- *Corresponding Author:
- Ling Jiang
Department of Geriatrics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province 430022, China
E-mail: xhger120@163.com
| Date of Received | 22 December 2022 |
| Date of Revision | 05 August 2023 |
| Date of Acceptance | 23 January 2024 |
| Indian J Pharm Sci 2024;86(1):353-360 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore whether oleuropein can mediate lipopolysaccharide-induced oxidative stress and apoptosis in alveolar epithelial cells induced by long non-coding ribonucleic acid Gm4419. Mouse alveolar type II epithelial cells were isolated and cultured, and divided into control, lipopolysaccharide, lipopolysaccharide+oleuropein, lipopolysaccharide+si-NC, lipopolysaccharide+si-Gm4419, lipopolysaccharide+oleuropein+plasmid cloning deoxyribonucleic acid, and lipopolysaccharide+oleuropein+plasmid cloning deoxyribonucleic acid Gm4419 groups. Determination of malondialdehyde content and superoxide dismutase activity in cells was undertaken using the indicated kits. Detection of apoptosis was done using flow cytometry. Protein levels were detected by Western blotting, and long non-coding ribonucleic acid Gm4419 expression was detected by reverse transcription quantitative polymerase chain reaction. Lipopolysaccharide stimulation elevated malondialdehyde content, reduced superoxide dismutase activity, and facilitated cell apoptosis in mouse alveolar type II epithelial cells. Furthermore, oleuropein treatment decreased lipopolysaccharide-induced oxidative stress and apoptosis in mouse alveolar type II epithelial cells. Moreover, lipopolysaccharide caused the upregulation of Gm4419 in mouse alveolar type II epithelial cells, and Gm4419 silencing weakened lipopolysaccharide-induced mouse alveolar type II epithelial cells oxidative stress and apoptosis. In addition, Gm4419 overexpression partly overturned oleuropein treatment-mediated effects on lipopolysaccharide induced mouse alveolar type II epithelial cells oxidative stress and apoptosis. Oleuropein may inhibit lipopolysaccharide induced alveolar epithelial cell oxidative stress and apoptosis by downregulating Gm4419.
Keywords
Oleuropein, Gm4419, alveolar epithelial cells, oxidative stress, sepsis
Sepsis is a systemic inflammatory response syndrome triggered by infection, which can cause acute respiratory distress syndrome or shock in severe cases, constituting a serious danger to human life and health[1]. One of the most susceptible organs to damage in the event of sepsis is the lungs[2]. A large amount of oxygen free radicals can be generated from pulmonary tissues in sepsis, leading to an excessive oxidative stress, thus contributing to alveolar epithelial cell apoptosis or necrosis, consequently leading to lung injury[3]. Clinical management of sepsis is very tricky, and it is mainly administered with nonspecific interventions (such as fluid resuscitation, lung protective ventilation, infection control, and improvement of hemodynamics) and symptomatic treatments (such as controlling organ failure and shock)[4]. Owing to the fact that there is no specific treatment available, its mortality rate remains as high as 38.5 %[5].
Oleuropein (OLEU), a non-toxic secoiridoid, is widely found in Olea europaea L[6]. OLEU possesses a broad pharmacological activity, such as blood pressure-lowering and antioxidant activities[6,7]. It was demonstrated that OLEU is useful for combating arthritis, relying on the modulation of the Nuclear Factor Kappa B (NF- κB) signaling pathway to inhibit Interleukin-1 Beta (IL-1β)-induced inflammatory responses in chondrocytes[8]. OLEU provided a protective action against pesticide-induced toxicity in human keratinocyte models[9]. In mouse models, OLEU protected against Lipopolysaccharide (LPS)- induced sepsis and attenuated the inflammatory response[10]. OLEU has beneficial effects on myocardial injury evoked by sepsis through mediation of the Glycogen Synthase Kinase 3 Beta (GSK3β) pathway[11]. OLEU may be an anti-inflammatory drug for managing Chronic Obstructive Pulmonary Disease (COPD) and asthma[12]. However, whether OLEU can attenuate sepsis-induced alveolar epithelial cell injury is uncharted.
Long non-coding RNAs (lncRNAs) are widely found in eukaryotes, and they participate in modulating physiological or pathological events such as apoptosis, oxidative stress, and inflammatory responses[13]. With regard toward sepsis, lncRNAs take part in the modulation of sepsis by engaging in promotion and suppression of immune functions[14]. For instance, the protective effect of lncRNA-AABR07066529.3 was identified in LPS-induced cardiomyocytes[15]. MALAT1-silenced mice exhibited notably longer survival in Cecal Ligation and Puncture (CLP)- induced sepsis[16]. Although a number of studies have inquired into the action of lncRNAs in the sepsis process, it is rarely published the mechanism by which OLEU mediates sepsis-induced alveolar epithelial cell injury through mediating lncRNAs.
The lncRNA Gm4419 has been evidenced to participate in several diseases[17-19]. In diabetic nephropathy, knocking down Gm4419 ameliorated NF-κB/NLRP3 inflammasome-mediated inflammation[20]. Repression of Dual-Specificity Phosphatase 5 (DUSP5) by Gm4419 through recruitment of Enhancer of Zeste Homolog 2 (EZH2) epigenetically activates Extracellular Signal-Regulated Kinase 1/2 (ERK1/2) pathwaymediated autophagy in myocardial ischemia/ reperfusion injury[21]. However, little research has been reported on the effects of Gm4419 on alveolar epithelial cell damage.
Therefore, we isolated and cultured mouse Alveolar Type II Epithelial Cells (AECII) and established LPS-induced AECII to elucidate the effects of OLEU and lncRNA Gm4419 on oxidative stress and apoptosis in this cell. Moreover, whether OLEU can regulate lncRNA Gm4419 to exert its role was also determined.
Materials and Methods
Reagents:
OLEU (purity >97 %) from FEIYUBIO (Nantong, China); M199 medium from Procell (Wuhan, China); Bicinchoninic Acid (BCA) protein assay and annexin V-Fluorescein Isothiocyanate (FITC)/Propidium Iodide (PI) kits from Solarbio (Beijing, China); LipofectamineTM 2000 kit from Invitrogen (Carlsbad, California, United States of America (USA)); Fetal Bovine Serum (FBS) from Sijiqing Biotechnology Materials Co., Ltd (Hangzhou, China); small interfering Ribonucleic Acid (RNA) for lncRNA Gm4419 (si-Gm4419) and overexpression plasmid for Gm4419 (plasmid cloning Deoxyribonucleic Acid (pcDNA)- Gm4419), negative control for si-Gm4419 (si- NC), empty vector (pcDNA), and quantitative Polymerase Chain Reaction (qPCR) primers from Sangon Biotech (Shanghai, China); Superoxide Dismutase (SOD) and Malondialdehyde (MDA) detection kits from Jiancheng Biotechnology Co., Ltd (Nanjing, China); RNA extraction kit, reverse transcription kit, and PCR kit from Takara (Dalian, China); antibodies for cleaved caspase-3 and cleaved caspase-9 from Abcam (China).
Methods:
Isolation and culture of mouse AECII: Isolation and culture of mouse AECII were performed according to the reference. Bilateral lungs were removed from Bagg Albino (BALB/c) mice killed by the cervical dislocation method, followed by soaking in 75 % ethanol for 5 min. Residual tracheal tissue and connective tissue were removed from bilateral lungs on an ultra-clean bench. After 2-3 washes with pre-cooled Phosphate Buffer Solution (PBS), lung tissues were minced and digested with trypsin solution at 37°. After terminating the digestion, the samples were sieved to collect the precipitate. The obtained precipitates were made into single-cell suspensions by adding M199 complete medium containing 10 % FBS, followed by incubation in an incubator after shifting to 25 cm2 flasks.
Cell transfection: The density of mouse AECII at the logarithmic stage was adjusted to 2.5×104 cells/ml, and 2.5 ml per well was inoculated into 6-well plates. 24 h post incubation, mouse AECII were transfected with si-NC, si-Gm4419, pcDNA, or pcDNA-Gm4419 into mouse AECII using lipofectamineTM 2000 reagent. After 12 h of transfection, the cells were switched to M199 complete medium containing 10 % FBS. After another 12 h of incubation, lncRNA Gm4419 expression was detected by RT-qPCR for verification of the transfection efficiency, and the cells were collected for subsequent experiments.
Cell treatment: The cell densities were all adjusted to 2.5×104 cells/ml and each well was inoculated with 2.5 ml of cell suspension in 6-well plates. For LPS treatment, mouse AECII were cultured with a complete medium containing 10 μg/ml of LPS for 24 h. Control cells were allowed to be cultured within complete medium. For OLEU treatment, cells were cultured in a complete medium containing Low (L, 50 μmol/l), Median (M, 100 μmol/l), and High (H, 200 μmol/l) concentrations of OLEU for 24 h. Three replicate wells were set up in each group, and the experiments were replicated three times.
Detection of MDA content and SOD activity in mouse AECII: The collected mouse AECII was washed twice with pre-cooled PBS solution, followed by lysing the cells through the cell lysate. Centrifugation was performed (3500 r/min, 5 min) for collection of the supernatants. MDA content and SOD activity in the supernatants were detected using MDA and SOD kits.
Detection of apoptosis by flow cytometry: The collected mouse AECII was adjusted to a density of 1.0×106 cells/ml. The cell suspension (1.0 ml) was taken and centrifuged (1000 r/min, 5 min). Following discarding the supernatant, the cells were resuspended in 500 μl of binding buffer, and apoptosis was detected using the Annexin V-FITC/ PI kit.
Protein blotting: The collected mouse AECII was incubated in Radioimmunoprecipitation Assay (RIPA) reagent to extract total protein. After measuring protein concentration by BCA method, electrophoresis, transferring membrane and blocking, the cells were incubated with cleaved caspase-3 (1:500), cleaved caspase-9 (1:500) and Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (1:1000) primary antibodies respectively in a refrigerator at 4° overnight. The membrane was washed and then incubated with goat antirabbit secondary antibody (1:2000) in a shaker. Developing solution was added, and the image was developed away from light. Image J software was used to analyze the bands.
RT-qPCR detection of Gm4419 expression: Precooled PBS was used to wash the cells in each group twice, followed by extraction of total RNA from the cells using an RNA extraction kit. After reverse transcription to complementary DNA, PCR amplification was performed. Sequences for the primers were as follows; Gm4419, 5’-GGAACCAAGCAGACCGAAGAC-3’(forward), 5’-CCCCCAACCCACAGGAACATAA-3’ (reverse) and β-actin, 5’-GGCACCCAGCACAATGAA-3’ (forward), 5’-TAGAAGCATTTGCGGTGG-3’ (reverse). Calculation of lncRNA Gm4419 expression relative to β-actin was done by the 2-ΔΔCt method.
Statistical analysis:
Experimental data were analyzed by Statistical Package for the Social Sciences (SPSS).22.0 software. Measurement information was expressed as mean±standard deviation. Comparisons between multiple groups were made with oneway Analysis of Variance (ANOVA). Comparisons between two groups were made by independent t-test. Differences were expressed as statistically significant with p<0.05.
Results and Discussion
Compared with the control group, mouse AECII in the LPS group had reduced SOD activity (p<0.05) and elevated MDA content (p<0.05). In comparison with the LPS group, a higher SOD activity (p<0.05) and a lower MDA content (p<0.05) were found in mouse AECII in the LPS+OLEU-L, LPS+OLEU-M, and LPS+OLEU-H groups (Table 1). And the comparison of MDA content and SOD activity among LPS+OLEU-L, LPS+OLEU-M, and LPS+OLEU-H groups exhibited significant differences (p<0.05) (Table 1).
| Group | MDA (nmol/mgprot) | SOD (U/mgprot) |
|---|---|---|
| Control | 2.58±0.24 | 148.58±12.48 |
| LPS | 9.22±0.77* | 31.49±3.11* |
| LPS+OLEU-L | 7.15±0.64# | 60.99±5.82# |
| LPS+OLEU-M | 5.44±0.47#& | 83.55±7.22#& |
| LPS+OLEU-H | 3.71±0.39#&$ | 111.74±12.43#&$ |
| F | 221.554 | 226.525 |
| p | 0.000 | 0.000 |
Note: *p<0.05 vs. control; #p<0.05 vs. LPS; &p<0.05 vs. LPS+OLEU-L and $p<0.05 vs. LPS+OLEU-M
Table 1: OLEU Undermined LPS-Induced Oxidative Stress in Mouse AECII (n=9)
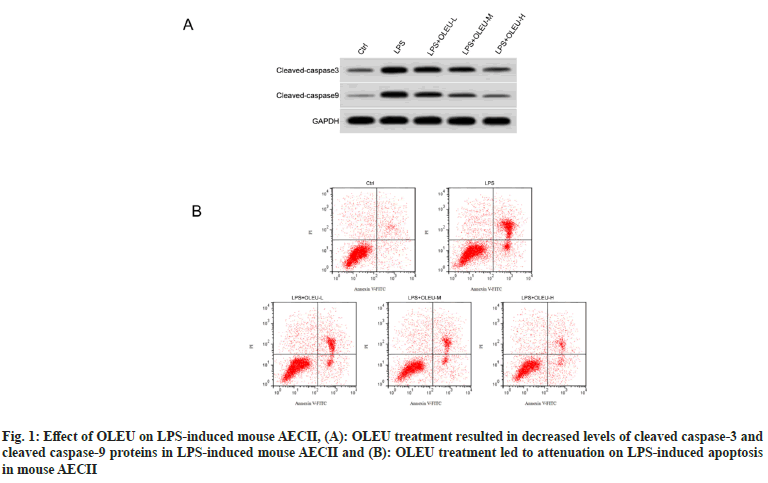
When comparing with the control group, an elevated apoptotic rate, as well as cleaved caspase-3 and cleaved caspase-9 protein levels, was observed in mouse AECII in response to LPS stimulation (p<0.05). Both apoptotic rate and cleaved caspase-3 and cleaved caspase-9 protein levels of mouse AECII were reduced in the LPS+OLEU-L, LPS+OLEU-M, and LPS+OLEU-H groups with respect to the LPS group (p<0.05). Moreover, a dose-dependent effect of OLEU on apoptosis rate and cleaved caspase-3 and cleaved caspase-9 protein levels was observed in LPS-induced mouse AECII (p<0.05) (Table 2, fig. 1A and fig. 1B).
| Group | Apoptosis rate (%) | Cleaved caspase-3 | Cleaved caspase-9 |
|---|---|---|---|
| Control | 5.82±0.52 | 0.21±0.02 | 0.12±0.02 |
| LPS | 32.58±3.16* | 0.78±0.06* | 0.61±0.05* |
| LPS+OLEU-L | 24.73±2.03# | 0.65±0.05# | 0.44±0.04# |
| LPS+OLEU-M | 17.12±1.22#& | 0.51±0.04& | 0.31±0.03#& |
| LPS+OLEU-H | 9.47±0.55#&$ | 0.31±0.04#&$ | 0.19±0.02#&$ |
| F | 333.817 | 256.175 | 299.716 |
| p | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. control; #p<0.05 vs. LPS; &p<0.05 vs. LPS+OLEU-L and $p<0.05 vs. LPS+OLEU-M
Table 2: Apoptosis was Attenuated by OLEU in LPS-Stimulated Mouse AECII
LPS stimulation forced an elevation in Gm4419 expression in mouse AECII vs. the control group (p<0.05). Treatment with OLEU brought about a decrease in Gm4419 expression in LPS-induced mouse AECII (p<0.05), with the decrease in a dose-dependent manner (Table 3).
| Group | Gm4419 |
|---|---|
| Control | 1.00±0.00 |
| LPS | 3.39±0.29* |
| LPS+OLEU-L | 2.71±0.21# |
| LPS+OLEU-M | 1.98±0.12#& |
| LPS+OLEU-H | 1.32±0.12#&$ |
| F | 277.094 |
| p | 0.000 |
Note: *p<0.05 vs. control; #p<0.05 vs. LPS; &p<0.05 vs. LPS+OLEU-L and $p<0.05 vs. LPS+OLEU-M
Table 3: OLEU Decreased GM4419 Expression in LPS-Induced Mouse AECII
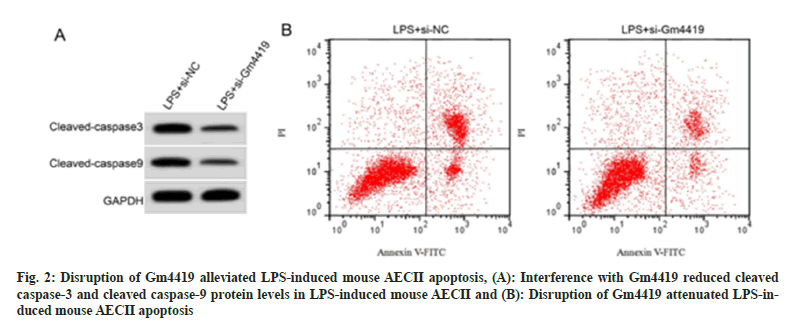
Transfection of si-Gm4419 showed a distinct decrease in Gm4419 expression than that in mouse AECII transfected with si-NC, indicating a successful construction of mouse AECII with interference of Gm4419 expression (Table 4). By comparing with the LPS+si-NC group, we observed a higher SOD activity (p<0.05) and lower MDA content (p<0.05) in mouse AECII within the LPS+si-Gm4419 group. Moreover, repression of Gm4419 decreased LPS-induced apoptosis and up-regulation of cleaved caspase-3 and cleaved caspase-9 proteins in mouse AECII (Table 5, fig. 2A and fig. 2B). Collectively, these outcomes suggested Gm4419 was associated with LPS-induced mouse AECII oxidative stress and apoptosis..
| Group | MDA (nmol/mgprot) | SOD (U/mgprot) |
|---|---|---|
| LPS+si-NC | 9.78±0.67 | 31.72±3.01 |
| LPS+si-Gm4419 | 4.25±0.36* | 89.28±6.58* |
| t | 21.812 | 23.865 |
| p | 0.000 | 0.000 |
Note: *p<0.05 vs. LPS+si-NC
Table 4: GM4419 Repression Impaired LPS-Induced Mouse AECII Oxidative Stress
| Group | Apoptosis rate (%) | Cleaved caspase-3 | Cleaved caspase-9 |
|---|---|---|---|
| LPS+si-NC | 34.61±3.18 | 0.79±0.06 | 0.62±0.04 |
| LPS+si-Gm4419 | 13.45±1.28* | 0.39±0.04* | 0.25±0.02* |
| t | 18.518 | 16.641 | 24.820 |
| p | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. LPS+si-NC
Table 5: GM4419 Silencing Weakened LPS-Induced Mouse AECII Apoptosis
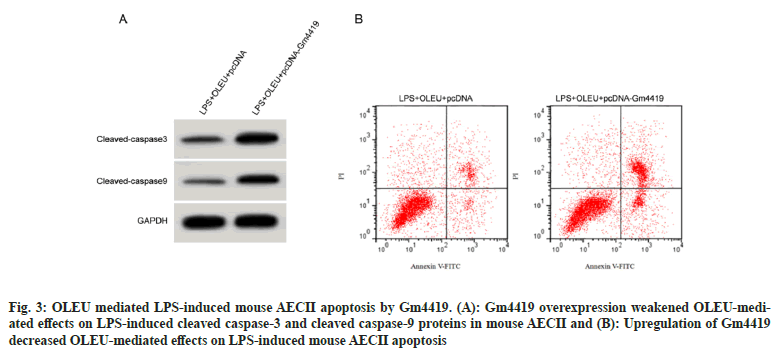
Mouse AECII transfected with pcDNA showed significantly higher expression of Gm4419 than those transfected with pcDNA, indicating that mouse AECII with overexpression of Gm4419 was constructed successfully. Compared with the LPS+OLEU+pcDNA group, mouse AECII in the LPS+OLEU+pcDNA-Gm4419 group showed reduced SOD activity (p<0.05) and elevated MDA content (p<0.05), accompanied with elevated apoptosis rate and cleaved caspase-3 and cleaved caspase-9 protein levels (p<0.05) (Table 6, fig. 3A and fig. 3B). These outcomes collectively demonstrated that OLEU mediated LPS-induced mouse AECII injury by Gm4419.
| Group | Gm4419 | MDA (nmol/mgprot) | SOD (U/mgprot) | Apoptosis rate (%) | Cleaved caspase-3 protein | Cleaved caspase-9 protein |
|---|---|---|---|---|---|---|
| LPS+OLEU+pcDNA | 1.00±0.00 | 3.59±0.33 | 113.84±7.16 | 9.34±0.76 | 0.32±0.03 | 0.18±0.02 |
| LPS+OLEU+pcDNA-Gm4419 | 2.66±0.23* | 8.12±0.73* | 42.96±4.18* | 22.28±2.11* | 0.67±0.04* | 0.49±0.04* |
| t | 21.652 | 16.964 | 25.648 | 17.309 | 21.000 | 20.795 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. LPS+OLEU+pcDNA
Table 6: GM4419 Upregulation Reduced OLEU-Mediated Effects on LPS-Induced Mouse AECII Injury
Fig. 3: OLEU mediated LPS-induced mouse AECII apoptosis by Gm4419. (A): Gm4419 overexpression weakened OLEU-mediated effects on LPS-induced cleaved caspase-3 and cleaved caspase-9 proteins in mouse AECII and (B): Upregulation of Gm4419 decreased OLEU-mediated effects on LPS-induced mouse AECII apoptosis
The pathological process of sepsis-induced lung injury is very complex, which involves alveolar epithelial cell oxidative stress, inflammatory response and apoptosis[22-24]. MDA can indirectly reflect the level of cellular oxidative stress[25]. SOD, an antioxidant enzyme, attenuates oxidative damage to body tissues caused by free radicals[26]. Excessive oxidative stress can further cause apoptosis of alveolar epithelial cells, exacerbating lung tissue damage[27]. Caspase cascade reactions participate in modulation of apoptosis, in which the initiator molecule caspase-9 is stimulated by apoptotic signals to be activated to generate cleaved caspase-9, which further transmits apoptotic signals to activate caspase-3 for generation of cleaved caspase-3, cutting a variety of intracellular substrates to induce apoptosis[28]. Our results showed that after induction with LPS, mouse AECII showed a marked decrease in SOD activity and an improvement in Dimethylamiloride (DMA) content, accompanied by increasing apoptosis rate and cleaved caspase-9 and cleaved caspase-3 protein levels, indicating that LPS mediated mouse AECII oxidative stress and apoptosis.
Natural plants or their active ingredients have shown great advantages and potential in sepsis treatment due to multiple targets of action, multiple routes of administration, and fewer side effects[29,30]. OLEU has a variety of pharmacological activities. A study showed that OLEU induced A549 cell apoptosis through mitochondrial apoptotic cascade[31]. Inflammatory responses triggered by LPS were attenuated by OLEU through stimulation of M2 macrophage polarisation[32]. Findings of the present study demonstrated that OLEU effectively increased the SOD activity as well as reduced MDA content, apoptosis rate, and cleaved caspase-9 and cleaved caspase-3 protein levels in mouse AECII induced by LPS in a dose-dependent manner, showing that OLEU suppressed the LPS-induced mouse AECII oxidative stress and inflammatory response, which was in line with the results reported by Dikmen et al.[33], suggesting that OLEU has a potential value for the treatment of septic lung injury.
Gm4419 is involved in the developmental process of multiple diseases. It was shown that lncRNA Gm4419 accelerated neuronal apoptosis by activating the NF-κB signaling pathway and promoting chemerin signaling, thus contributing to the deterioration of cerebral atherosclerosis in hypertension. Inflammatory injury and astrocyte apoptosis in traumatic brain tissues could be promoted by Gm4419, which bund to microRNA (miR)-466I to up-regulate Tumor Necrosis Factor- Alpha (TNF-α) expression, making Gm4419 as a possible molecular target for traumatic brain injury treatment. Results of our study showed that LPS promoted Gm4419 expression in mouse AECII, and interfering with Gm4419 expression decreased MDA content, apoptosis rate, and cleaved caspase-3 and cleaved caspase-9 protein levels and elevated SOD activity, suggesting that disruption of Gm4419 repressed LPS-induced mouse AECII oxidative stress and apoptosis, implying that Gm4419 may be a therapeutic target for sepsisinduced lung injury. In addition, OLEU dosedependently inhibited Gm4419 expression in LPSinduced mouse AECII, whereas overexpression of Gm4419 reduced the inhibitory effect of OLEU on LPS-induced oxidative stress and apoptosis in mouse AECII, suggesting that olive OLEU may inhibit LPS-induced mouse AECII injury.
In conclusion, OLEU could effectively inhibit LPSinduced oxidative stress and apoptosis in alveolar epithelial cells, and its mechanism of action may be related to the down-regulation of Gm4419 expression in the cells, offering a potential value for therapy of sepsis-induced lung injury.
Conflict of interests:
The authors declared no conflict of interests.
References
- Liu D, Huang SY, Sun JH, Zhang HC, Cai QL, Gao C, et al. Sepsis-induced immunosuppression: Mechanisms, diagnosis and current treatment options. Mil Med Res 2022;9(1):56.
[Crossref] [Google Scholar] [PubMed]
- Kumar V. Pulmonary innate immune response determines the outcome of inflammation during pneumonia and sepsis-associated acute lung injury. Front Immunol 2020;11:1722.
[Crossref] [Google Scholar] [PubMed]
- Xu S, Li L, Wu J, An S, Fang H, Han Y, et al. Melatonin attenuates sepsis-induced small-intestine injury by upregulating SIRT3-mediated oxidative-stress inhibition, mitochondrial protection, and autophagy induction. Front Immunol 2021;12:625627.
[Crossref] [Google Scholar] [PubMed]
- Ronco C, Chawla L, Husain-Syed F, Kellum JA. Rationale for Sequential Extracorporeal Therapy (SET) in sepsis. Crit Care 2023;27(1):50.
- Bauer M, Gerlach H, Vogelmann T, Preissing F, Stiefel J, Adam D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019—results from a systematic review and meta-analysis. Crit Care 2020;24(1):239.
[Crossref] [Google Scholar] [PubMed]
- Ahamad J, Toufeeq I, Khan MA, Ameen MS, Anwer ET, Uthirapathy S, et al. Oleuropein: A natural antioxidant molecule in the treatment of metabolic syndrome. Phytother Res 2019;33(12):3112-28.
[Crossref] [Google Scholar] [PubMed]
- Zheng Y, Liu Z, Yang X, Liu L, Ahn KS. An updated review on the potential antineoplastic actions of oleuropein. Phytother Res 2022;36(1):365-79.
[Crossref] [Google Scholar] [PubMed]
- Feng Z, Li X, Lin J, Zheng W, Hu Z, Xuan J, et al. Oleuropein inhibits the IL-1β-induced expression of inflammatory mediators by suppressing the activation of NF-κB and MAPKs in human osteoarthritis chondrocytes. Food Function 2017;8(10):3737-44.
[Crossref] [Google Scholar] [PubMed]
- Leri M, Vasarri M, Barletta E, Schiavone N, Bergonzi MC, Bucciantini M, et al. The protective role of oleuropein aglycone against pesticide-induced toxicity in a human keratinocytes cell model. Int J Mol Sci 2023;24(19):14553.
[Crossref] [Google Scholar] [PubMed]
- Alsharif KF, Almalki AA, Al-Amer O, Mufti AH, Theyab A, Lokman MS, et al. Oleuropein protects against lipopolysaccharide-induced sepsis and alleviates inflammatory responses in mice. IUBMB Life 2020;72(10):2121-32.
[Crossref] [Google Scholar] [PubMed]
- Xing C, Xu L, Yao Y. Beneficial role of oleuropein in sepsis-induced myocardial injury. Possible involvement of GSK-3β/NF-kB pathway. Acta Cir Bras 2021;36(1):e360107.
[Crossref] [Google Scholar] [PubMed]
- Kim YH, Choi YJ, Kang MK, Lee EJ, Kim DY, Oh H, et al. Oleuropein curtails pulmonary inflammation and tissue destruction in models of experimental asthma and emphysema. J Agric Food Chem 2018;66(29):7643-54.
[Crossref] [Google Scholar] [PubMed]
- Anchesi I, Schepici G, Mazzon E. LncRNAs and circRNAs as strategies against pathological conditions caused by a hypoxic/anoxic state. Biomolecules 2023;13(11):1622.
[Crossref] [Google Scholar] [PubMed]
- Yue L, Gu Y, Xu J, Liu T. Roles of noncoding RNAs in septic acute kidney injury. Biomed Pharmacother 2023;165:115269.
[Crossref] [Google Scholar] [PubMed]
- Wen R, Zhang TN, Zhang T, Tong YJ, Song WL, Liu YP, et al. A novel long noncoding RNA—lncRNA-AABR07066529. 3 alleviates inflammation, apoptosis, and pyroptosis by inhibiting MyD88 in lipopolysaccharide-induced myocardial depression. FASEB J 2023;37(8):e23063.
[Crossref] [Google Scholar] [PubMed]
- Chen J, Tang S, Ke S, Cai JJ, Osorio D, Golovko A, et al. Ablation of long noncoding RNA MALAT1 activates antioxidant pathway and alleviates sepsis in mice. Redox Biol 2022;54:102377.
[Crossref] [Google Scholar] [PubMed]
- Xia WQ, Niu GZ, Yin CG, Lu S, Bu XY. Effects of lncRNA gm4419 on rats with hypertensive cerebral atherosclerosis through NF-κB pathway. Eur Rev Med Pharmacol Sci 2019;23(24):10976-81.
[Crossref] [Google Scholar] [PubMed]
- Yu Y, Cao F, Ran Q, Wang F. Long non-coding RNA Gm4419 promotes trauma-induced astrocyte apoptosis by targeting tumor necrosis factor α. Biochem Biophys Res Commun 2017;491(2):478-85.
[Crossref] [Google Scholar] [PubMed]
- Zhao G, Hailati J, Ma X, Bao Z, Bakeyi M, Liu Z. LncRNA Gm4419 regulates myocardial ischemia/reperfusion injury through targeting the miR-682/TRAF3 axis. J Cardiovasc Pharmacol 2020;76(3):305-12.
[Crossref] [Google Scholar] [PubMed]
- Yi H, Peng R, Zhang LY, Sun Y, Peng HM, Liu HD, et al. LncRNA-Gm4419 knockdown ameliorates NF-κB/NLRP3 inflammasome-mediated inflammation in diabetic nephropathy. Cell Death Dis 2017;8(2):e2583.
[Crossref] [Google Scholar] [PubMed]
- Zeng M, Wei X, He YL, Chen JX, Lin WT, Xu WX. EGCG protects against myocardial I/RI by regulating lncRNA Gm4419-mediated epigenetic silencing of the DUSP5/ERK1/2 axis. Toxicol Appl Pharmacol 2021;433:115782.
[Crossref] [Google Scholar] [PubMed]
- Gong C, Jin Y, Wang X, Mao J, Wang D, Yu X, et al. Lack of S1PR2 in macrophage ameliorates sepsis-associated lung injury through inducing IL-33-mediated type 2 immunity. Am J Respir Cell Mol Biol 2023.
[Crossref] [Google Scholar] [PubMed]
- Tao Y, Xu X, Yang B, Zhao H, Li Y. Mitigation of sepsis-induced acute lung injury by BMSC-derived exosomal miR-125b-5p through STAT3-mediated suppression of macrophage pyroptosis. Int J Nanomed 2023;18:7095-113.
- Xie W, Deng L, Lin M, Huang X, Qian R, Xiong D, et al. Sirtuin1 mediates the protective effects of echinacoside against sepsis-induced acute lung injury via regulating the NOX4-Nrf2 axis. Antioxidants 2023;12(11):1925.
[Crossref] [Google Scholar] [PubMed]
- Tsikas D. Assessment of lipid peroxidation by measuring Malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem 2017;524:13-30.
[Crossref] [Google Scholar] [PubMed]
- Petrov D, Daura X, Zagrovic B. Effect of oxidative damage on the stability and dimerization of superoxide dismutase 1. Biophys J 2016;110(7):1499-509.
[Crossref] [Google Scholar] [PubMed]
- Yao M, Li F, Xu L, Ma L, Zhang S. 24-Dehydrocholesterol reductase alleviates oxidative damage-induced apoptosis in alveolar epithelial cells via regulating phosphatidylinositol-3-kinase/protein Kinase B activation. Bioengineered 2022;13(1):155-63.
[Crossref] [Google Scholar] [PubMed]
- Yue G, Chen C, Bai L, Wang G, Huang Y, Wang Y, et al. Knockdown of long noncoding RNA DLEU1 suppresses the progression of renal cell carcinoma by downregulating the Akt pathway. Mol Med Rep 2019;20(5):4551-7.
[Crossref] [Google Scholar] [PubMed]
- Pak S, Thapa B, Lee K. Decursinol angelate mitigates sepsis induced by methicillin-resistant Staphylococcus aureus infection by modulating the inflammatory responses of macrophages. Int J Mol Sci 2021;22(20):10950.
[Crossref] [Google Scholar] [PubMed]
- Zhang W, Jiang H, Huang P, Wu G, Wang Q, Luan X, et al. Dracorhodin targeting CMPK2 attenuates inflammation: A novel approach to sepsis therapy. Clin Transl Med 2023;13(10):e1449.
[Crossref] [Google Scholar] [PubMed]
- Cao S, Zhu X, Du L. P38 MAP kinase is involved in oleuropein-induced apoptosis in A549 cells by a mitochondrial apoptotic cascade. Biomed Pharmacother 2017;95:1425-35.
[Crossref] [Google Scholar] [PubMed]
- Mirsanei Z, Heidari N, Hazrati A, Asemani Y, Niknam B, Yousefi Z, et al. Oleuropein reduces LPS-induced inflammation via stimulating M2 macrophage polarization. Biomed Pharmacother 2023;163:114857.
[Crossref] [Google Scholar] [PubMed]
- Dikmen N, Cellat M, Etyemez M, Işler CT, Uyar A, Aydın T, et al. Ameliorative effects of oleuropein on lipopolysaccharide-induced acute lung injury model in rats. Inflammation 2021;44(6):2246-59.
[Crossref] [Google Scholar] [PubMed]