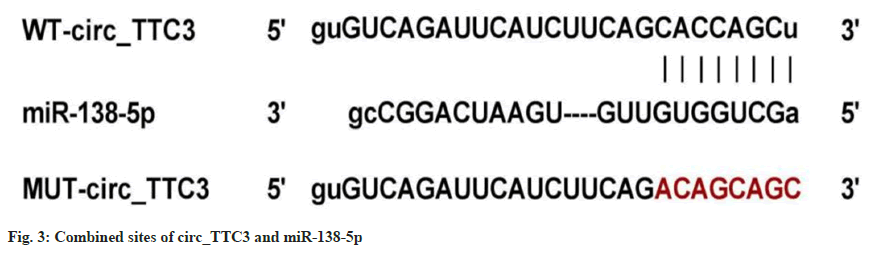- *Corresponding Author:
- Na Zhao
Department of Traditional Chinese Medicine and Health, Changchun Humanities and Sciences College, Changchun, Jilin Province 130119, China
E-mail: zhaona84@126.com
| Date of Received | 03 June 2023 |
| Date of Revision | 08 December 2023 |
| Date of Accepted | 13 February 2024 |
| Indian J Pharm Sci 2024;86(1):278-285 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect and its mechanism of oleuropein on the oxidative damage and apoptosis of astrocytes induced by oxygen-glucose deprivation, and its effect on circ_TTC3 and microRNA-138-5p. Astrocytes were divided into control group, oxygen-glucose deprivation group, oxygen-glucose deprivation+oleuropein 25 μmol/l, 50 μmol/l, 100 μmol/l groups, oxygen-glucose deprivation+si-negative control, si-circ_TTC3 group, oxygen-glucose deprivation+oleuropein+plasmid cloning deoxyribonucleic acid plasmid cloning deoxyribonucleic acid and plasmid cloning deoxyribonucleic acid-circ_TTC3 group. In comparison with the control group, the inhibiting rate of cell growth, apoptosis rate, malondialdehyde, lactate dehydrogenase levels and circ_TTC3 expression of astrocytes in the oxygen-glucose deprivation group were increased, while superoxide dismutase activity and miR-138-5p expression were declined (p<0.05). Compared to the oxygen-glucose deprivation group, the proliferative inhibiting rate, apoptosis rate, malondialdehyde, lactate dehydrogenase levels and circ_TTC3 expression of astrocytes in the oxygen-glucose deprivation+oleuropein 25 μmol/l group, oxygen-glucose deprivation+oleuropein 50 μmol/l group, and oxygen-glucose deprivation+oleuropein 100 μmol/l group were all weakened, superoxide dismutase activity and microRNA-138-5p expression were enhanced (p<0.05), and all were concentration-dependent. After silencing circ_TTC3, circ_TTC3 expression, malondialdehyde, lactate dehydrogenase levels, cell proliferation inhibition rate and apoptosis rate in astrocytes in the oxygen-glucose deprivation+si-circ_TTC3 group were all lower than those in oxygen-glucose deprivation+si-negative control group, while the superoxide dismutase activity was higher than that in oxygen-glucose deprivation+si-negative control group (p<0.05). Circ_TTC3 targeted and regulated microRNA-138-5p. Circ_TTC3 level, malondialdehyde and lactate dehydrogenase levels, cell proliferation inhibition rate, and apoptosis rate in astrocytes in the oxygen-glucose deprivation+oleuropein+ plasmid cloning deoxyribonucleic acid-circ_TTC3 group were all higher than those in oxygen-glucose deprivation+oleuropein+plasmid cloning deoxyribonucleic acid group, and there was a decrease of superoxide dismutase activity in the oxygen-glucose deprivation +oleuropein+plasmid cloning deoxyribonucleic acid-circ_TTC3 group compared with oxygen-glucose deprivation+oleuropein+plasmid cloning deoxyribonucleic acid group (p<0.05). Oleuropein could reduce oxidative stress and apoptosis, thereby protecting astrocytes from damage induced by oxygen-glucose deprivation by upregulating circ_TTC3 to target microRNA-138-5p.
Keywords
Astrocytes, oleuropein, oxygen-glucose deprivation, oxidative stress, apoptosis, circ_TTC3, microRNA-138-5p
Stroke is a main reason of physical disabilities and the second-major reason for death worldwide[1,2], therein approximately 87 % of these cases are attributed to ischemia[3]. During the past decades, researchers have primarily concentrated upon neurons to treat ischemic injuries. Ischemic injury usually involves multiple pathological processes, including oxidative stress and apoptosis[4,5]. There is increasing evidence about the main function of astrocytes in ischemic damage. Astrocytes can supply the support for neurons at nutritional, structural and metabolic levels and they are the richest sort of neuroglial cells in the brain[6]. Aside from these physical functions, these astrocytes also can play neuroprotective role in fighting ischemic damage[7,8]. Injury and unbalance of the astrocytes will compromise the survival of neurons after ischemia. Hence, protecting and functionally regulating astrocytes is crucial tactic for preventing neuronal injury ascribed to ischemia.
Oleuropein is a phenolic compound from olive leaf extract. According to literature reports, oleuropein has been reported to have good therapeutic effects on the damage caused by myocardial ischemiareperfusion[9], cerebral ischemia-reperfusion[10] and renal ischemia-reperfusion[11] through antioxidant or anti-apoptotic mechanisms. However, the role of oleuropein in ischemia-damaged astrocytes is not yet clear.
Some circular RNAs (circRNAs) and microRNAs (miRNAs) have been identified as key regulatory Ribonuclic Acid (RNAs) participated in the occurrence and evolution of ischemic stroke[12,13]. Circ_TTC3 was mentioned to promote cerebral ischemia-reperfusion damage and inhibit neural stem cell function in cerebral infarction through miR-372-3p/Toll-Like Receptor 4 (TLR4) axis[14].
miR-138-5p was low expressed in PC-12 cells which was treated with Oxygen-Glucose Deprivation (OGD)/reoxygenation[15], and can also protect astrocytes from damage induced by OGD[16]. However, the relation of circ_TTC3 and miR-138- 5p and whether it is involved in the functional mechanism of oleuropein-induced ischemic injury of astrocytes are still unclear. In view of this, this research concentrate upon investigating the effects of oleuropein on apoptosis and oxidative stress damage of astrocytes induced by OGD, and combined with its regulatory effects on circ_TTC3 and miR-138-5p, furtherly to determine the latent molecular mechanism underlying the efficacy of oleuropein.
Materials and Methods
Cells and reagents:
Human astrocytes were purchased from two Biotech Co., Ltd (Shenzhen, China). Oleuropein (≥98 %), Superoxide Dismutase (SOD), Malondialdehyde (MDA) and Lactate Dehydrogenase (LDH) kits were bought from Beyotime (Shanghai, China).
Cell culture and OGD injury:
The astrocytes were fostered at 37° in Dulbecco’s Modified Eagle Medium (DMEM) added with 10 % Fetal Bovine Serum (FBS) and 1 % penicillin/ streptomycin antibiotics in 5 % Carbon dioxide (CO2) incubator. For OGD injury, the astrocytes were quickly cleaned with Phosphate Buffer Solution (PBS) followed by the addition of sugarfree medium, and later injured in 37° atmosphere with 5 % CO2, 1 % Oxygen (O2) and 94 % Nitrogen (N2) for 6 h[17].
Experimental grouping and treatment:
The experiment was split into multiple groups; control, OGD, OGD+oleuropein 25 μmol/l, OGD+oleuropein 50 μmol/l, OGD+oleuropein 100 μmol/l, OGD+si-Negative Control (si-NC), OGD+si-circ_TTC3, OGD+oleuropein+plasmid cloning Deoxyribonucleic Acid (pcDNA), OGD+oleuropein+pcDNA-circ_TTC3 group. MiR-NC or miR-138-5p mimic, si-circ_TTC3, si- NC, pcDNA-circ_TTC3, pcDNA were purchased from Ribobio (Guangzhou, China). Among them, the astrocytes in the control group did not undergo any treatment, and the cells in the OGD group were damaged by oxygen and glucose deprivation.
Oleuropein was dissolved in PBS and prepared as 10 mmol/l mother solution. During the experiment, astrocytes were treated with (25, 50, or 100 μmol/l) oleuropein diluted by culture medium for 24 h and oxygen-sugar deprivation injury was performed. Astrocytes in the groups of OGD+si-NC, OGD+si-circ_TTC3, OGD+oleuropein+pcDNA, and OGD+oleuropein+pcDNA-circ_TTC3 were planted into 6-well plates (1×105 cells). According to the instruction manual of Lipofectamine 2000, si-NC, si-circ_TTC3, pcDNA and pcDNAcirc_ TTC3 were transfected into cells with 60 % confluence. After (6-8) h, above cells were replaced with complete medium or 100 μmol/l oleuropeinadded medium for 24 h, followed by OGD injury.
Cell Counting Kit-8 (CCK-8) assay:
Astrocytes were planted in 96-well plate and managed as mentioned in experimental grouping and treatment. Later, CCK-8 reagent (10 μl/well, Beyotime) was supplied for the cells and put into a 5 % CO2 incubator for 2 h. Synergy H1 microplate reader was used for measuring the (Optical Density (OD)=450 nm) value. The proliferation inhibition rate of astrocytes was 100 %-(OD value ratio of treatment group/control group)×100 %.
Analysis of apoptosis:
The astrocytes were grouped and processed as described in experimental grouping and treatment. Following the wash using pre-cooling PBS, 1×105 cells were gathered and dyed for 15 min using Annexin V-Fluorescein Isothiocyante (FITC) and Propidium Iodide (PI) in a gloomy environment following apoptotic kit (Beyotime). The apoptotic percentage was measured via flow cytometry and cell quest software.
Detection of oxidative stress levels:
The astrocytes were grouped according to experimental grouping and treatment and the supernatant of the cells was respectively collected according to the operating instructions of the SOD, MDA, and LDH detection kits, and later the SOD activity, MDA and LDH levels of astrocytes were tested by corresponding kits.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR):
The astrocytes were grouped and processed as described in experimental grouping and treatment. Trizol (Invitrogen, Carlsbad, CA, United States of America (USA)) was used for isolating total RNA, and multi scribe RT kit (Invitrogen) was applied for reverse transcription. The levels of circ_TTC3 and miR-138-5p was respectively tested via SYBR Green Master Mix (TaKaRa, Dalian, China) and miRNA qPCR kit (Ribobio). Relative expression was evaluated through 2− ΔΔCt strategy. The primers are listed as, forward circ_TTC3, 5'-CACGATTGCATCCCTGTGTG-3'; reverse 5'-ACTGTCACGTTTCAAGATCACT-3'; forward Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH), 5'-AAGCCACCCCACTTCTCTCTA A - 3 ' ; reverse 5'-AATGCTATCACCTCCCCTGTGT-3'; forward miR-138-5p, 5'-GCCGGATAAGTGTTGTGGTCGA-3'; reverse 5'-ACTGAGCAAGCACTACCACCAGCA-3'; forward U6, 5'-CTCGCTTCGGCAGCACA-3' and reverse 5'-AACGCTTCACGAATTTGCGT-3'.
Dual-luciferase reporter assay:
Starbase software forecasted the binding sequences of circ_TTC3 and miR-138-5p. The Wild-Type (WT) and Mutant (MUT) circ_TTC3 sequences with miR-138-5p combinative sites were synthesized and fused with the luciferase reporter plasmid psiCHECK2 to construct WTcirc_ TTC3 and MUT-circ_TTC3 plasmids. The astrocytes were inoculated into 6-well plates, and WT-circ_TTC3 or MUT-circ_TTC3 was subjected to the co-transfection of miR-NC or miR-138-5p mimics according to the manual of Lipofectamine 2000. 48 h later, the activity of luciferase was tested through an exploratory system of dualluciferase reporter gene. In addition, si-NC, sicirc_ TTC3, pcDNA or pcDNA-circ_TTC3 was respectively transfected into astrocytes, and 48 h later, miR-138-5p level in astrocytes was tested as described in qRT-PCR.
Statistical analysis:
Data are appeared as mean (x̄±s) standard deviation. Statistical Package for the Social Sciences (SPSS) 22.0 software was used to evaluate statistical significance. Comparing the difference of multiple groups or between two groups was conducted using one-way analysis of variance or independent sample t-test. p<0.05 was be deemed to have statistical significance.
Results and Discussion
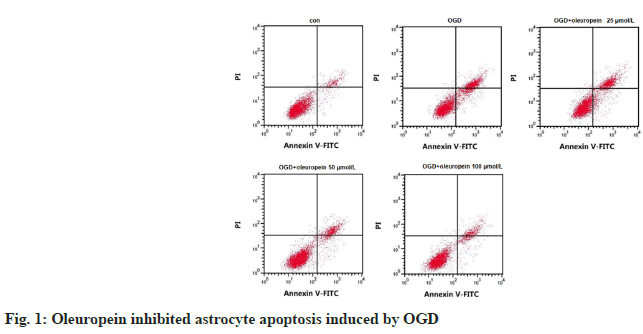
Compared with control group, the rate of proliferation inhibition and apoptosis of astrocytes in OGD group was upregulated (p<0.05). While the rate in OGD+oleuropein (containing 25, 50, or 100 μmol/l oleuropein) was declined vs. OGD group (p<0.05) in a concentration-related way (Table 1 and fig. 1).
| Group | Inhibition rate/% | Apoptosis rate/% |
|---|---|---|
| Control | 0.00±0.00 | 7.32±0.79 |
| OGD | 56.26±1.80* | 24.44±1.65* |
| OGD+oleuropein 25 μmol/l | 44.78±2.16 # | 21.39±1.22# |
| OGD+oleuropein 50 μmol/l | 31.93±1.15#△ | 17.08±1.20#△ |
| OGD+oleuropein 100 μmol/l | 21.11±1.45#△□ | 11.72±1.05#△□ |
| F | 1873.976 | 296.369 |
| p | <0.05 | <0.05 |
Note: *p<0.05 vs. control group; #p<0.05 vs. OGD group; △p<0.05 vs. OGD+leuropein 25 μmol/l group; □p<0.05 vs. OGD+leuropein 50 μmol/l group
Table 1: The Influence of Oleuropein on Apoptosis of Astrocytes (X̄±S, N=9)
SOD activity of astrocytes after OGD treatment was reduced vs. control group, MDA and LDH levels were incremental (p<0.05). In comparison with OGD group, SOD activity of astrocytes after the treatment of OGD and different concentration of oleuropein (25, 50, or 100 μmol/l) was increased, while MDA and LDH levels were decreased (p<0.05), and all in a concentration-related way Table 2.
| Group | SOD/U·l-1 | MDA/nmol·ml-1 | LDH/U·l-1 |
|---|---|---|---|
| Control | 508.47±32.95 | 38.44±3.59 | 155.89±15.37 |
| OGD | 140.73±13.03* | 410.90±21.66* | 751.37±37.38* |
| OGD+oleuropein 25 μmol/l | 246.88±13.56# | 350.64±11.55# | 633.77±28.32# |
| OGD+oleuropein 50 μmol/l | 371.21±21.85#△ | 264.34±13.44#△ | 471.52±35.76#△ |
| OGD+oleuropein 100 μmol/l | 434.65±18.51#△□ | 150.35±12.57#△□ | 281.82±20.34#△□ |
| F | 431.666 | 1069.020 | 652.529 |
| p | <0.05 | <0.05 | <0.05 |
Note: *p<0.05 vs. control group; #p<0.05 vs. OGD group; △p<0.05 vs. OGD+leuropein 25 μmol/l group; □p<0.05 vs. OGD+leuropein 50 μmol/l group
Table 2: Effects of Oleuropein On Sod, Mda And Ldh In Astrocytes (X̄±S, N=9)
Compared with control group, circ_TTC3 level in astrocytes of OGD group was increased, while miR-138-5p level was declined (p<0.05). While the expression of circ_TTC3 in astrocytes of OGD and 25, 50, or 100 μmol/l oleuropein coprocessing group was decreased vs. OGD group, and the expression of miR-138-5p was increased (p<0.05), and both of them were concentration dependent (Table 3).
| Group | circ_TTC3 | miR-138-5p |
|---|---|---|
| Control | 1.00±0.00 | 1.00±0.00 |
| OGD | 3.75±0.11* | 0.13±0.02* |
| OGD+oleuropein 25 μmol/l | 3.19±0.08# | 0.33±0.04# |
| OGD+oleuropein 50 μmol/l | 2.46±0.10#△ | 0.55±0.05#△ |
| OGD+oleuropein 100 μmol/l | 1.52±0.08#△□ | 0.78±0.07#△□ |
| F | 1670.841 | 574.263 |
| p | <0.05 | <0.05 |
Note: *p<0.05 vs. control group; #p<0.05 vs. OGD group; △p<0.05 vs. OGD+leuropein 25 μmol/l group; □p<0.05 vs. OGD+leuropein 50 μmol/l group
Table 3: Effects of Oleuropein on Expression of circ_TTC3 and Mir-138-5p in Astrocytes (X̄±S, N=9)
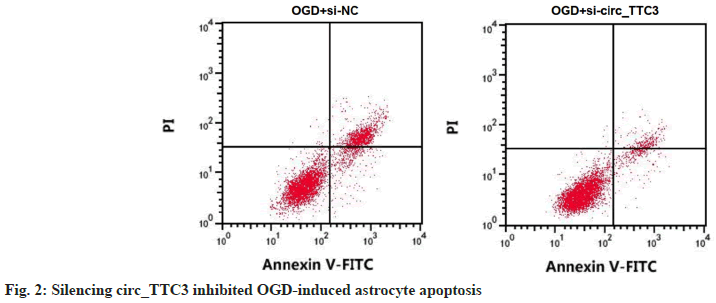
After circ_TTC3 was silenced, circ_TTC3 level in OGD-induced astrocytes was approximately 0.68 times lower than that in si-NC transfection group, and the activity of SOD was elevated vs. OGD+si- NC group, while MDA and LDH levels, inhibition rate of cell growth and the rate of apoptosis were all decreased vs. OGD+si-NC group (p<0.05) (Table 4 and fig. 2).
| Group | circ_TTC3 | SOD/U.l-1 | MDA/nmol.ml-1 | LDH/U.l-1 | inhibition rate/% | apoptosis rate/% |
|---|---|---|---|---|---|---|
| OGD+si-NC | 1.00±0.00 | 139.21±16.42 | 411.71±26.47 | 737.02±43.50 | 56.40±2.08 | 24.23±1.75 |
| OGD+si-circ_TTC3 | 0.32±0.04* | 460.79±28.97* | 99.16±6.43* | 230.35±18.62* | 14.21±0.81* | 9.12±0.58* |
| t | 51.000 | 28.971 | 34.422 | 32.122 | 56.703 | 24.588 |
| p | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
Note: *p<0.05 vs. OGD+si-NC group
Table 4: Detection of OGD-Induced Apoptosis and Oxidative Stress of Astrocytes by Silenced circ_TTC3 (X̄±S, N=9)
The sites between circ_TTC3 and miR-138- 5p binding was predicted by star base (fig. 3). The luciferase activity of WT-circ_TTC3 cotransfected with miR-138-5p was dramatically declined vs. miR-NC transfection (p<0.05), while there was no marked difference in the luciferase activity of MUT-circ_TTC3 in miR-138-5p and miR-NC groups (p=0.604) (Table 5). The level of miR-138-5p was 4.25±0.13 in si-circ_TTC3 group in astrocytes, which was higher than 1.00±0.00 in si-NC group (t=75.000, p<0.05), and 0.21±0.02 in pcDNA-circ_TTC3 group, which was lower than 1.00±0.00 in pcDNA group (t=118.500, p<0.05).
| Group | WT-circ_TTC3 | MUT-circ_TTC3 |
|---|---|---|
| miR-NC | 1.00±0.12 | 0.98±0.11 |
| miR-138-5p | 0.46±0.05* | 0.95±0.13 |
| t | 12.462 | 0.528 |
| p | <0.05 | 0.604 |
Note: *p<0.05 vs. miR-NC group
Table 5: Dual-Luciferase Reporter Assay (X̄±S, N=9)
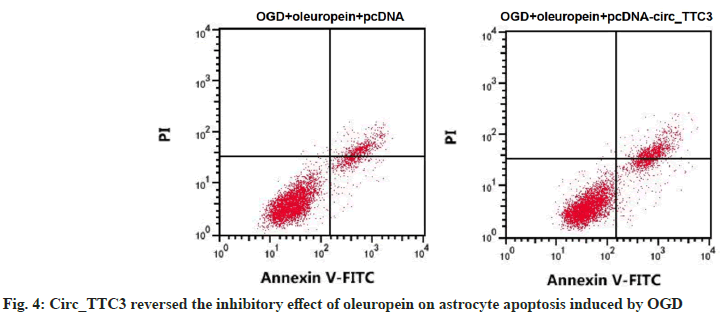
Circ_TTC3 level in astrocytes of OGD+oleuropein+pcDNA-circ_TTC3 group was about 1.89 times higher compared to OGD+oleuropein+pcDNA group and SOD activity was lower vs. OGD+oleuropein+pcDNA group. Moreover, MDA and LDH levels, inhibition rate of cell growth and apoptosis rate in astrocytes of OGD+oleuropein+pcDNA-circ_TTC3 group were all elevated compared to OGD+oleuropein+pcDNA group (p<0.05) (Table 6 and fig. 4).
| Group | circ_TTC3 | SOD/U·l-1 | MDA/nmol·ml-1 | LDH/U·l-1 | inhibition rate/% | apoptosis rate/% |
|---|---|---|---|---|---|---|
| OGD+oleuropein pcDNA | 1.00±0.00 | 432.84±19.76 | 146.10±16.98 | 276.43±24.37 | 21.07±1.48 | 11.92±1.23 |
| OGD+oleuropein+pcDNA-circ_TTC3 | 2.89±0.16* | 200.14±11.56* | 378.61±19.53* | 670.59±34.32* | 48.64±3.19* | 22.55±1.50* |
| t | 35.438 | 30.494 | 26.953 | 28.093 | 23.520 | 15.960 |
| p | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
Note: *p<0.05 vs. OGD+oleuropein+pcDNA group
Table 6: The Effect of Circ_TTC3 on OGD-Induced Apoptosis and Oxidative Stress of Astrocytes was Attenuated by Oleuropein (X̄±S, N=9)
Astrocytes are the richest glial cells existed in the central nervous system[18,19]. Astrocytes are demonstrated to exert a crucial function in protecting neurons after stroke in many ways, including antioxidant activity, ion homeostasis, energy transfer, and neurotransmitter transport[20,21]. Thus, alleviating astrocyte damage is a promising direction for preventing excessive neuronal injury after stroke. In vitro OGD models of astrocytes have been widely used to simulate ischemic damage occurring in stroke[22]. The main factors causing ischemic stroke include oxidative damage, apoptosis, autophagy, inflammation, energy metabolism disorders, and so on[23]. Cellular defense in the brain involves endogenous protective enzymes, such as SOD, which are produced by astrocytes to protect neurons from oxidative stress[24]. In addition, oxidative stress markers LDH and MDA can also be used as indicators for ischemic injury. Here, we showed that OGD caused the damage of astrocytes. Compared with control group, the astrocytes treated by OGD showed higher cell proliferation inhibition rate, higher apoptosis rate, higher MDA, LDH levels and lower SOD activity. This result was similar to previous studies[25,26], which indicated that the OGD model used in this study successfully simulated ischemic injury.
Oleuropein, a phenolic component of olive, has multifarious biologic activities, including antioxidation, anti-dyslipidemia, weight loss, antidiabetes, anti-atherosclerosis, anti-hypertension, anti-inflammatory and liver protection[27,28]. Also, oleuropein was reported to have suppressive effects on neurodegenerative and neuropsychiatric disorders, through playing neuroprotective role in induction of apoptosis and autophagy[29]. According to the reports, oleuropein can ameliorate the enhancement of LDH and MDA and the reduction of SOD activity in the cerebral tissues of infarcted rats in a stroke rat model, providing neuroprotective effects on ischemic stroke through its antioxidant and antithrombotic activities[30]. Oleuropein improves cognitive function after stroke by promoting histone acetylation and phosphorylation of cyclic Adenosine 3′,5′-Monophosphate (cAMP) responsive element binding protein in middle cerebral artery occlusion rats[31]. In a rat intracerebral hemorrhage model established by collagenase injection, oleuropein treatment has shown to have overall relief of neurologic deficits and cerebral edema associated with intracerebral hemorrhage in a dose-dependent manner[32]. In addition, oleuropein inhibited cell apoptosis through the Tyrosine Kinase B (Akt)/ Glycogen Synthase Kinase 3 Beta (GSK-3β) pathway and upregulated neurotrophic factors after cerebral ischemia/reperfusion damage in rats, thereby producing a tutelar influence on cerebral ischemia[33]. In this study, oleuropein with various doses (25, 50 and 100 μmol/l) could reduce the astrocyte damage induced by OGD in a dosedependent way through promoting proliferation, inhibiting apoptosis and oxidative stress damage, which was in line with the former study. These results indicated that oleuropein could reverse OGD-induced increased astrocyte apoptosis and oxidative stress, and had a protective function against ischemic injury.
The present results showed that OGD up-regulated circ_TTC3 expression and down-regulated miR- 138-5p expression of astrocytes, while oleuropein treatments with different concentrations reversed this result in a concentration-reliant manner, suggesting that the protective role of oleuropein in OGD-damaged astrocytes was related to circ_ TTC3 and miR-138-5p. Previously, circ_TTC3 has been shown to be highly expressed in astrocytes treated with hypoxia and glucose deficiency, and the loss of circ_TTC3 inhibited the apoptosis and LDH level of astrocytes induced by hypoxia and glucose deficiency and the mechanism was related to sponging miR-372-3p[14]. Circ_TTC3 had distinct elevated level in ischemic myocardium and cardiomyocytes suffering from hypoxic injury, and could absorb miR-15b-5p to isolate and block its liveness to adjust cardiac function after myocardial infarction[34]. In hypoxia-induced HaCaT cells, circ_TTC3 overexpression improved the growth of HaCaT cells and diminished apoptosis by downregulating miR-449a[35]. The results of this experiment were similar to the study of Yang et al.[14], namely, circ_TTC3 silencing could improve the proliferation of astrocytes induced by OGD and inhibit apoptosis and oxidative stress. Besides, miR-138-5p upregulation has been reported to promote the growth and inhibit apoptosis of astrocytes damaged by OGD, along with lessened levels of LDH and inflammatory factors[16], certifying that miR-138-5p could serve as a potential target in fighting ischemic stroke. In the mechanism study of this experiment, circ_ TTC3 targeted miR-138-5p and had an in vs. regulating effect on miR-138-5p level. The effects of oleuropein on astrocyte proliferation, apoptosis and oxidative stress induced by OGD could be reversed by circ_TTC3 overexpression. These results suggested that circ_TTC3 could target miR-138-5p, and the two may be a new mechanism of oleuropein to protect astrocyte damage induced by OGD.
In conclusion, oleuropein inhibited oxidative stress injury of astrocytes induced by OGD through upregulation of circ_TTC3 and inhibiting miR- 138-5p, providing new insights into the regulation of oleuropein in ischemic injury.
Conflict of interests:
The authors declared no conflict of interests.
References
- Paul S, Candelario-Jalil E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp Neurol 2021;335:113518.
[Crossref] [Google Scholar] [PubMed]
- Markus HS. Reducing disability after stroke. Int J Stroke 2022;17(3):249-50.
[Crossref] [Google Scholar] [PubMed]
- Zhu T, Wang L, Feng Y, Sun G, Sun X. Classical active ingredients and extracts of Chinese herbal medicines: pharmacokinetics, pharmacodynamics, and molecular mechanisms for ischemic stroke. Oxid Med Cell Longev 2021;202:8868941.
[Crossref] [Google Scholar] [PubMed]
- Yang J, Shao C, Li W, Wan H, He Y, Yang J. Protective effects of Astragaloside IV against oxidative injury and apoptosis in cultured astrocytes by regulating Nrf2/JNK signaling. Exp Brain Res 2021;239(6):1827-40.
[Crossref] [Google Scholar] [PubMed]
- Wu L, Xiong X, Wu X, Ye Y, Jian Z, Zhi Z, et al. Targeting oxidative stress and inflammation to prevent ischemia-reperfusion injury. Front Mol Neurosci 2020;13:28.
[Crossref] [Google Scholar] [PubMed]
- Wang S, Cao X, Duan Y, Zhang G. Delta opioid peptide [d-Ala2, d-Leu5] enkephalin (DADLE) exerts a cytoprotective effect in astrocytes exposed to oxygen-glucose deprivation by inducing autophagy. Cell Transpl 2019;28(6):775-82.
[Crossref] [Google Scholar] [PubMed]
- Hirayama Y, Koizumi S. Astrocytes and ischemic tolerance. Neurosci Res 2018;126:53-9.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Chopp M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Progr Neurobiol 2016;144:103-20.
[Crossref] [Google Scholar] [PubMed]
- Jin HX, Zhang YH, Guo RN, Zhao SN. Inhibition of MEK/ERK/STAT3 signaling in oleuropein treatment inhibits myocardial ischemia/reperfusion. Int J Mol Med 2018;42(2):1034-43.
[Crossref] [Google Scholar] [PubMed]
- Yu H, Liu P, Tang H, Jing J, Lv X, Chen L, et al. Oleuropein, a natural extract from plants, offers neuroprotection in focal cerebral ischemia/reperfusion injury in mice. Eur J Pharmacol 2016;775:113-9.
[Crossref] [Google Scholar] [PubMed]
- Nasrallah H, Aissa I, Slim C, Boujbiha MA, Zaouali MA, Bejaoui M, et al. Effect of oleuropein on oxidative stress, inflammation and apoptosis induced by ischemia-reperfusion injury in rat kidney. Life Sci 2020;255:117833.
[Crossref] [Google Scholar] [PubMed]
- Lu M, Dong X, Zhang Z, Li W, Khoshnam SE. Non-coding RNAs in ischemic stroke: Roles in the neuroinflammation and cell death. Neurotox Res 2020;38(3):564-78.
[Crossref] [Google Scholar] [PubMed]
- Xie H, Huang Y, Zhan Y. Construction of a novel circRNA-miRNA-ferroptosis related mRNA network in ischemic stroke. Sci Rep 2023;13(1):15077.
[Crossref] [Google Scholar] [PubMed]
- Yang B, Zang LE, Cui J, Wei L. Circular RNA TTC3 regulates cerebral ischemia-reperfusion injury and neural stem cells by miR-372-3p/TLR4 axis in cerebral infarction. Stem Cell Res Ther 2021;12(1):125.
[Crossref] [Google Scholar] [PubMed]
- Li H, Tang C, Wang D. LncRNA H19 promotes inflammatory response induced by cerebral ischemia–reperfusion injury through regulating the miR-138-5p–p65 axis. Biochem Cell Biol 2020;98(4):525-36.
[Crossref] [Google Scholar] [PubMed]
- Deng Y, Chen D, Gao F, Lv H, Zhang G, Sun X, et al. Exosomes derived from microRNA-138-5p-overexpressing bone marrow-derived mesenchymal stem cells confer neuroprotection to astrocytes following ischemic stroke via inhibition of LCN2. J Biol Eng 2019;13(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Guo H, Liu ZQ, Zhou H, Wang ZL, Tao YH, Tong Y. P2Y1 receptor antagonists mitigate oxygen and glucose deprivation‑induced astrocyte injury. Mol Med Rep 2018;17(1):1819-24.
[Crossref] [Google Scholar] [PubMed]
- Linnerbauer M, Rothhammer V. Protective functions of reactive astrocytes following central nervous system insult. Front Immunol 2020;11:573256.
[Crossref] [Google Scholar] [PubMed]
- Kimelberg HK, Norenberg MD. Astrocytes. Sci Am 1989;260(4):66-77.
- Fan Y, Zhu S, Wang J, Zhao Y, Wang X. Propofol protects against oxygen/glucose deprivation‑induced cell injury via gap junction inhibition in astrocytes. Mol Med Rep 2020;22(4):2896-904.
[Crossref] [Google Scholar] [PubMed]
- Yao ZM, Sun XR, Huang J, Chen L, Dong SY. Astrocyte-neuronal communication and its role in stroke. Neurochem Res 2023;48(10):2996-3006.
- Zhu C, Zhou Q, Luo C, Chen Y. Dexmedetomidine protects against oxygen–glucose deprivation-induced injury through inducing astrocytes autophagy via TSC2/mTOR pathway. Neuromol Med 2020;22(2):210-7.
[Crossref] [Google Scholar] [PubMed]
- Campbell BC, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, et al. Ischaemic stroke. Nat Rev Dis Primers 2019;5(1):70.
- Revuelta M, Elicegui A, Scheuer T, Endesfelder S, Bührer C, Moreno-Cugnon L, et al. In vitro P38MAPK inhibition in aged astrocytes decreases reactive astrocytes, inflammation and increases nutritive capacity after oxygen-glucose deprivation. Aging (Albany NY). 2021;13(5):6346.
[Crossref] [Google Scholar] [PubMed]
- Duan X, Song N, Ma K, Tong Y, Yang L. The effects of protein-rich extract from Rhizoma Gastrodiae against cerebral ischemia/reperfusion injury via regulating MAPK and PI3K/AKT signaling pathway. Brain Res Bull 2023;203:110772.
[Crossref] [Google Scholar] [PubMed]
- Li CX, Wang XQ, Cheng FF, Yan X, Luo J, Wang QG. Hyodeoxycholic acid protects the neurovascular unit against oxygen-glucose deprivation and reoxygenation-induced injury in vitro. Neural Regen Res 2019;14(11):1941-9.
[Crossref] [Google Scholar] [PubMed]
- Ahamad J, Toufeeq I, Khan MA, Ameen MS, Anwer ET, Uthirapathy S, et al. Oleuropein: A natural antioxidant molecule in the treatment of metabolic syndrome. Phytother Res 2019;33(12):3112-28.
[Crossref] [Google Scholar] [PubMed]
- Zheng Y, Liu Z, Yang X, Liu L, Ahn KS. An updated review on the potential antineoplastic actions of oleuropein. Phytother Res 2022;36(1):365-79.
[Crossref] [Google Scholar] [PubMed]
- Butt MS, Tariq U, Naz A, Rizwan M. Neuroprotective effects of oleuropein: Recent developments and contemporary research. J Food Biochem 2021;45(12):e13967.
[Crossref] [Google Scholar] [PubMed]
- Mnafgui K, Ghazouani L, Hajji R, Tlili A, Derbali F, da Silva FI, et al. Oleuropein protects against cerebral ischemia injury in rats: Molecular docking, biochemical and histological findings. Neurochem Res 2021;46(8):2131-42.
[Crossref] [Google Scholar] [PubMed]
- Gao Y, Li X, Xu R, Guo Y, Yin H, Tan R, et al. Oleuropein improved post cerebral stroke cognitive function by promoting histone acetylation and phosphorylation of cAMP response element-binding protein in MCAO rats. Dose Response 2020;18(3):1559325820950102.
[Crossref] [Google Scholar] [PubMed]
- Shi J, Wu G, Zou X, Jiang K. Oleuropein protects intracerebral hemorrhage-induced disruption of blood-brain barrier through alleviation of oxidative stress. Pharmacol Rep 2017;69:1206-12.
[Crossref] [Google Scholar] [PubMed]
- Zhang W, Liu X, Li Q. Protective effects of oleuropein against cerebral ischemia/reperfusion by inhibiting neuronal apoptosis. Med Sci Monit 2018;24:6587.
[Crossref] [Google Scholar] [PubMed]
- Cai L, Qi B, Wu X, Peng S, Zhou G, Wei Y, et al. Circular RNA Ttc3 regulates cardiac function after myocardial infarction by sponging miR-15b. J Mol Cell Cardiol 2019;130:10-22.
[Crossref] [Google Scholar] [PubMed]
- Yu L, Wang Q, Liu N, Zhao J, Yu J, Tao S. Circular RNA circ‐Ttc3 protects HaCaT cells from hypoxic injury by downregulation of miR‐449a. IUBMB Life 2020;72(3):505-14.
[Crossref] [Google Scholar] [PubMed]