used as received from commercial suppliers. Mannitol (Merck India Ltd, Mumbai, India), colloidal silicon dioxide, croscarmellose sodium (S. D. Fine Chem., Mumbai, India), microcrystalline cellulose PH101 (Avicel® FMC Corporation, Philadelphia, USA) and glipizide (Aristo Pharmaceuticals Ltd, India). All other chemicals and reagents were of analytical reagent grade.
Preparation of co-processed particles
Feed suspensions of spray drying process were prepared with mannitol and microcrystalline cellulose to have ratios of 1:1, 1.25:1, 2:1, 3:1, 4:1, 1:1.25, 1:2, 1:3, and 1:4. The required proportions of powdered mannitol and microcrystalline cellulose, which were previously passed through sieve no. 80 were dispersed in absolute alcohol to have the final solid content of feed suspension of 10%w/w. Then, suspensions were mixed thoroughly using a magnetic stirrer for 10 min to obtain homogeneous feed dispersion. The dispersions were spray dried with a JISL mini lab spray drier to prepare the co-processed excipients. The process parameters were set as follows; inlet temperature (120º); outlet temperature (60º); aspirator setting (12-18); pump setting (10 ml/min); air pressure 2 kg/cm2.
Thermal analysis
Differential scanning calorimetry (DSC) was performed on mannitol, microcrystalline cellulose, spray dried mixture of co-processed excipients and physical mixture of the excipients in the same ratio. DSC measurements were done on a Shimadzu DSC- 60 and samples were heated at the rate of 10° min-1. The samples were heated up to 250°.
Fourier transform infrared (FTIR) spectral studies
Fourier transform infrared (FTIR) spectral data were taken on a Shimadzu (model FTIR -8300) instrument to find out the chemical stability of the excipients. FTIR spectra of the mannitol, microcrystalline cellulose, spray dried mixture of co-processed excipients and physical mixture of the excipients in the same ratio were obtained. All the samples were crushed with potassium bromide to get pellets at 1 ton/cm2. Spectral scanning was done in the range between 4000-500 cm-1.
Size and size distribution
The microspheres suspended in liquid paraffin were examined using an optical microscope. Photomicrographs of the co-processed excipients were taken. The size and size distribution of the microspheres were determined from a total of 100 microspheres.
Flowability of powders
The static angle of repose was measured according to the fixed funnel and free standing cone method [9]. The bulk density of the mixed powders before compression was calculated by determining the Hausner’s ratio and Carr’s index from the poured and tapped bulk densities of a known weight of sample using a measuring cylinder and the following formula [10-11]; Hausner’s ratio = Dp÷Dt, Carr’s index= [(Dp-Dt)÷Dp]×100], where Dp (poured density)= weight÷Vp (poured volume), Dt (tapped density)= weight ÷Vt (tapped volume).
Angle of Spatula [12]
This is a readily determined property that gives a relative angle of internal friction (or angle of rupture) for a dry material. Spatula with a 5×1/3 ‘‘blade, was inserted parallel to the bottom of the container and then lifted straight up and out of the dry material. A free flowing material will form one angle of rupture. A non-free flowing material will form a number of irregular angles on the blade. In either case the angles to the horizontal were measured and an average value was taken. Then the spatula was tapped gently, producing a lower angle or angles of spatula that were measured and averaged again. The average of these two measurements was used in the flow computation and is defined as the angle of spatula.
Preparation of tablets
The composition of tablet is presented in Table 1. The composition was compressed into flat tablets with 10 mm diameter using a single punch tablet machine at a fixed compression force. The punches and die were lubricated with a small amount of magnesium stearate using a cotton swab preceding compression. The tablets were stored at 25° and 34% relative humidity for one week in a desiccator. The relative humidity of the desiccator was controlled by the use of a saturated solution of magnesium chloride hexahydrate.
| Ingredient (mg/tablet) | Batch | |||
|---|---|---|---|---|
| B1 | B2 | B3 | B4 | |
| Glipizide | 5.0 | 5.0 | 5.0 | 5.0 |
| Sodium starch glycolate (disintegrant) | 20.0 | 5.0 | 20.0 | 5.0 |
| Corn starch (anti-adherent) | 25.0 | 25.0 | 25.0 | 25.0 |
| Aspartame sodium (sweetening agent) | 15.0 | 15.0 | 15.0 | 15.0 |
| Strawberry (flavouring agent) | 10.0 | 10.0 | 10.0 | 10.0 |
| Talc (glidantflow aid) | 4.0 | 8.0 | 8.0 | 4.0 |
| Magnesium stearate (lubricant) | 3.0 | 3.0 | 3.0 | 3.0 |
| Fast dissolving co-processed excipients | ||||
| ML: MCC (1.25:1) | 250.0 | 250.0 | 250.0 | 250.0 |
| Total | 332.0 | 321.0 | 336.0 | 317.0 |
B1 - BATCH 1, B2– - BATCH 2, B3 -– BATCH 3, B4 - – BATCH 4.
Table 1: Formulation Of Fast Dissolving Glipizide Tablet Prepared By Direct Compression
Measurement of tablet tensile strength and friability
The tablet crushing load, which is the force required to break a tablet into halves by compression in the diametric direction, was measured using a Pfizer tablet hardness tester. Tablet’s friability was measured using Roche friabilator (USP) at 25 rpm for 4 min.
Measurement of disintegration time in vitro and in vivo
The disintegration test was performed using an I.P.’85 disintegration apparatus, with distilled water at 37±0.5°. All tablet property values are shown as averages of five determinations. The complete disintegration time in the mouth was measured in five healthy volunteers. The end point for the disintegration in the mouth is the time when the tablet placed on the tongue disintegrates until no lumps remain. While testing, the volunteers kept the tablets motionless on their tongues [13].
Measurement of tablet porosity
The porosity of the tablet was calculated from bulk and true tablet volume. It was calculated from the measured tablet diameter, thickness, true density of powder using the following equation E= 100 (1-Vt/ Vb) [14]. The diameter and thickness of the tablet were measured with a micrometer. The true density of the powder was determined using a helium pycnometer (AccuPyc 1330, Micrometitics Instrument Inc., Norcross, GA).
Wetting time and water absorption ratio
A piece of tissue paper folded twice was placed in a small petridish (i.d.= 6.5 cm) containing 6 ml of water. A tablet was placed on the paper and the time required for complete wetting was then measured. The water absorption ratio, R, was determined using the following equation, R=Wa-Wb÷ Wb×100, where, Wb is the weight of the tablet before water absorption and Wa is the weight of the tablet after water absorption [15]. The results were the average of five measurements.
Dissolution test
Dissolution test was carried out in 900 ml of 0.1 N HCl at 37±0.5° in a dissolution tester USP XXІV with a paddle rotation at 50 rpm. An aliquot of dissolution medium was withdrawn at various time intervals and analyzed by HPLC. Analysis was performed using an HPLC method with a mobile phase of methyl alcohol: tetrahydrofuran:potassium dihydrogen phosphate buffer adjusted to pH 2.6 with concentrated orthophosphoric acid at a flow rate of 0.8 ml/min. The column used was novapack 300×3.9 i.d. C18, 4 µm and the detection wavelength was 226 nm [16]. An equal volume of the dissolution medium was added to the beaker to maintain sink condition. Dissolution was carried out for all designed formulations and conventional marketed tablet [17].
In vivo oral absorption test
In vivo test was performed to study the oral absorption of the prepared glipizide hydrochloride after each formulation was administered to three healthy volunteers, by keeping the tablet in the oral cavity until disintegration18. The subjects then rinsed their mouth with an aliquot of distilled water. It was quantified by using UV spectrophotometer to determine the amount remaining in the oral cavity. The amount of drug absorbed through the oral mucosa was calculated by subtracting the amount from the initial amount.
Results and Discussion
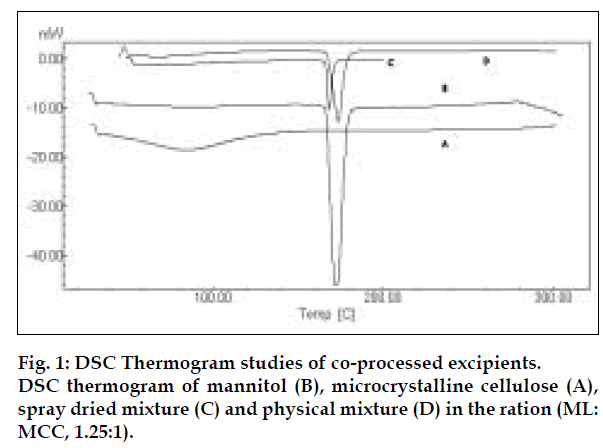
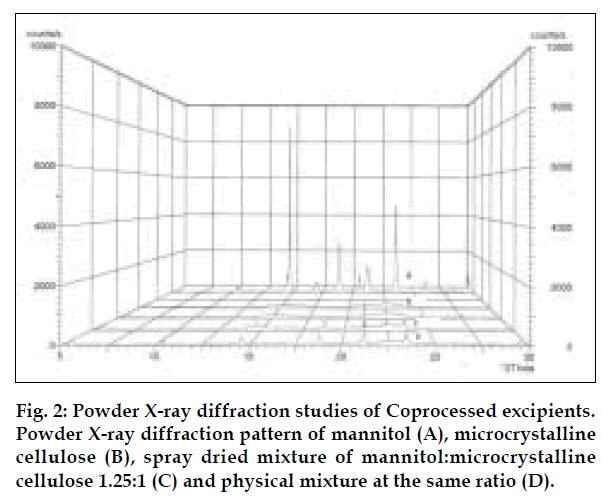
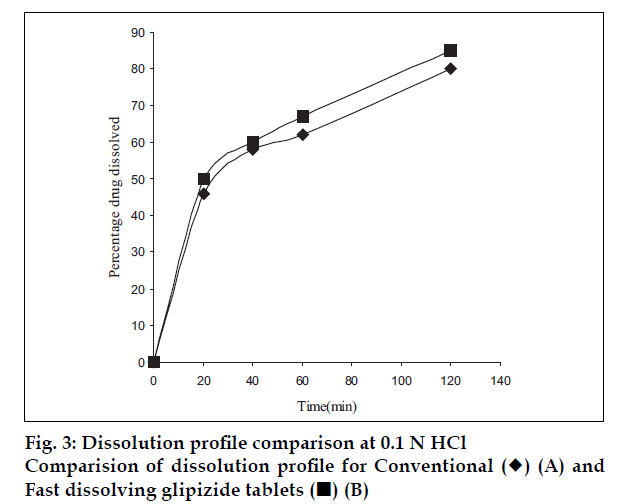
Co-processed excipients were prepared by incorporating one excipient into the particle structure of another excipient using a process such as co-drying. Thus they are simple physical mixtures of two or more existing excipients mixed at the particle level. We have found that co-processing of mannitol and microcrystalline cellulose leads to the formation of excipients granulates with superior properties compared to physical mixtures of components or with individual components. Since mannitol has good aqueous solubility, negative heat of solution and good wetting properties we have combined it with highly compressible microcrystalline cellulose which is having good wicking and absorbing capacity. These attributes improve the binding of the tablet, increase the water uptake and thereby decrease the disintegration time of the tablets. Maarschalk has inferred that co-processing works well with a large amount of brittle materials and a small amount of plastic material as exemplified by cellactose [19]. This combination prevents the storage of too much elastic energy during compression, which results in a small amount of stress relaxation and a reduced tendency for capping and lamination. A combination of plastic and brittle materials is necessary for optimum tableting performance by selectively overcoming the individual disadvantages [8]. Such combination can help improve functionalities such as compaction performance, flow properties, strain rate sensitivity, lubricant sensitivity or sensitivity to moisture, or reduced hornification. FTIR studies showed, the presence of prominent peak of mannitol at 2340 cm-1 in the spray dried and physical mixture, thereby indicating that there is no interaction between the two excipients. DSC analysis indicated that melting endotherm peak of mannitol at 173.22° is intact in spray dried and physical mixtures (fig. 1). Spray dried mixture shows heat energy value of –161.52 mJ or –80.76 J/g but physical mixture shown a value of –527.66 mJ or –195.43 J/g indicating that amorphization has occurred in the spray dried mixture. The absence of chemical changes helps to reduce a company’s regulatory concerns during the development phase. The X-ray diffraction patterns of mannitol, microcrystalline cellulose, spray dried mixture and physical mixture respectively (fig. 2) shows that, with the increasing amount of amorphous material, the base line increases too, whereas the peaks, which are a measure for the crystalline content, decrease. The peak at 23.45°2θ was chosen as analytical peak because it showed fewer variations than other peaks. Furthermore, most of the polymorphic forms of mannitol have a diffraction peak at this region [20,21]. Intensity of the peak at 23.45°2θ showed the maximum relative intensity for physical mixture and decreased intensity for mannitol crystallinity for spray dried mixture. Table 2 has shown the particle size distribution of mannitol, microcrystalline cellulose, physical and spray dried mixtures. Particle size of mannitol was decreased in spray dried mixture compared to physical mixtures. Table 3 shows the physical properties of tablet prepared from different mixing ratios of spray dried mannitol and microcrystalline cellulose using constant compressive load. There was not much change in the diameter, thickness and friability of the tablets prepared from these mixtures. As seen from Table 4, increase in microcrystalline cellulose imparted greater compressibility but flowability decreased as indicated by angle of repose and angle of spatula experiments. The percentage cohesion and flowability values indicated that the co-processed material has appropriate properties and its performance can be improved by adding glidant; like talc and magnesium stearate. With the absence of a chemical change during processing, the above co-processed excipients can be generally regarded as safe (GRAS) since the parent excipients have also GRAS certified by the regulatory agencies therefore these excipients do not require additional toxicological studies [4]. Glidant and super disintegrant amount were changed at two level and various properties of the tablet were evaluated. The disintegration time was very short without any non wetting properties at the core of the tablet. There is a good correlation between in vivo disintegration time and wetting time. Dissolution efficiency at sixty minutes values were calculated for various selected formulation batches. It was found to have consistent values between the batches. Dissolution studies for comparative evaluation of marketed and fast dissolving tablets were conducted and showed no differences between release rates (fig. 3). In comparison to direct compression tablets containing the physical mixtures of mannitol and microcrystalline cellulose, a faster drug release was observed from the tablets made from spray dried excipient mixture which might be attributed to increased porosity. Flowability of the preparation was improved considerably by adding talc and magnesium stearate. In vivo absorption was around 3% and there was a slight improvement in the bioavailability (Table 5). Finally sensory study on disintegration time and mouth feel attributes ranked the present formulation based on grittiness, chalkiness and overall preference as the best (Table 6). It can be concluded from the present work that co processed excipients of mannitol and microcrystalline cellulose are superior to physical mixtures of mannitol and microcrystalline cellulose used in fast dissolving tablets.
| Material | D10* | D50* | D90* | Spar (D90-D10)/D50 |
|---|---|---|---|---|
| ML | 500 (0.12) | 900 (0.11) | 1100(0.01) | 0.66(0.12) |
| MCC | 14.56 (0.13) | 30.3 (0.11) | 56 (0.13) | 1.367(0.12) |
| Physical mixtureof ML and MCC(1.25:1) | 42.1(0.11) | 90.4 (0.14) | 120 (0.11) | 0.8617(0.14) |
| Spray driedmixture of MLand MCC (1.25:1) | 34 (0.04) | 60.3 (0.06) | 71 (0.07) | 0.613(0.11) |
Particle size distribution studies of co-processed excipients. Mannitol (ML), microcrystalline cellulose (MCC), and physical and spray dried mixture of the excipients ML and MCC in the same ratio (1.25:1). *Mean (SD).
Table 2: Particle Size Distribution Studies Of Coprocessed Excipients
| Material | Hardness* (Kg/cm2) |
Diameter* (mm) |
Thickness* (mm) |
Friability* (%) |
Disintegration time* (min) |
|---|---|---|---|---|---|
| ML | < 1 | 10.1 | 3.0 | >5 | 0.55 |
| MCC | ŧ | 10.4 | 3.4 | 0.4 | 5.2 |
| Physical mixture ML:MCC 1.25:1 | 8.0 | 9.8 | 3.0 | 0.5 | 4.1 |
| Spray dried mixtures ML: MCC 1:1 | 10.0 | 9.9 | 3.0 | 0.3 | 1.3 |
| 1.25:1 | 9.8 | 9.8 | 3.0 | 0.3 | 0.55 |
| 2:1 | 8.9 | 9.7 | 3.0 | 0.4 | 0.54 |
| 3:1 | 8.0 | 9.7 | 3.0 | 0.4 | 0.53 |
| 4:1 | 7.9 | 9.6 | 3.0 | 0.5 | 0.54 |
| 1:1.25 | 10.2 | 10.1 | 3.0 | 0.2 | 2.3 |
| 1:2 | 10.2 | 10.3 | 3.1 | 0.2 | 2.8 |
| 1:3 | 10.4 | 10.4 | 3.1 | 0.2 | 3.2 |
| 1:4 | 10.5 | 10.4 | 3.2 | 0.2 | 3.3 |
Physical properties of tablets made by co-processed excipients using compression force of equal load. Mannitol (ML) and microcrystalline cellulose (MCC). Mean (SD). !Very hard tablets (could not be measured by hardness tester)
Table 3: Physical Properties Of Tablets Made By Coprocessed Excipients
| Material | Angle of repose*(θ) | Angle of spatula*(θ) | Compressibility* (%) | Cohesion* (%) | Flowability index* |
|---|---|---|---|---|---|
| ML | ŧ | ŧ | 45.7 | ŧ | 0.9 |
| MCC | 48.2 (0.6) | 54.2 (1.8) | 39.7 (1.8) | 0.89 (0.5) | 49.4 (1.1) |
| Physical mixture of ML: MCC1.25:1 | 34.5 (2.1) | 51.8 (0.9) | 21.1 (0.54) | 9.2 | 64.6 (1.4) |
| Spray dried mixture of ML: MCC 1:1 | 31.4 (1.1) | 56.4 (1.2) | 19.2 (0.12) | 8.8 (0.12) | 63.5 (0.8) |
| 1.25:1 | 33.4 (1.2) | 57.7 (1.4) | 19.3 (0.34) | 8.7 (0.34) | 65.4 (0.6) |
| 2:1 | 33.7 (1.4) | 57.8 (1.6) | 19.4 (0.41) | 8.9 (0.14) | 63.4 (0.5) |
| 3:1 | 34.1 (2.1) | 58.1 (1.7) | 19.8 (0.46) | 9.2 (0.14) | 64.3 (0.7) |
| 4:1 | 34.7 (2.8) | 57.7 (1.9) | 21.1 (0.11) | 9.8 (0.16) | 66.1 (1.5) |
| 1:1.25 | 31.3 (1.1) | 55.5 (0.78) | 20.6 (0.21) | 9.0 (0.21) | 68.3 (2.1) |
| 1:2 | 30.9 (0.6) | 54.9 (1.2) | 20.7 (0.34) | 8.7 (0.19) | 69.5 (1.9) |
| 1:3 | 29.9 (1.0) | 55.3 (0.78) | 20.9 (0.23) | 8.5 (0.23) | 68.4 (1.5) |
| 1:4 | 29.7 (0.97) | 56.2 (0.72) | 21.1 (0.21) | 8.5 (0.15) | 67.1 (1.1) |
Powder characteristics of starting materials and composite particles of co-processed excipients. Mannitol (ML), microcrystalline cellulose (MCC). *Mean (SD) ‡Could not able to determine due to poor fl uidity
Table 4: powder characteristics of starting materials and coprocessed excipients.
| Properties | Batch B1 | Batch B2 | Batch B3 | Batch B4 | |
|---|---|---|---|---|---|
| Angle of repose(θ) | Mean | 25.86 | 22.94 | 26.54 | 21.76 |
| CV | 0.528 | 0.127 | 0.114 | 0.138 | |
| Compressibility (%) | Mean | 24.67 | 26.76 | 27.32 | 28.32 |
| CV | 0.256 | 0.327 | 0.227 | 0.238 | |
| Friability (%) | Mean | 0.215 | 0.189 | 0.101 | 0.167 |
| CV | 0.014 | 0.013 | 0.039 | 0.013 | |
| Porosity (%) | Mean | 12.32 | 12.13 | 12.11 | 12.98 |
| CV | 0.049 | 0.022 | 0.015 | 0.023 | |
| Wetting time (sec) | Mean | 17.0 | 28.0 | 15.0 | 23.0 |
| CV | 1.56 | 1.89 | 2.76 | 2.98 | |
| Water absorption ratio (sec) | Mean | 37.56 | 36.96 | 39.93 | 32.12 |
| CV | 4.67 | 5.32 | 6.76 | 4.32 | |
| In vivo disintegration time (sec) | Mean | 17.0 | 25.0 | 18.0 | 22.0 |
| CV | 4.43 | 3.98 | 4.12 | 3.27 | |
| % glipizide absorbed from buccal cavity | Mean | 3.12 | 2.58 | 2.57 | 2.03 |
| Dissolution efÞciency 60 min (%) | Mean | 69.17 | 68.99 | 67.98 | 69.90 |
| CV | 0.65 | 0.34 | 0.22 | 0.12 |
Friability, Porosity, Wetting time, Water absorption ratio, In vivo disintegration time and Dissolution efficiency of prepared tablets
Table 5: Friability, Porosity, Wetting Time, Water Absorption Ratio And In Vivo Disintegration Time Of Prepared Tablets
| Formulation code | B1 | B2 | B3 | B4 |
|---|---|---|---|---|
| Time to dissolve (sec) | 2 | 2 | 3 | 1 |
| Grittiness | 2 | 2 | 3 | 1 |
| Chalkiness | 2 | 2 | 3 | 2 |
| Overall preference | 2 | 2 | 3 | 1 |
Sensory studies were done as per the ranking values: 1=BEST, 4=WORST
Table 6: Sensory Study On Disintegration Time And Mouth Feel Attributes Of Prepared Tablets
References
- Hanawa T. New oral dosage form for elderly patients: Preparation and characterization of silk Þbroin gel. Chem Pharm Bull 1995;47:284-8.
- York P. Crystal engineering and particle design for the powder compaction process. Drug Develop Ind Pharm 1992;18:677-722.
- Armstrong NA, Palfrey LP. The effect of machine speed on the consolidation of four directly compressible tablet diluents. J Pharm Pharmacol 1989;41:149-51.
- Moreton RC. Tablet Excipients to the year 2001: A look into the crystal ball. Drug Develop Ind Pharm 1996;22:11-23.
- Reimerdes D. The near future of tablet Excipients. Manuf Chem 1993;64:14-5.
- Dev KM. Coprocessed microcrystalline cellulose and calcium carbonate and its preparation US Patent No., 4744987, 1988.
- Bolnius GK, Chowhan RT. Materials for direct compaction in pharmaceutical powder compaction technology. In; Alderborn G, Nystrom C, editors. Powder compaction technology. Marcel Dekker Inc: New York;1996. p. 419-29.
- Casohoursat L, Lemogen G, Larrowture D. . Drug Develop Ind Pharm 1988;14:2179-82.
- Train D. Some aspects of the property of angle of repose of powders. J Pharm Pharmacol 1958;10:127-35.
- Carr RL. Evaluating flow properties of solids. Chem Eng 1965;72:163-8.
- Hausner HH. Friction conditions in a mass of metal powder. Int J Metall 1967;3:7-13.
- Terzaghi T, Peck S. In; Theoretical soil mechanics in engineering practice. Wiley J: New York; 1948. p. 112-5.
- Koizumi K. New method of preparing high porosity saliva soluble compressed tablets using mannitol with camphor. Int J Pharm 1997;152:127-31.
- Marshall K. In; Lachman L, Liberman HA, Kanig JI, editors. The theory and practice of industrial pharmacy, 3rd ed. Verghese Publishing House: Mumbai; 1987. p. 234-6.
- Bi YX, Sunada H, Yonezawa Y, Danjo K. Evaluation of rapidly disintegrating tablets prepared by a direct compression method. Chem Pharm Bull 1996;44:2121-5.
- Tracqui A, Kintz P, Mangin P, Systematic toxicological analysis using HPLC/DAD. J Forensic Sci 1995;40:254-62.
- The United States Pharmacopoeia-24/National Formulary-19, 2000, Asian Edition; US Pharmacopeial Convention. Inc: Rockville, MD; 1945-1946.
- Ishikawa T, Watanabe Y, Utoguchi N, Matsumoto N. Preparation and evaluation of tablets rapidly disintegrating in saliva containing bitter taste masked granules by the compression method. Chem Pharm Bull 1999;47:1451-6.
- Maarschalk KS, Bolhius GK. Improving properties of material for direct compression. Pharm Technol 1999;23:34-41.
- Kim AI, Akers MJ, Nail SL, The physical state of mannitol after freeze-drying: Effects of mannitol concentration, freezing rate and a non crystallizing cosolute. J Pharm Sci 1998;87:931-5.
- Yu L, Milton N, Groleau EG, Mishra DS, Vanisickle RE, Existence of mannitol hydrate during freeze-drying and practical implications. J Pharm Sci 1999;88:196-9.