- *Corresponding Author:
- G. R. Mudunuri
Department of Pharmacology, Gokaraju Rangaraju College of Pharmacy, Bachupally, Hyderabad, Telangana 500090, India
E-mail: mgrpharma@gmail.com
| Date of Received | 16 March 2021 |
| Date of Revision | 20 September 2021 |
| Date of Acceptance | 05 August 2022 |
| Indian J Pharm Sci 2022;84(4):1041-1050 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Insulin sensitivity is a symptom of diabetes, which is caused by insulin resistance, decreasing insulin production and ultimately pancreatic cancer. The current study is focused on the anti-diabetic and antioxidant properties of Chrysanthemum indicum flower extract and to scientifically validate the claim by in silico docking studies of natural compounds present in the extract for their respective sites against selected proteins. Pharmacological evaluations were done using 200 and 400 mg/kg body weight of the extract. Upon treatment with methanolic extract of Chrysanthemum indicum at 200 mg/kg body weight, 400 mg/kg body weight and glibenclamide 0.5 mg/kg body weight, significant reduction in blood glucose levels was observed. The extract was screened against reducing power assay and hydrogen peroxide scavenging assay for its anti-oxidant potential and the results were compared with the standard ascorbic acid. In both assays, the findings clearly demonstrated the extract's ability to scavenge free radicals. Docking studies were performed for natural compounds present in the extract such as epigallactocatechin, quercetin, kaempferol, apigenin, myrcetin, rutin, luteolin and standard drug glibenclamide against protein data bank ID: 1VA5, protein data bank ID: 1Z2C, protein data bank ID: 5WUA using Mcule software. The statistical verification of the model was evaluated with PROCHEK; a structure verification program relies on Ramachandran plot which determines the quality of the predicted structures. The results revealed that epigallactocatechin and rutin had shown highest glide scores which indicates a stronger receptor-ligand binding affinity among the various phytochemical constituents present in the extract and this might be the possible mechanism by which the extract showed antidiabetic and antioxidant activities. From the above it is concluded that the extract possess anti-diabetic and anti-oxidant activity.
Keywords
Antidiabetic, Antioxidant, Chrysanthemum indicum, docking, glibenclamide
Insulin sensitivity as a result of insulin resistance, decreasing insulin production and subsequent pancreatic beta (β) cell failure characterize Diabetes Mellitus (DM) [1]. Majority of people with type 2 diabetes are obese, with central visceral adiposity. It is a potentially morbid condition with high prevalence worldwide and currently global prevalence of the disease is around 200 million and would increase to 300 million by 2025. In spite of important developments in the management of DM with prescription medicines, the quest for natural antidiabetic plants as an alternate continues. Free radicals can play a part in the pathogenesis of DM and its complications, according to experimental evidence. As a result, plants that can neutralize free radicals can help to avoid diabetes and reduce the incidence of diabetic complications[2]. Phenolics and flavonoids can exert antioxidant activity through a variety of mechanisms, including quenching free radicals, chelating metal ions, and inhibiting enzymatic systems that generate free radicals[3].
Glibenclamide is a sulphonylurea of the second generation that is used as a standard medication. It primarily works by inducing insulin release from pancreatic β cells in the islets of Langerhans and by increasing the sensitivity of peripheral tissues to insulin[4]. The flowers of Chrysanthemum indicum of the family Compositae is native to India, commonly called as chaamanthi in telugu. The whole plant is used as anti-inflammatory action, in oriental medicine, antidiabetic, antihyperlipidemic, anti-oxidant, diabetic nephropathy[5-10]. The leaves are used for migraine as they are deporative and essential oil used for antimicrobial activity[11]. The various phytochemicals present in the flowers of Chrysanthemum indicum are flavonoids, phenolic compounds, steroids, triterpenoids, glycosides, carbohydrates, volatile oils and saponins.
Materials and Methods
Plant collection and drying:
The florae of Chrysanthemum indicum were collected from Bachannapet village Jangaon district, Telangana in the month of January was identified and authenticated. The flowers were cleaned, reduced to small fragments, dried under shade for about 6 d and coarsely powdered in a mixer grinder. The powdered material was stored or taken up for extraction process.
Preparations of methanolic flower extract Chrysanthemum indicum:
Soxhlet extraction is a continuous extraction method in which the same solvent is pumped repeatedly through the extractor. This procedure entails solvent extraction accompanied by evaporation. The solvent vapours are collected in a condenser and the dissolved liquid is returned to the substance for further extraction[12]. Large amounts of drug can be extracted with a much smaller quantity of solvent.
Preliminary phytochemical analysis of the extract:
The extract was subjected to preliminary phytochemical screening to identify various phytoconstituents present in Chrysanthemum indicum.
Acute toxicity testing:
The acute toxicity studies were carried out using Organization for Economic Co-operation and Development (OECD) 425 guidelines. Present study was carried out in Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) approved animal house of Gokaraju Rangaraju College of Pharmacy, Bachupally, Hyderabad, India. (Registered No. 1175/PO/ERe/S/08/CPCSEA).
Animal housing:
The Albino mice and Wistar Albino rats were held in poly acrylic cages with a maximum of six animals per cage and a 12 h light/12 h dark period. Animals have unrestricted access to a standard diet and unlimited water. The animals were given a week to acclimate to the laboratory environment before the experiment began. The care and maintenance of the animals were carried out as per the approved guidelines of the CPCSEA.
Anti-Diabetic Activity
Dexamethasone induced diabetic rat model:
30 Wistar albino rats weighing 250–300 g of either sex were selected for the present study. These rats were divided into 5 groups of 6 animals in each group. Dexamethasone (10 mg/kg, body weight (bd.wt.) p.o) was used to induce diabetes. The Methanolic Extract of Chrysanthemum indicum (MECI) (200 mg/kg, p.o and 400 mg/kg, p.o) is used for anti-diabetic effect and compared with standard glibenclamide (0.5 mg/kg, p.o). Group-I as normal vehicle control received (saline). Group II as disease control group received dexamethasone (10 mg/kg bd.wt. p.o) for 10 d, Group-III and IV as test groups received MECI (200 mg/kg, p.o) and MECI (400 mg/kg, p.o) for 10 d along with dexamethasone (10 mg/kg bd.wt. p.o) and Group-V as standard group received dexamethasone (10 mg/kg bd.wt. p.o) for 10 d along with glibenclamide (0.5 mg/kg, p.o). The blood glucose levels in the treated groups were compared with that of diabetic control group[13].
Antioxidant Activity
Reducing power assay:
The theory behind this approach is that a decrease in the absorbance of reaction mixtures implies an increase in antioxidant activity. Substances with reduction potential react with potassium Ferricyanide (Fe3+) to form potassium Ferrocyanide (Fe2+), which then interacts with ferric chloride to form ferric ferrous complex with a 700 nm absorption limit. 2.5 ml potassium Fe3+ (1 % w/v) and 2.5 ml phosphate buffer pH 6.6 were applied to 1 ml of test and normal compounds and incubated at 50° for 30 min. 2.5 ml distilled water and 0.5 ml ferric chloride solution (0.1 % w/v) were applied to 2.5 ml of the above supernatant solvent. The absorbance of ferric ferrous complex was measured at 700 nm using a ultravioletvisible spectrophotometer with phosphate buffer pH 6.6 as a control and the increase in absorbance was calculated. The following equation was used to measure the percent (%) increase in reducing power.
Percentage increase in reducing power (%)=(Abs control)-(Abs sample)/(Abs control)×100
Where, Abs (control): Absorbance of the control and Abs (sample): Absorbance of the extracts/standard[14].
Hydrogen peroxide radical scavenging assay:
Hydrogen peroxide is a weak oxidizing agent and can inactivate a few enzymes directly, usually by oxidation of essential Thiol (-SH) groups. Hydrogen peroxide can cross cell membranes rapidly, once inside the cell, hydrogen peroxide can probably react with Fe2+, and possibly Copper (Cu2+) ions to form hydroxyl radical and this may be the origin of many of its toxic effects. It is therefore biologically advantageous for cells to control the amount of hydrogen peroxide that is allowed to accumulate. A solution of hydrogen peroxide (2 mmol/l) was prepared in phosphate buffer (pH 7.4) and test compounds (10-100 μg/M) were added to hydrogen peroxide solution (0.6 ml). Absorbance of hydrogen peroxide at 230 nm was determined after 10 min against a blank solution containing phosphate buffer without hydrogen peroxide and compared with ascorbic acid, the reference compound.
Percent Hydrogen peroxide activity=(Abs control)-(Abs sample)×100
(Abs control)
Where, Abs (control): Absorbance of the control; Abs (sample): Absorbance of the extract/standard[14].
Molecular docking by Mcule:
Molecular docking is the study of how two or more molecular structures (e.g., drug and enzyme or protein) fit together. In a simple definition, docking is a molecular modeling technique that is used to predict how a protein (enzyme) interacts with small molecules (ligands). The ability of a protein (enzyme) and nucleic acid to interact with small molecules to form a supra molecular complex plays a major role in the dynamics of the protein, which may enhance or inhibit its biological function[15]. To understand the interactions between the ligands and proteins and to explore their binding mode, docking studies were performed using Mcule software. In the present study the docking studies were performed for natural compounds such as epigallocatechin, quercetin, kampherol, apigenin, myrcetin, rutin, luteolin present in the extract and standard drug glibenclamide against selected proteins. The statistical verification of the model was evaluated with PROCHEK; a structure verification program relies on Ramachandran plot which determines the quality of the predicted structures.
Selection of proteins:
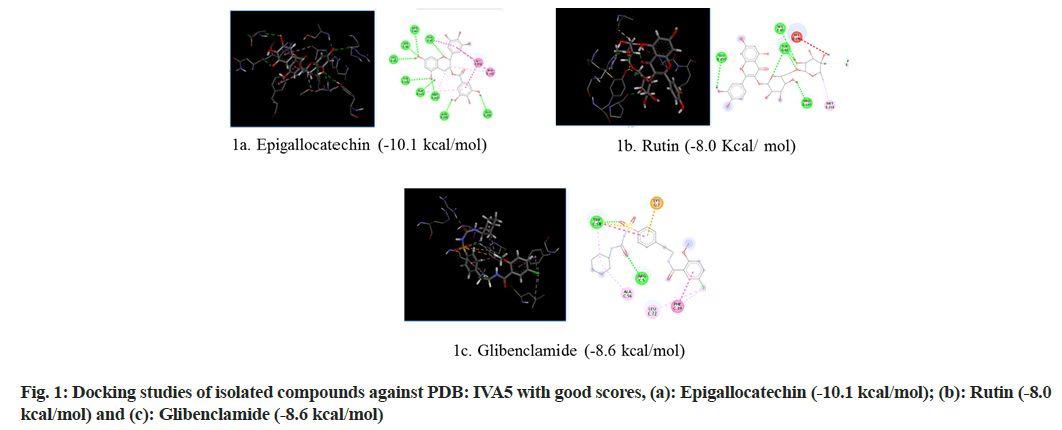
Selection of Protein Data Bank (PDB) ID-1VA5: Glucokinase (GK) is an enzyme that regulates glucose metabolism in the liver. Diabetes is related to the inactivation of GK, while hypoglycemia is linked to an increase in GK operation. The possibility of controlling GK activity with small chemical compounds acting as allosteric activators has piqued scientists' interest in studying the process of new allosteric GK activators are being developed[16]. The first phase in the complicated mechanism that regulates GK function is the interaction of GK with ligands. Molecular docking was used to investigate the interaction of the GK promoter (PDB ID: 1VA5) with natural compounds contained in the extract and the standard drug glibenclamide in this study (fig. 1).
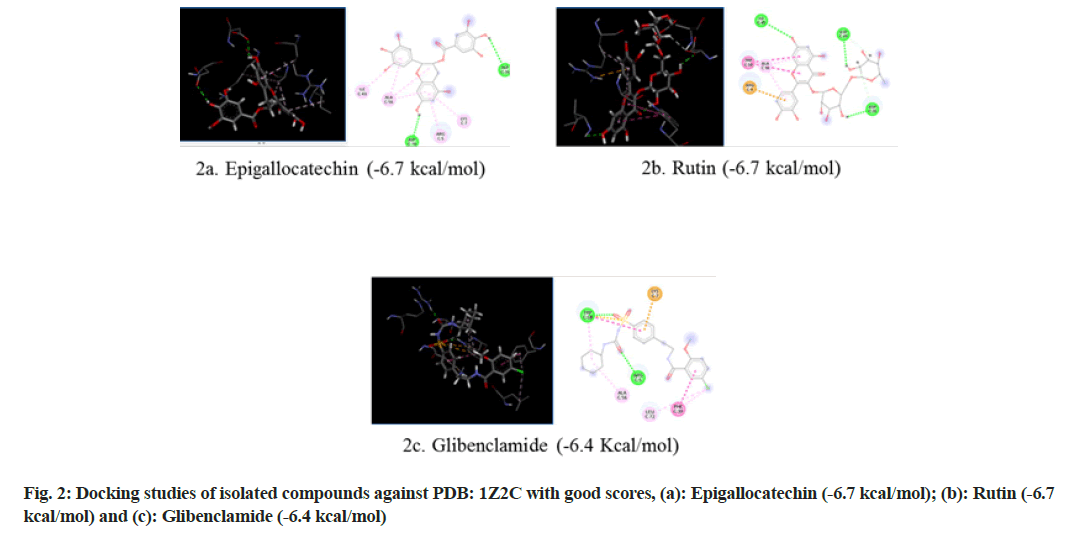
Selection of PDB ID-1Z2C: When proteins are exposed to reducing sugars, they undergo nonenzymatic glycation, which results in Advanced Glycation End products (AGEs). Glycation causes enzymatic function loss, protein crosslinking and aggregation. The accumulation of AGEs plays an important role in many health disorders including DM, immunoinflammation, cardiovascular and neurodegenerative diseases. AGEs mediate their pathological effects by activating signaling cascades via the Receptor for Advanced Glycation End products (RAGE), a 45 kDa transmembrane receptor of the immunoglobulin superfamily prevalent at low concentrations in a variety of healthy human tissues, including the lungs, kidneys, liver, cardiovascular, nervous and immune systems[17]. As a receptor for AGE and other proinflammatory ligands, RAGE has been investigated as a potential biomarker of numerous pathological conditions. So in the present study RAGE inhibitor (PDB ID: IZ2C) was selected and subjected to molecular docking (fig. 2).
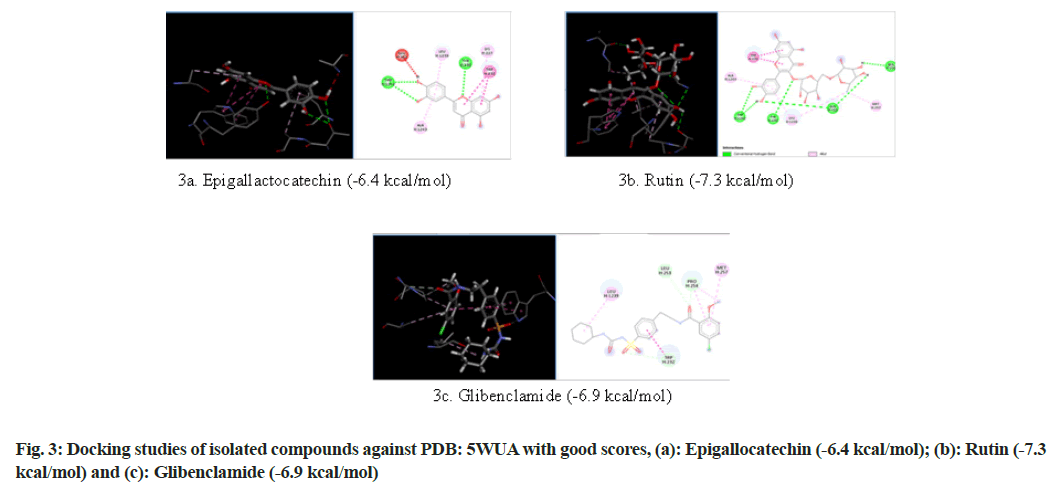
Selection of PDB ID-5WUA: The Adenosine Triphosphate (ATP)-sensitive K+ channel (KATP channel) in the pancreatic cell is critical for coupling membrane excitability with Glucose-Stimulated Insulin Secretion (GSIS)[18]. Increased glucose metabolism causes an increase in the intracellular [ATP]/[ Adenosine Diphosphate (ADP)] ratio, KATP channel closure and membrane depolarization. Consequent activation of voltage-dependent Calcium (Ca2+) channels causes a rise in Ca2+, which stimulates insulin release. Conversely, a decrease in the metabolic signal is predicted to open KATP channels and suppress the electrical trigger of insulin secretion. Sulfonylurea drugs promote and diazoxide suppresses, insulin secretion by binding to the regulatory Sulfonylurea Receptor-1 (SUR1) subunit and, inhibiting or activating KATP channel current respectively[19]. So in the present study an ATP sensitive K+ channel inhibitor protein (PDB ID: 5WUA) is selected and subjected to molecular docking (fig. 3).
Results and Discussion
MECI was screened for its anti-diabetic activity and antioxidant activity. All the results obtained in this study were included. The MECI was prepared by Soxhlation technique. The percent yield of the extract was calculated by using the following formula.
Percent yield of extract=Amount of powder used (gm)/ Amount of extract obtained (gm)×100
=60/500×100
=12 % w/w
MECI showed the presence of flavonoids, phenolic compounds, steroids, triterpenoids, glycosides, carbohydrates, proteins and volatile oils. The results are showed in Table 1.
| Phytochemical constituents | Results |
|---|---|
| Flavonoids | ++ |
| Phenolic compounds | ++ |
| Steroids | ++ |
| Triterpenoids | + |
| Glycosides | + |
| Carbohydrates | + |
| Volatile oils | + |
| Saponins | - |
Note: (+): Present and (-): Absent
Table 1: Preliminary Phytochemical Analysis
Acute toxicity studies of MECI were performed as per OECD 425 guidelines. The oral administration of MECI did not exhibit any signs of toxicity and mortality even upto 2000 mg/kg. bd. wt. All animals were safe even after 14 d of observation.
The MECI was tested to determine its effects on blood glucose level in dexamethasone induced diabetic rat model.
Results obtained in dexamethasone induced diabetic rat model were tabulated in the Table 2. From the results, it is clear that the blood glucose levels of diabetic rats were elevated when compared to normal rats. Upon treatment with MECI at 200 mg/kg, bd. wt, 400 mg/kg; bd. wt. and glibenclamide 0.5 mg/kg, bd. wt. significant reduction in blood glucose levels was observed.
| Treatment | Blood glucose level (gm/dl) | ||
|---|---|---|---|
| 0th d | 5th d | 10th d | |
| Normal control | 84.5±1.124 | 92.33± 0.565 | 90.167±0.723 |
| Negative control (dexamethasone 10 mg/kg) | 154.5±0.964a,** | 162.5±0.5652a,** | 234.5±0.905a,** |
| MECI (200 mg/kg) | 152.62±0.451a,** | 144.5±0.807A,a,** | 125.83±0.796A,a,** |
| MECI (400 mg/kg) | 151.67±0.4513a,B,** | 137.17±0.280A,a,** | 104.33±0.652A,a,** |
| Glibenclamide (0.5 mg/kg) | 152.5±0.656A,** | 107.67±0.693A,** | 94.5±0.697A,** |
Note: Values are expressed as mean±standard error of mean, (n=6). Statistical analysis was performed by using analysis of variance followed by Dunnett’s test. Results were compared with control group (**p<0.01), diabetic control (Ap<0.01, Bp<0.05) and standard (ap<0.01)
Table 2: Anti-Diabetic Activity Of MECI on Dexamethasone Induced Diabetic Rats
The MECI was subjected to in vitro antioxidant activity by reducing power assay and hydrogen peroxide scavenging assay.
The in vitro antioxidant activity was performed using reducing power assay. The half-maximal Inhibitory Concentration (IC50) value of the MECI was 36 μg/ ml and the standard drug ascorbic acid was 30 μg/ml respectively. From the results it is clear that the MECI showed antioxidant activity. The results were expressed in Table 3.
| S. No | Compound | Concentration (µg/ml) | % Inhibition | IC50 value (µg/ml) |
|---|---|---|---|---|
| I | MECI | 10 | 10.79±1.352 | 36 |
| 20 | 31.80±0.637 | |||
| 30 | 45.39±0.202 | |||
| 40 | 56.55±0.129 | |||
| 50 | 68.97±0.066 | |||
| II | Ascorbic acid | 10 | 18.46±1.576 | 30 |
| 20 | 35.59±0.562 | |||
| 30 | 54.37±0.157 | |||
| 40 | 66.78±0.330 | |||
| 50 | 72.71±0.348 |
Note: Values are expressed as mean±standard error of mean
Table 3: Anti-Oxidant Activity of MECI on Reducing Power Assay Method
The MECI has shown antioxidant activity in reduced power assay. The reducing ability of a compound generally depends on the presence of reductants which have been exhibiting antioxidative potential by breaking the free radical chain and donating a hydrogen atom.
The in vitro antioxidant activity was performed using hydrogen peroxide scavenging assay. The IC50 value of the MECI was 20 μg/ml and ascorbic acid was 15 μg/ml respectively. From the results it is clear that the MECI showed antioxidant activity. The results were expressed in Table 4. The MECI has shown antioxidant activity in hydrogen peroxide radical scavenging assay. The scavenging capacity of a compound may serve as a significant indicator of its potential antioxidant activity[14].
| S. No | Compound | Concentration (µg/ml) | % Inhibition | IC50 value (µg/ml) |
|---|---|---|---|---|
| I | MECI | 10 | 40.23±0.340 | 20 |
| 20 | 48.23±0.388 | |||
| 30 | 56.49±0.173 | |||
| 40 | 63.77±0.129 | |||
| 50 | 74.60±0.07 | |||
| II | Ascorbic acid | 10 | 46.14±0.421 | 15 |
| 20 | 53.61±0.183 | |||
| 30 | 64.14±0.461 | |||
| 40 | 73.31±0.490 | |||
| 50 | 77.06±0.117 |
Table 4: Anti-Oxidant Activity of MECI on Hydrogen Peroxide Radical Scavenging Activity
Dexamethasone, a corticosteroid, is similar to a natural hormone produced by your adrenal glands. Glucocorticoids are known to oppose insulin action at the level of the liver by promoting gluconeogenesis and at the periphery, especially in muscle, by inhibiting glucose uptake and reducing glycogen synthesis and glucose storage. In addition the glucocorticoids are believed to attenuate insulin secretion, particularly the extent of the anticipated hyperinsulinaemic response to the insulin resistant state. Together these mechanisms are postulated to cause the development of glucose intolerance and diabetes observed in patients exposed to glucocorticoid therapy[14] (Table 5).
| Components | Glide score (Kcal/mol) | ||
|---|---|---|---|
| PDB ID: 1VA5 | PDB ID: 1Z2C | PDB ID: 5WUA | |
| Glibenclamide | -8.6 | -6.4 | -6.9 |
| Epigallactocatechin | -10.1 | -6.7 | -6.4 |
| Kampherol | -7 | -6 | -6.2 |
| Rutin | -8 | -6.7 | -7.3 |
| Apigenin | -7.4 | -6.3 | -6.3 |
| Quercetin | -7.6 | -6 | -6.2 |
| Myrcetin | -7.5 | -6 | -6.4 |
| Luteolin | -7.7 | -6.1 | -6.3 |
Table 5: Glide Score for Different Natural Components and Glibenclamide
In the present study phytochemical screening has revealed the presence of flavonoids, phenolic compounds, steroids, triterpenoids, glycosides, carbohydrates, volatile oils as major chemical constituents. Flavonoids like epigallactocatechin, quercetine, myricetin, luteolin, apigenin, kaempferol, rutin and vitex are majorly present in the extract as reported in Gas Chromatography– Mass Spectrometry (GC-MS) analysis[20]. Quercetin is 3,5,7,3’,4’-pentahydroxyflavone or quercetin dihydrate (C15H10O7) is the most abundant flavonoid in human dietary nutrition. It is another epidrug present in citrus fruits and buckwheat. This drug acts as a DNA Methyltransferase 1 (DNMT1) inhibitor (via the repression of Tumor Necrosis Factor (TNF) induced Nuclear Factor-kappa B (NF-κB) transcription factor) and promotes Fas ligand-related apoptosis via histone H3 acetylation and potential Histone Deacetylase Inhibitors (HDACi). Quercetin was shown to be involved in the stimulation of glucose uptake through MAPK insulin-dependent mechanism. This is accomplished in the muscle via the translocation of Glucose Transporter-4 (GLUT4) transporters and in the liver via the downregulation of key gluconeogenesis enzymes. Treating Streptozotocin (STZ) induced diabetic rats with quercetin decreases the activity of GK, hyperglycemia stimulating GLUT4, hepatic gluconeogenesis and glycogenolysis while it increases glucose liver uptake[21]. Quercetin supplementation for 2 w lowered the blood glucose level, upregulated the expression of genes involved in cell survival and proliferation in a liver, and enhanced the serum insulin in STZ induced diabetic mice[22]. Quercetin blocks the activities of a tyrosine kinase inhibitor, which has shown an effect against diabetes. The regulatory effect of quercetin to NF-κB also helps in improving glucose stimulated insulin secretion[23]. The co-treatment of quercetin and sitagliptin (a selective dipeptidyl peptidase-IV inhibitor) demonstrated an improvement in its oxidative and inflammatory status, metabolic profile, glycemic control, β cells function and islet structure in STZ induced DM in rats[24]. Quercetin intake is inversely associated with the prevalence of Type 2 DM (T2DM) in the Chinese population which suggests its preventive activity against T2DM.
Rutin is also known as glycosylated quercetin, sophorin and quercetin-3-O-rutinoside. The anti-diabetic effects of rutin includes the reduction of carbohydrates absorption from the small intestine, the improvement of glucose uptake by tissues, the suppression of tissue gluconeogenesis, the activation of insulin secretion from β-cells and the protection of the islets of Langerhans from degenerative changes. Rutin also lowers the formation of reactive oxygen species, advanced glycation end-product precursors, sorbitol and pro-inflammatory cytokines[25]. The oral or Intraperitoneal administration of rutin (50 mg/kg, bd.wt or 100 mg/kg, bd. wt) into a STZ model of type 1 diabetic rats significantly decreased glycated hemoglobin/A Hemoglobin A1c (HbA1c) and Fasting Blood Glucose (FBG)[26].
Kaempferol (3,4' ,5,7-tetrahydroxyflavone) is a natural flavonol, found in a variety of plants and plant-derived foods, has several antidiabetic effects, like improving Adenosine Monophosphate (AMP) activated cellular protein expression and activation, reducing cellular apoptosis by suppressing caspase 3 activities, and increasing the production and secretion of insulin from β-cells. The extracts of kaempferol from Bauhinia forficate leaves reduce hyperglycemia and enhance glucose uptake, mimicking the action of insulin[27]. In vitro studies confirmed that treating with 10 μM of kaempferol enhances cellular viability and represses apoptosis. Apigenin is a trihydroxyflavone that is flavone substituted by hydroxy groups at positions 4', 5 and 7. The administration of apigenin (4 mg/kg, bd. wt) in STZ induced diabetic rats, showed a significant anti-hyperglycemic effect[28]. Apigenin treatment could prevent induced apoptosis through the inhibition of NF-κB activation. In alloxan induced insulin dependent diabetic mice, the oral administration of apigenin for 10 d reduced hepatic antioxidants, like catalase, glutathione and superoxide dismutase. The treatment with apigenin helps in reducing hyperglycemia, serum cholesterol and Glucose-6-Phosphatase (G6Pase) activities in the liver[29]. Luteolin 3' ,4' ,5,7-tetrahydroxyflavone, is a common flavonoid and was reported to initiate insulin action and to enhance the expression of Peroxisome Proliferator-Activated Receptor gamma (PPAR-γ) target genes in primary mouse adipose cells. In damaged pancreatic cells, β cells, this flavonoid improves insulin secretion in uric acid by decreasing Micro-Autologous Fat Transplantation (MAFT), a transactivator of insulin gene through NF-κB signaling pathway[30].
Myricetin is a hexahydroxyflavone that is flavone substituted by hydroxy groups at positions 3, 3', 4', 5, 5' and 7. It has been isolated from the leaves of Myrica rubra and other plants. It has a role as a cyclooxygenase 1 inhibitor, an antineoplastic agent, an antioxidant, a plant metabolite, a food component and a hypoglycemic agent. Myricetin has been observed to increase the activity of glycogen synthase 1 in the hepatocytes of rats with diabetes[31]. Recent evidence suggests that myricetin administration might be beneficial for increasing insulin sensitivity and inhibiting islet β-cell apoptosis[32]. Epigallocatechin Gallate (EGCG), also known as epigallocatechin-3-gallate, is the ester of epigallocatechin and gallic acid, and is a type of catechin. Hepatic glucose production was regulated by epigallocatechin through Phosphoinositide 3-Kinase (PI3K) dependent manner[33]. L-EGCG could markedly inhibit the activity of a-glucosidase in vitro as well as in vivo and therefore exerted its significant effect on postprandial hyperglycemia improvement. Glibenclamide acts to increase secretion of insulin from the pancreas, probably by interacting with SURs on β cells or by interfering with ATP-sensitive potassium channels on pancreatic β cells, which increases secretion of insulin. These drugs also may increase sensitivity of existing insulin receptors.
The results have indicated that MECI at 200 mg/kg, bd. wt. and 400 mg/kg, bd. wt. significantly reduced the elevated FBG levels in diabetic animals. The possible mechanism by which MECI reduced the elevated blood glucose levels might be due to the presence of flavonoids and phenolics. These phytochemical constituents might be increasing either the pancreatic secretion of insulin from β cells of islets of Langerhans or increased peripheral utilization of glucose[34]. Like the standard drug glibenclamide, the flavonols like kaempferol, rutin and quercetin were able to reduce blood glucose levels by acting as insulin secretagogue. The presence of these flavonols in the extract might have worked synergistically in the reduction of blood glucose levels.
Free radical scavenging action is considered to be one among the various mechanisms for antioxidation. The MECI has shown antioxidant activity in reducing power assay and hydrogen peroxide radical scavenging assay.
Reducing power assay method is based on the principle that substances, which have reduction potential, react with potassium Fe3+ to form potassium Fe2+, which then reacts with ferric chloride to form ferric–ferrous complex that has an absorption maximum at 700 nm.
The reducing capacity of a compound can be known by measuring Fe3+-Fe2+ complex due to electron donating activity of MECI, which is an important mechanism of antioxidant action and this might be due to the presence of flavonoids and phenols in the extract with adequate number of hydroxyl groups[35].
Hydrogen peroxide is a weak oxidizing agent that inactivates a few enzymes directly and cross cell membranes rapidly; once inside the cell, it can probably react with Fe2+ and possibly Cu2+ ions to form hydroxyl radicals and this may be the origin of many of its toxic effects. The scavenging capacity of a compound may serve as a significant indicator of its potential antioxidant activity. The MECI have shown reducing capacity in reducing power, assay hydrogen peroxide and good scavenging ability which was comparable to ascorbic acid. It might be due to the presence of flavonoids and phenols in the extract with adequate number of hydroxyl groups as reported[36].
The application of antioxidants especially of natural origin is one of the strategies in treating DM[37]. Triterpenes play an important role as plant antioxidants. Ursolic acid exhibited hydroxyl radical scavenging activity, perhaps through its hydrogen donating ability. Some triterpene compounds possess the ability to suppress formation of AGEs and are promising agents in the prevention and treatment of DM.
MECI have shown reducing capacity in reducing power assay and also scavenged the toxic hydroxyl free radical produced from hydrogen peroxide. And this may be used as an indicator of the potential anti-oxidant activity.
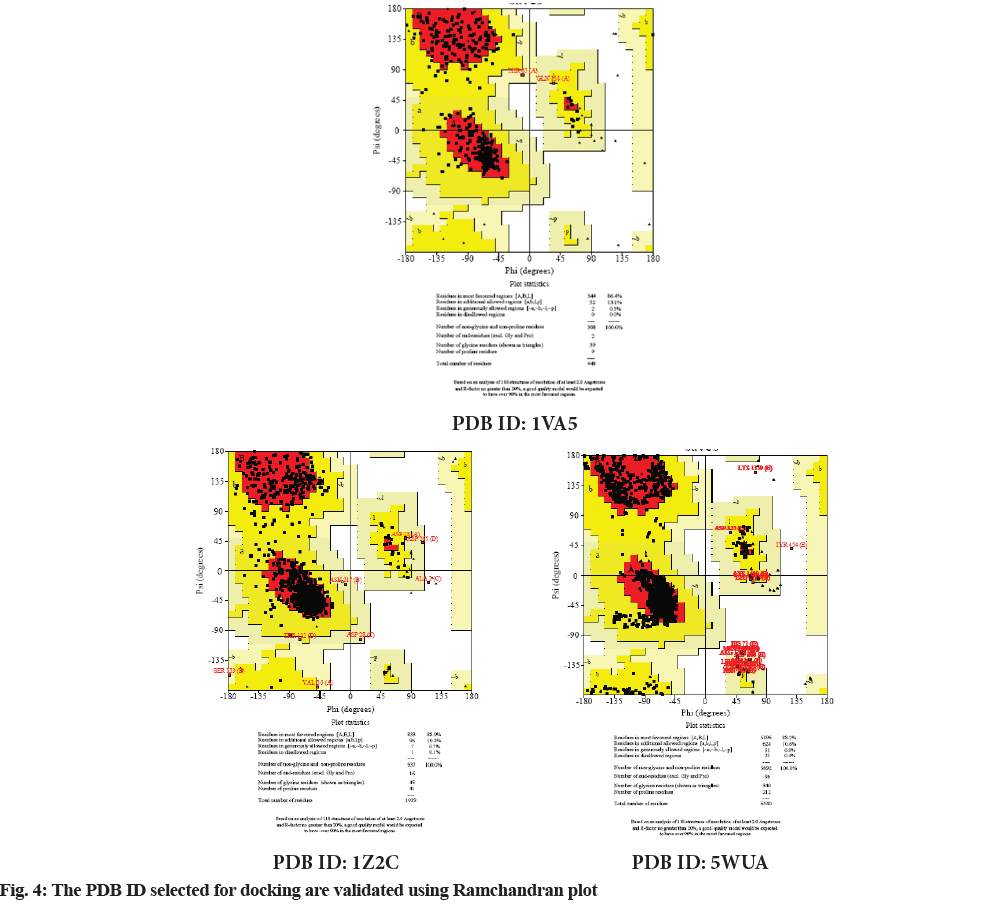
Molecular docking continues to holds great promise in the field of computer based drug design which screens small molecules by orienting and scoring them in the binding site of a protein. The docking analysis of isolated compounds from MECI and standard drug like glibenclamide were carried out using Mcule database. The various constituents identified in the plant extract are epigallocatechin, quercetin, kampherol, apigenin, myrcetin, rutin, luteolin and standard drug glibenclamide were subjected to docking against PDB ID: 1VA5, PDB ID: 1Z2C and PDB ID: 5WUA. The proteins identified namely PDB ID: 1VA5, PDB ID: 1Z2C and PDB ID: 5WUA are modeled and the qualities of the 3D model were evaluated using the PROCHECK program and assessed using the Ramachandran plot (fig. 4). It is evident from the Ramachandran plot that predicted models have most favorable regions, additionally allowed regions, generally allowed regions and disallowed regions. Such a percentage distribution of the protein residues determined by Ramachandran plot shows that the predicted models are of good quality. According to Ramachandran plot, a good quality model would be expected to have over 90 % in most favored regions. So in the present study also, all the proteins models selected showed more than 90 % most favored regions clearly indicating that these models are of good quality.
The results revealed that epigallocatechin and rutin had shown highest glide scores among the various phytochemical constituents present in the extract. The highest glide scores were observed with epigallocatechin, glibenclamide and rutin against PDB ID: 1VA5 and PDB ID: 1Z2C whereas the rutin, epigallocatechin and glibenclamide against PDB ID: 5WUA. The glide scores of the epigallocatechin and rutin were found to be similar with the glide score of standard drug glibenclaimde stating that the compounds have same affinity to bind to the proteins. These results clearly indicate that the chemical constituents’ epigallocatechin and rutin might have shown similar mechanism to that of the standard drug glibenclamide in reducing diabetes. The MECI possess anti-diabetic and anti-oxidant activity in rodent models.
Acknowledgements:
The authors are grateful to the management of the Gokaraju Rangaraju College of pharmacy, for the constant support and encouragement during the course of the work.
Conflict of interests:
All authors have no conflicts of interest to declare.
References
- Fujioka K. Pathophysiology of type 2 diabetes and the role of incretin hormones and beta-cell dysfunction. JAAPA 2007;20(12):3-8.
[Crossref] [Google Scholar] [Pub Med]
- Rani MS, Pippalla RS, Mohan GK, Gangaraju M. Anti-diabetic activity of methanolic and ethyl acetate extracts of Wrightia tinctoria R. Br. fruit. Int J Pharm Sci Res 2012;3(10):3861-6.
- Firuzi O, Lacanna A, Petrucci R, Marrosu G, Saso L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim Biophys Acta 2005;1721(1-3):174-84.
[Crossref] [Google Scholar] [Pub Med]
- Jacobs DB, Hayes GR, Lockwood DH. In vitro effects of sulfonylurea on glucose transport and translocation of glucose transporters in adipocytes from streptozocin-induced diabetic rats. Diabetes 1989;38(2):205-11.
[Crossref] [Google Scholar] [Pub Med]
- Choi G, Yoon T, Cheon MS, Choo BK, Kim HK. Anti-inflammatory activity of Chrysanthemum indicum extract in acute and chronic cutaneous inflammation. J Ethnopharmacol 2009;123(1):149-54.
[Crossref] [Google Scholar] [Pub Med]
- Kim IS, Ko HM, Koppula S. Protective effect of Chrysanthemum indicum against 1-methyl-4-phenylpridine ion and lipopolysaccharide-induced cytotoxicity in cellular model parkinson’s disease. Food Chem Toxicol 2011;49(4):963-73.
- Sharma A, Sharma AK, Chand T, Khardiya M, Yadav KC. Antidiabetic and antihyperlipidemic activity of Cucurbita maxima Duchense (pumpkin) seeds on streptozotocin induced diabetic rats. J Pharmacogn Phytochem 2013;1(6):108-6.
- Patel DK, Kumar R, Prasad SK, Sairam K, Hemalatha S. Antidiabetic and in vitro antioxidant potential of Hybanthus enneaspermus (Linn) F. Muell in streptozotocin–induced diabetic rats. Asian Pac J Trop Biomed 2011;1(4):316-22.
[Crossref] [Google Scholar] [Pub Med]
- Pandit R, Phadke A, Jagtap A. Antidiabetic effect of Ficus religiosa extract in streptozotocin-induced diabetic rats. J Ethnopharmacol 2010;128(2):462-6.
[Crossref] [Google Scholar] [Pub Med]
- Kumar AY, Nandakumar K, Handral M, Talwar S, Dhayabaran D. Hypoglycaemic and anti-diabetic activity of stem bark extracts Erythrina indica in normal and alloxan-induced diabetic rats. Saudi Pharm J 2011;19(1):35-42.
[Crossref] [Google Scholar] [Pub Med]
- Shunying Z, Yang Y, Huaidong Y, Yue Y, Guolin Z. Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J Ethnopharmacol 2005;96(2):151-8.
[Crossref] [Google Scholar] [Pub Med]
- Rangari DV. Pharmacognosy and Phytochemistry. 1st ed. Nashik (Maharashtra): Career publication; 2004.
- Raju MG, Reddy THS. Aristolochia bracteolate-Anti hyperglycemic and antihyperlipidemic activity in dexamethasone induced diabetic rat model. Asian J Pharm Clin Res 2017;10(8):75-7.
- Ganga Raju M, Kumara Swamy K. Anti-inflammatory and antiradical potential of methanolic extract of Cajanus cajan. Asian J Pharm Pharmacol 2018;4(6):860-4.
- Roy K, Kar S, Das RN. Understanding the basics of QSAR for applications in pharmaceutical sciences and risk assessment. 1st ed. Academic press; 2015. p. 357-425.
- Ermakova E. Structural insight into the glucokinase-ligands interactions. Molecular docking study. Computational Biol Chem 2016;64:281-96.
[Crossref] [Google Scholar] [Pub Med]
- Bongarzone S, Savickas V, Luzi F, Gee AD. Targeting the receptor for advanced glycation endproducts (RAGE): A medicinal chemistry perspective. J Med Chem 2017;60(17):7213-32.
[Crossref] [Google Scholar] [Pub Med]
- Ashcroft FM, Rorsman P. ATP-sensitive K+ channels: A link between B-cell metabolism and insulin secretion. Biochem Soc Trans 1990;18(1):109-11.
[Crossref] [Google Scholar] [Pub Med]
- Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev 1999;20(2):101-35.
[Crossref] [Google Scholar] [Pub Med]
- Liang-Yu W, Hong-Zhou G, Xun-Lei W, Jian-Hui Y, Jian-Liang L, Yue-Rong L. Analysis of chemical composition of Chrysanthemum indicum flowers by GC/MS and HPLC. J Med Plants Res 2010;4(5):421-6.
- Kobori M, Masumoto S, Akimoto Y, Takahashi Y. Dietary quercetin alleviates diabetic symptoms and reduces streptozotocin‐induced disturbance of hepatic gene expression in mice. Mol Nutr Food Res 2009;53(7):859-68.
[Crossref] [Google Scholar] [Pub Med]
- Eid HM, Nachar A, Thong F, Sweeney G, Haddad PS. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn Mag 2015;11(41):74-81.
[Crossref] [Google Scholar] [Pub Med]
- Eitah HE, Maklad YA, Abdelkader NF, El Din AA, Badawi MA, Kenawy SA. Modulating impacts of quercetin/sitagliptin combination on streptozotocin-induced diabetes mellitus in rats. Toxicol Appl pharmacol 2019;365:30-40.
[Crossref] [Google Scholar] [Pub Med]
- Dai X, Ding Y, Zhang Z, Cai X, Li Y. Quercetin and quercitrin protect against cytokine‑induced injuries in RINm5F β-cells via the mitochondrial pathway and NF-κB signaling. Int J Mol Med 2013;31(1):265-71.
[Crossref] [Google Scholar] [Pub Med]
- Ghorbani A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed Pharmacother 2017;96:305-12.
[Crossref] [Google Scholar] [Pub Med]
- Niture NT, Ansari AA, Naik SR. Anti-hyperglycemic activity of rutin in streptozotocin-induced diabetic rats: An effect mediated through cytokines, antioxidants and lipid biomarkers. Indian J Exp Biol2014;52(7):720-7.
- An G, Gallegos J, Morris ME. The bioflavonoid kaempferol is an Abcg2 substrate and inhibits Abcg2-mediated quercetin efflux. Drug Metab Dispos 2011;39(3):426-32.
[Crossref] [Google Scholar] [Pub Med]
- Rauter AP, Martins A, Borges C, Mota‐Filipe H, Pinto R, Sepodes B, et al. Antihyperglycaemic and protective effects of flavonoids on streptozotocin–induced diabetic rats. Phytother Res 2010;24(S2):S133-8.
[Crossref] [Google Scholar] [Pub Med]
- Kim EK, Kwon KB, Song MY, Han MJ, Lee JH, Lee YR, et al. Flavonoids protect against cytokine-induced pancreatic β-cell damage through suppression of nuclear factor κB activation. Pancreas 2007;35(4):e1-9.
[Crossref] [Google Scholar] [Pub Med]
- Ding Y, Shi X, Shuai X, Xu Y, Liu Y, Liang X, et al. Luteolin prevents uric acid-induced pancreatic β-cell dysfunction. J Biomed Res 2014;28(4):292.
[Crossref] [Google Scholar] [Pub Med]
- Kandasamy N, Ashokkumar N. Protective effect of bioflavonoid myricetin enhances carbohydrate metabolic enzymes and insulin signaling molecules in streptozotocin–cadmium induced diabetic nephrotoxic rats. Toxicol Appl Pharmacol 2014;279(2):173-85.
[Crossref] [Google Scholar] [Pub Med]
- Lin CY, Ni CC, Yin MC, Lii CK. Flavonoids protect pancreatic beta-cells from cytokines mediated apoptosis through the activation of PI3-kinase pathway. Cytokine 2012;59(1):65-71.
[Crossref] [Google Scholar] [Pub Med]
- Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK. Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem 2002;277(38):34933-40.
[Crossref] [Google Scholar] [Pub Med]
- Prince PS, Kannan NK. Protective effect of rutin on lipids, lipoproteins, lipid metabolizing enzymes and glycoproteins in streptozotocin‐induced diabetic rats. J Pharm Pharmacol 2006;58(10):1373-83.
[Crossref] [Google Scholar] [Pub Med]
- Deepa B, Prema G, Sai KB. Antioxidant and free radical scavenging activity of triphala determined by using different in vitro models. J Med Plants Res 2013;7(39):2898-905.
- Nishaa S, Vishnupriya M, Sasikumar JM, Hephzibah PC, Gopalakrishnan VK. Antioxidant activity of ethanolic extract of Maranta arundinacea L. tuberous rhizomes. Asian J Pharm Clin Res 2012;5(4):85-8.
- Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother 2005;59(7):365-73.
[Crossref] [Google Scholar] [Pub Med]