- *Corresponding Author:
- S. Cuervo Escobar
Facultad de Ciencias, Universidad de Ciencias Aplicadas y Ambientales U.D.C.A, Calle 72 No. 14-20, Bogotá, Colombia
E-mail: scuervo@udca.edu.co
| Date of Submission | 22 June 2016 |
| Date of Revision | 11 February 2017 |
| Date of Acceptance | 19 July 2017 |
| Indian J Pharm Sci 2017;79(5): 731-739 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In this study, development and validation of a high performance liquid chromatographic method without an internal standard, for simultaneous determination of acetaminophen and caffeine in tablets was reported. This analysis was carried using a LaChrom Elite chromatograph and diode array detector. The separation was carried out in a RP column (C18), at room temperature, 275 nm detection, 1.5 ml/min flow, and water-methanol-acetic acid (69:28:3, v/v/v) as the mobile phase. No chromatographic interference was found. The linearity of the method proposed was assessed in the range 60-140 µg/ml and 7.9-18 µg/ml for acetaminophen and caffeine, respectively, being linear relationships in these ranges and validated the correlation coefficient (r2=0.9989 acetaminophen; r2=0.9982 caffeine), the slope and the intercept. Precision, measured as repeatability and as intermediate precision, and assessed through the coefficient of variation was less than 2.0% for both analytes. Recovery percentages were 99.53% and 99.12% for acetaminophen and caffeine, respectively. Reproducibility of this method was proven through an interlaboratory assay. Elimination of the internal standard for this method does not affect the quantification of the active principles significantly in finished product batches against the method using such internal standard. The outcome of this assay proves that proposed methodology is simple, fast, precise, accurate, and it may be confidently used for the determination of acetaminophen and caffeine in tablets.
Keywords
Acetaminophen, caffeine, HPLC, validation
Combination of acetaminophen and caffeine is an analgesic-antipyretic formulation with known therapeutic efficacy; acetaminophen works by inhibiting prostaglandin synthesis in the central nervous system (CNS)[1,2] and caffeine increases the analgesic efficacy because of its stimulating effect on the CNS, relieving frequent pain-associated depression[3-6]. They are used as treatment for pain in conditions such as muscle, menstrual, dental pain, arthritis, headaches (migraine included), backache, cold, sinusitis, among others. However, an overdose of these drugs in combination may induce nausea, vomit, diarrhoea, seizures, tachycardia and even hepatic problems[7-12]. Therefore, their determination as the main components in a pharmaceutical preparation and at the trace level is paramount. Additionally, international regulations establish that all manufacturers must conduct a thorough technical evaluation of their products in order to provide evidence regarding the quality, safety, and efficacy of their formulations so that they can be distributed and commercialized[13-15]. Methodologies, based on instrumental techniques and applied to the individual determination of each of these drugs[16-23], as well as their determination when are mixed, have been previously described. Instrumental techniques in high performance liquid chromatography (HPLC)[24-26], UV/Vis and infrared (IR) absorption spectrophotometry, fluorescence spectrophotometry[27-32] and electrochemical techniques have also been described to that purpose[33-36]. However, there are few reliable methods to simultaneously determine these active principles. The use of official monographs for the analysis of drugs is probably the most reliable way to determine whether the active principles of the formulations mentioned meet the parameters established for therapeutic use, so that the product may be confidently released to the market. Nonetheless, official methods most commonly used to this purpose-result in very long analysis times and because of their conditions, may not be robust enough under certain circumstances[24,25]. This is why the aim of this study was to optimize and validate a more robust method, from the methodology established by the United Stated Pharmacopoeia (USP)[24], which allowed the simultaneous determination of acetaminophen and caffeine in tablets, and could be used for the identification and determination of their contents in a fast and reliable way.
Materials and Methods
All water and reagents used in this assay were HPLC or analytical grade. Methanol and glacial acetic acid were obtained from Merck (Merck, Germany). Acetaminophen (batch K0I244; 99.8% purity), and caffeine (batch K0K210; 99.9% purity) standards were primary reference standards obtained from the USP (USP, USA).
Instrumentation and analytical conditions
Chromatographic analysis was conducted in a LaChrom Chromatograph (Merck-Hitachi, Germany-Japan), equipped with a quaternary pump, online degassing system and a diode array detector (DAD, UV/Vis). Separation was conducted through a 250×4.6 mm and 5 μm Luna C18 reverse phase column (Phenomenex, USA) at room temperature, at a 1.5 ml/min flow rate and 275 nm detection. The injection volume, both for the samples and for the standards, was 10 μl. Water-methanol-glacial acetic acid (69:28:3, v/v/v), respectively, were prepared as the mobile phase.
Preparation of standards and samples
Among the conditions that were optimized to the level of samples and standards, regarding the USP method, are: elimination of the internal standard and the last dilution of standards in the mobile phase, instead of the suggested solvent. For the sample, tablets preparation instructions-set by the USP-were followed, except for the addition of the internal standard. A mixture was made out of the common excipients, used in the acetaminophen-caffeine tablet formulation to be used as placebo. Spiked placebo was obtained from the USP standards and from the placebo just mentioned, and all solutions used in the validation were the result of the mixtures just mentioned and the pure standards.
Validation parameters assessed
Method was validated following the Technical Requirements for Registration of Pharmaceuticals for Human Use of the International Conference on Harmonisation (ICH), recognized by the FDA for the validation of analytical procedures[37]. Concentration ranges used in this method assessment assay were: acetaminophen 60-140 μg/ml and caffeine 7.8-18.2 μg/ml. Validation included the assessment of the following parameters: specificity, linearity, repeatability, intermediate precision, reproducibility, accuracy and uncertainty.
Hundred microgram per millilitre and 13 μg/ml concentration standards, placebo, and spiked placebo solutions were prepared in acetaminophen and caffeine, respectively, to evaluate the specificity and resolution capability of the method. In order to determine that the introduced changes in the method would not affect the specificity, in presence of probable degradation products, standards, placebo and spiked placebo samples were treated for 10 min in a water bath at 60°, with specific reagents to independently generate acid (HCl 0.1 M), basic (NaOH 0.1 M) and oxidative (H2O2, 3%) conditions. After this, samples were neutralized and diluted with the mobile phase. Additionally, independent samples were exposed to heating and UV radiation. Resulting samples are analysed with HPLC following the established method.
The method linearity was assessed through weighing and preparation, according the procedure established and independently, of five spiked placebo samples covering the concentration ranges previously established. Three preparations were made for each concentration level and each of these preparations was injected three times in the chromatograph. The method precision was assessed at 3 levels: repeatability, intermediate precision and reproducibility.
As for the repeatability or intra-assay precision, it was determined through the assessment of the measurements; dispersion, three times, from the 5 concentration levels selected for the linearity assay and after variance homogeneity assessment with Cochran´s C test. For the assessment of the intermediate precision, spiked placebo samples were analysed three times, by two different analysts, in two different days, and at three different concentration levels, following the established method.
Finally, reproducibility was assessed through interlaboratory assays, with laboratories accredited for the conduction of this type of assays, on a commercial batch of acetaminophen-caffeine tablets, which was distributed to all participants under the same conditions and making sure all of them received the same instructions regarding the method of analysis established. The interlaboratory assay was designed and developed pursuant to the guidelines established by regulations ISO/IEC 17043:2010 and ISO 13528:2005[38,39]. The assay was also developed and coordinated by Mol Labs, an institution accredited by standard ISO/IEC 17043:2010[38], with the capacity to conduct this type of assays. Three laboratories, with technical measurement competence were invited to participate. Each laboratory measured the samples in conditions of intermediate precision. The sample was provided by UDCA. Tablets of a commercial lot of acetaminophen-caffeine were used. The stated value by manufacturer to acetaminophen is 500 mg and the value of caffeine is 65 mg/tablet (g). The assays of homogeneity and stability of the sample was determined according to the provisions of standard ISO 13528:2005[39]. The homogeneity and stability tests were carried out under repeatability conditions by trained personnel and methods that met requirements of ISO/IEC 17025:2005[40].
Accuracy was assessed by calculating the recovery percentage, obtained from the relation between the quantity found and the real quantity contained in the spiked placebo in nine determinations, corresponding to three different concentration levels (within the range used in the linearity), assessed three times and interpolated in a calibration curve with USP standards. The measurement of the estimation of uncertainty was completed following the model described in the Guide to the Evaluation of Measurement Uncertainty ISO/IEC Guide 98-3:2008[41]. For the estimation, the a priori type B components were included, and the repeatability of the chromatogram areas was taken into account as type A components.
The contents of acetaminophen and caffeine were determined from the method developed in a commercial batch of tablets, in triplicate (two injections per sample). Results were compared between the modified and validated method and the official USP method. For this assay, tablets of a commercial lot of acetaminophencaffeine were used. Statistical analysis was conducted using GraphPad. Differences are statistically significant when P<0.05.
Results and Discussion
According to the method established by the USP[24], there are conditions which may be optimized in order to reduce analysis times, preserve the lifespan of the equipment and the columns, and use equipment that does not allow for or when column-heating is not desirable, which results in a faster and more robust methodology. Another important aspect at the optimization level of the method was the change in the solvent of the last standard dilution. According to the original methodology, this dilution must be done in a mixture of solvents (methanol 95% and glacial acetic acid 5%) which, though important for the solubilisation of the analytes, may be too acidic to preserve their non-protonated form, generating a balance between the neutral and the ion species which could be a source of asymmetries and “shoulders” in the peaks under certain circumstances, especially if the method is performed at room temperature. On the other hand, in the mobile phase (which was the solvent that was changed), though acetic acid is present, it is less in proportion (3%), which contributes to the stabilization of molecules without significantly contributing to their protonation. It is also worth mentioning that the last dilution of the sample, suggested by the USP monograph, is conducted in the mobile phase, which supports the validity of the change even more, since, at the analytical level and in the use of methods that use reference substances, it is important to preserve the same preparation conditions, both with the samples and with the standard, which was not being followed in the method reported and is, as mentioned earlier, one of the changes made to this optimization.
Additionally, it is important to point out that, for the HPLC analyses, the analytes should dissolve in the mobile phase, or at least their last dilution should be made in the last phase, in order to minimize the presence of “ghost” peaks in the chromatogram and even the precipitation of said analytes, condition which is met at the sample level but not at the standards level of the USP methodology and which reinforces even more the validity of the change made in the solvent mentioned in the last dilution. All these start to support the validity of the change in the last dilution of the standard.
The following are the concentration ranges used in the validation assay: acetaminophen 60-140 μg/ml and caffeine 7.8-18.2 μg/ml (corresponding to 60-140% of the analysis concentration). Hundred millilitres of stock solutions were prepared from the USP standards of the placebo and the spiked placebo in the solvents mixture (glacial acetic acid-methanol). These stock solutions contained 0.5 mg/ml acetaminophen and 0.065 mg/ml caffeine (in mixture) approximately and an equivalent amount in excipients for the placebo.
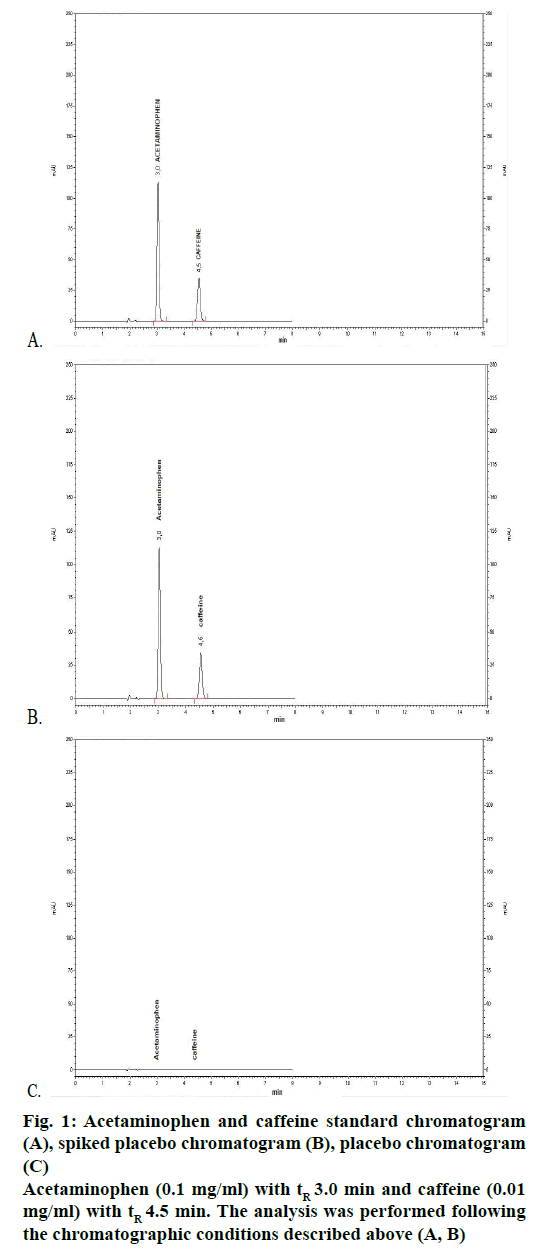
Specificity evaluates the capability of the analytical method to accurately quantify and identify the specific analytes in the presence of other substances, that may be present in the sample or that may be generated from the reaction of the components and the environment. The specificity of the method was demonstrated through the comparison of the chromatograms obtained for the excipients of the formulation (placebo, prepared from all the excipients of the product), spiked placebo (finished product) and standards (active principles of the formulation). First, we observed that there was a proper resolution of the acetaminophen and caffeine peaks (resolution >9.0 and asymmetries <1.2, for each one of the signals) with retention times (tR) of 3.0 min and 4.5 min, respectively (Figure 1A). This comparison also demonstrates that the excipients does not interfere in the identification and quantification of the active principles, since they does not show any type of peak at the retention time of the peaks under study (acetaminophen and caffeine) (Figure 1C vs. A). Finally, the spiked placebo sample acts exactly as the standards (peaks were observed at the same retention times) (Figure 1B vs. A), which demonstrates there was no influence of the placebo on the retention of the analytes. This demonstrates that the methods developed and standardized were specific to the identification and quantification of acetaminophen and caffeine (in mixture) in solid pharmaceutical preparations (tablets).
Additionally in order to evaluate the specificity of the methods in presence of possible degradation products, an artificial degradation assay was conducted. No peaks that may interfere with the peaks of the analytes were observed in the chromatograms obtained from the solutions subject to the different treatments with agents and UV light, which makes this method appropriate for use in stability assays. In addition, the DAD used in this study allowed us to compare the UV spectra of the signals of each treatment against those spectra of the standards without treatment. Such comparisons enable us to evaluate the peak purity and allowed to observe that each signal corresponded exclusively to that obtained for the pure substance.
A series of 15 solutions was prepared taking the spiked placebo as previously described. Worked concentrations levels are shown in Table 1. Each solution was analysed three times following the procedure described above to evaluate linearity. An adjustment or linear regression was made to the data obtained from the linearity analyses (area vs. concentration). The linear regression coefficient, the slope and the intercept were calculated from this adjustment (Table 2). Validity of the parameters in the straight line mentioned above was evaluated through hypothesis tests and t tests (Table 2). Additionally, an analysis of the variance (ANOVA) of the regression was carried out to confirm correlation between the two variables (Table 2). The correlation coefficient showed that calculated t (tc)>Table t (tt); therefore, there was a correlation between the variables analysed; also, r2>0.998 for the concentration ranges validated. In the beta coefficient calculations for the slope, tc>tt, and intercept tc<tt, which proves that the slope was statistically different from zero and the intercept was not.
| Level | Number of samples | Number of readings | Concentration of acetaminophen (µg/ml) | Concentration of caffeine (µg/ml) |
|---|---|---|---|---|
| 1 | 3 | 3 | 60.0 | 7.8 |
| 2 | 3 | 3 | 80.0 | 10.4 |
| 3 | 3 | 3 | 100.0 | 13.0 |
| 4 | 3 | 3 | 120.0 | 15.6 |
| 5 | 3 | 3 | 140.0 | 18.2 |
Table 1: Preparation of Solutions for Method Linearity Evaluation
| Statistical analysis | ANOVA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Value | Degree of freedom | tc | Compa-rison of t | Source | Degree of freedom | Fc | Ft | ||||
| Acetaminophen | Acetaminophen | |||||||||||
| Correlation coefficient | r2=0.9989 | 43 | 202.6 | tc>tt | Regression | 1 | 41060 | 4.07 | ||||
| Slope | b=23259 | 43 | 202.6 | tc>tt | Residue | 43 | ||||||
| Intercept | a=-6511 | 43 | 0.545 | tc<tt | Total | 44 | ||||||
| Caffeine | Caffeine | |||||||||||
| Correlation coefficient | r2 = 0.9982 | 43 | 155.1 | t>tt | Regression | 1 | 24070 | 4.07 | ||||
| Slope | b = 72894 | 43 | 155.1 | tc>tt | Residue | 43 | ||||||
| Intercept | a = -5017 | 43 | 0.792 | tc<tt | Total | 44 | ||||||
Table 2: Statistical Analysis of the Linearity of Acetaminophen and Caffeine
In the ANOVA, calculated F (Fc)>table F (Ft) for the regression, which means there was a linear correlation between the two variables. These results allowed us to conclude there was linearity in the concentration ranges (acetaminophen 60.0-140.0 μg/ml and caffeine 7.8-18.2 μg/ml) used for the quantification of acetaminophen and caffeine in tablets.
Assessing precision makes possible to measure the level of consistency between the results of single tests, when the method was repeatedly applied to multiple readings of a homogeneous sample, determining the dispersion of the measures around the mean or central value. It is mathematically expressed as the coefficient of variation (CV) in a series of measurements. Precision was evaluated in three levels. A 100% concentration solution was prepared, in the same way the linearity of the system was prepared; and then, 9 consecutive injections of this solution were introduced following the conditions established. The CV obtained from the 9 determinations cannot be higher than 2.0% for the system to be considered precise (system repeatability). Table 3 summarizes the results obtained for the CV in the evaluation of the precision of the system. It can be observed, for all cases, that the CV is lower than the limit established for this type of methods and concentration levels, which corresponds to 2.0%. Hence, it can be concluded that the methodology shows system precision at level of the two analytes, which means the system is appropriate for the analysis of acetaminophen and caffeine in tablets. The repeatability of the method was evaluated for 5 concentration levels, including the analysis concentration. Data dispersion was evaluated through the calculation of a weighted CV from the coefficients of variation at each concentration level and evaluating the homogeneity of variances through the Cochran C test. This CV was compared to the maximum coefficient allowed for this type of methods (2.0%), to determine whether the parameter has been validated. Table 3 summarizes the results obtained from the homogeneity test of variances and of the weighted CV in the evaluation of the precision of the method for both methodologies. It can be observed, for all cases, that the CV is lower than the limit established for this type of methodologies and concentration levels, which corresponds to 2.0%, which demonstrates homogeneity within the different data groups and that the dispersion and variation observed was only related to random causes and not to errors introduced by the use of the methodology. Hence, it can be concluded that the analytical methodology showed method precision at the level of the two analytes and across the range of concentrations studied.
| Repeatability of system | CV (%) | Method repeatability | Cochran test (Cc) | Weighted coefficient of variation (%) |
|---|---|---|---|---|
| Acetaminophen | 0.464 | Acetaminophen | 0.112 | 1.03 |
| Caffeine | 0.457 | Caffeine | 0.314 | 1.24 |
Table 3: Coefficients of Variation for the Evaluation of Method and System Repeatability
The intermediate precision was evaluated when the variations resulting in the laboratory (different days, different analysts, etc.) show significant dispersion errors within the use of the methodology. In order to evaluate this parameter, three samples (S) of spiked placebo were analysed by two different analysts (A), in two different days (D) and at three concentration levels (C), following the analysis methodology previously described. The results were presented in the percentage related to the expected quantity of analyte in the spiked placebo (Table 4). An ANOVA was conducted with the results in order to determine whether any of the variables introduced (analyst, day, or sample) had a significant effect on the analytes in the corresponding methodology (Table 5). According to the ANOVA results for intermediate precision where, both for acetaminophen and caffeine, Fc was lower than Ft, it is possible to conclude that there is no significant influence in the dispersion of the data when the methodology was used by different analysts on different days.
| Day-analyte/ sample | Acetaminophen | Caffeine | ||||
|---|---|---|---|---|---|---|
| C1 (%) | C2 (%) | C3 (%) | C1 (%) | C2 (%) | C3 (%) | |
| D1-A1/S1 | 98.8 | 97.6 | 98.0 | 97.8 | 96.6 | 97.0 |
| D1-A1/S2 | 99.5 | 98.3 | 99.3 | 98.7 | 97.3 | 98.2 |
| D1-A1/S3 | 99.4 | 98.6 | 99.0 | 98.6 | 97.6 | 98.0 |
| D2-A2/S1 | 99.2 | 98.8 | 98.5 | 98.1 | 97.8 | 97.4 |
| D2-A2/S2 | 99.1 | 98.8 | 98.9 | 98.0 | 97.7 | 97.8 |
| D2-A2/S3 | 99.3 | 98.7 | 98.8 | 98.1 | 97.7 | 97.8 |
Table 4: Analysis of the Spiked Placebo Samples for the Evaluation of Intermediate Precision
| ANOVA | Intermediate precision | Reproducibility | ||||
|---|---|---|---|---|---|---|
| Source | Degree of freedom | Fc | Ft | Degree of freedom | Fc | Ft |
| Acetaminophen | ||||||
| Between groups | 5 | 3.07 | 3.11 | 2 | 2.91 | 4.26 |
| Within groups | 12 | 9 | ||||
| Total | 17 | 11 | ||||
| Caffeine | ||||||
| Between groups | 5 | 3.09 | 3.11 | 2 | 3.94 | 4.26 |
| Within groups | 12 | 9 | ||||
| Total | 17 | 11 | ||||
Table 5: Anova of Intermediate Precision and Reproducibility
Reproducibility was evaluated through interlaboratory or aptitude assays with domestic and internationally known laboratories or institutions, experts on this type of assays and on a commercial batch of the product acetaminophen-caffeine tablets. Reproducibility allows assessing the precision under conditions different to the ones found in the laboratory where the methodology was developed, since it is performed in the facilities of other laboratories, which permitted a more demanding challenge to that methodology in terms of precision. Table 8 lists the results of the assay of four samples (S), for the three participating laboratories (L), in terms of percentage of the active principle found in the stated amount. The CV found for the set of measurements of the two analytes was lower than 2.0% (Table 6) which shows reproducibility in the determination of the analytes. Additionally, when performing an ANOVA to determine whether there is influence from the factor laboratory for the quantification of the actives, it was found that the Fc was lower than the Ft, which demonstrates that the use of the methodology at the interlaboratory level does not introduce a significant error in the determination of the analytes, which in turn demonstrates reproducibility in its use and that the methodology can be reliably used in any laboratory with a technical capability for this type of analysis (Table 5).
| Laboratory/sample | Acetaminophen (%) | Caffeine (%) |
|---|---|---|
| L1/S1 | 98.43 | 101.43 |
| L1/S2 | 98.21 | 101.21 |
| L1/S3 | 99.63 | 102.63 |
| L1/S4 | 100.02 | 103.02 |
| L2/S1 | 98.31 | 101.98 |
| L2/S2 | 97.69 | 100.42 |
| L2/S3 | 97.58 | 101.77 |
| L2/S4 | 97.44 | 99.89 |
| L3/S1 | 98.80 | 100.61 |
| L3/S2 | 97.49 | 99.77 |
| L3/S3 | 99.48 | 99.95 |
| L3/S4 | 97.76 | 101.17 |
| Average | 98.40 | 101.15 |
| Standard deviation (SD) | 0.90 | 1.07 |
| CV | 0.91 | 1.06 |
Table 6: Results of Different Laboratories
The accuracy of an analytical methodology expresses the level of consistency between the value taken as true or accepted value of reference and the value found. Accuracy was verified through the calculation of the percentage of recovery obtained from the relation between the quantity found and the real quantity contained in the spiked placebos in nine determinations, corresponding to three different levels of concentration (80, 100 and 120%), evaluated three times and after the evaluation of homogeneity of variance through the Cochran test. In order to do this, the results obtained from the linearity of the method were used after being interpolated on the calibration curve of corresponding standard and comparing the quantity recovered and the real quantity contained. The percentage of recovery must be between 98 and 102% for each analyte with a CV lower than 2.0% and through a t test hypothesis test, it was demonstrated that said recovery does not significantly differ from 100%. The results obtained show that recovery was not lower than 98% for any case or for any of the concentration levels (Table 7). In the t test, tc was lower than tt, which evidences no statistically significant difference between the percentage of recovery and the 100% (Table 7), which established that the quantity of analyte recovered or found for the use of the methodology does not significantly differ from the real quantity contained in the sample. Additionally, the dispersion, measured as CV across the results of the different measurements, was below 2.0% (Table 7). This leads to the conclusion that the standardized methodology was appropriate for the quantitative determination both of acetaminophen and caffeine in tablets, without systematic errors which may affect the recovery of the analytes mentioned.
| Concentration level (%) | Acetaminophen recovery (%) | Caffeine recovery (%) | Statistical evaluation | Acetaminophen | Caffeine |
|---|---|---|---|---|---|
| 80 | 99.44 | 99.75 | n | 9 | 9 |
| 100 | 99.39 | 98.69 | tt | 2.306 | 2.306 |
| 120 | 99.76 | 98.92 | SD | 0.957 | 1.156 |
| Recovery average (%) | 99.53 | 99.12 | CV | 0.961 | 1.166 |
| tc | 1.465 | 2.261 |
Table 7: Statistical Evaluation of Percent Recovery for Acetaminophen and Caffeine
In addition, the uncertainty associated to the measurement of each analyte with the modified methodology was estimated in this essay. The combined uncertainty uc it expanded to the k factor of coverage appropriate to reach a confidence level of 95%[41]. The result of the estimation of measurement uncertainty, taking into account the result of the measurement in terms of percentage of the amount declared, was 0.90% and 1.15% for acetaminophen and caffeine, respectively.
The contents of acetaminophen and caffeine were determined from the method developed in a commercial batch of tablets, in triplicate. The results were compared between the modified and validated method and the official USP method. For this assay, tablets of a commercial lot of acetaminophen-caffeine were used. The results are expressed as a percentage of the amount declared (Table 8). The result of the t test states there is no significant difference between the two methodologies for determining acetaminophen and caffeine in tablets, given that tc is lower than tt (Table 8).
| USP methodology | Modified and validated methodology | |||
|---|---|---|---|---|
| Sample | Acetaminophen (%) | Caffeine (%) | Acetaminophen (%) | Caffeine (%) |
| S1 | 95.55 | 97.8 | 94.94 | 95.91 |
| S2 | 95.63 | 97.48 | 96.84 | 97.46 |
| S3 | 94.72 | 97.46 | 95.88 | 97.74 |
| Average | 95.3 | 97.58 | 95.89 | 97.03 |
| SD | 0.50 | 0.19 | 0.95 | 0.99 |
| CV | 0.53 | 0.20 | 0.99 | 1.02 |
| tc | 0.947 | 0.939 | ||
| tt | 2.776 | 2.776 | ||
Table 8: Comparative Evaluation of a Batch of Acetaminophen-Caffeine Tablets Using USP Methdology and Modified and Validated Methodology
Overall, we can say that the validated methodology meets the specificity, linearity, repeatability, intermediate precision, reproducibility and accuracy requirements, and therefore may be reliably used to qualitatively and quantitatively determine the active principles acetaminophen and caffeine in tablets, and used in the regular quality control to stability assays and even to dissolution assays. This methodology, without the use of the internal standard, is faster since the analysis time was approximately reduced in half without affecting any of the characteristics or validation parameters mentioned above. Additionally, the validated methodology was equivalent to USP official methodology to quantitatively determine the analytes mentioned before.
Acknowledgements
The authors would like to acknowledge the financial and technical support from Universidad de Ciencias Aplicadas y Ambientales, U.D.C.A.
Conflict of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Smith HS. Potential analgesic mechanisms of acetaminophen. Pain Physician 2009;12:269-80.
- Swierkosz TA, Jordan L, McBride M, McGough K, Devlin J, Botting RM. Actions of paracetamol on cyclooxygenases in tissue and cell homogenates of mouse and rabbit. Med SciMonit 2002;8:496-503.
- Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Rev 1992;17:139-70.
- Liguori A, Hughes JR, Grass JA. Absorption and subjective effects of caffeine from coffee, cola and capsules. PharmacolBiochemBehav 1997;58:721-6.
- Straube A, Aicher B, Fiebich BL, Haag G. Combined analgesics in (headache) pain therapy: shotgun approach or precise multi-target therapeutics? BMC Neurology 2011;11:1-15.
- Fiebich BL, Candelario-Jalil E, Mantovani M, Heinzmann M, Akundi RS, Hüll M, et al. Modulation of catecholamine release from rat striatal slices by the fixed combination of aspirin, paracetamol and caffeine. Pharmacol Res 2006;53:391-6.
- Graham GG, Scott KF. Mechanism of action of paracetamol. Am J Ther 2005;12:46-55.
- Benson GD. Acetaminophen in chronic liver disease. ClinPharmacolTher 1983;33:95-101.
- Bessems JG, Vermeulen NP. Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol 2001;31:55-138.
- Olaleye MT, Rocha BT. Acetaminophen-induced liver damage in mice: effects of some medicinal plants on the oxidative defense system. ExpToxicolPathol 2008;59:319-27.
- Mazer M, Perrone J. Acetaminophen-induced nephrotoxicity: pathophysiology, clinical manifestations and management. J Med Toxicol 2008;4:2-6.
- Kerrigan S, Lindsey T. Fatal caffeine overdose: two case reports. Forensic SciInt 2005;153:67-9.
- https://www.fda.gov/downloads/drugs/guidances/ucm070305.pdf.
- Karmakar P, Kibria MG. In vitrocomparative evaluation of quality control parameters between paracetamol and paracetamol/caffeine tablets available in Bangladesh. IntCurr Pharm J 2012;1:7.
- da Cruz AG, Cenci SA, Maia MCA. Quality assurance requirements in produce processing. Trends Food SciTechnol 2006;17:406-11.
- Kamberi M, Riley CM, Ma X, Huang CWC. A validated, sensitive HPLC method for the determination of trace impurities in acetaminophen drug substance. J Pharm Biomed Anal 2004;34:123-8.
- Marin A, Barbas C. CE versus HPLC for the dissolution test in a pharmaceutical formulation containing acetaminophen, phenylephrine and chlorpheniramine. J Pharm Biomed Anal 2004;35:769-77.
- Barfield M, Spooner N, Lad R, Parry S, Fowles S. Application of dried blood spots combined with HPLC-MS/MS for the quantification of acetaminophen in toxicokinetic studies. J Chromatogr B AnalytTechnol Biomed Life Sci 2008;870:32-7.
- Bui M-PN, Li CA, Han KN, Pham XH, Seong GH. Determination of acetaminophen by electrochemical co-deposition of glutamic acid and gold nanoparticles. Sens Actuators B Chem 2012;174:318-24.
- Dawidowicz AL, Wianowska D. PLE in the analysis of plant compounds: Part I. The application of PLE for HPLC analysis of caffeine in green tea leaves. J Pharm Biomed Anal 2005;37:1155-9.
- Huck CW, Guggenbichler W, Bonn GK. Analysis of caffeine, theobromine and theophylline in coffee by near infrared spectroscopy (NIRS) compared to high-performance liquid chromatography (HPLC) coupled to mass spectrometry. AnalyticaChimicaActa 2005;538:195-203.
- Liotta E, Gottardo R, Seri C, Rimondo C, Miksik I, Serpelloni G, et al. Rapid analysis of caffeine in "smart drugs" and "energy drinks" by microemulsionelectrokinetic chromatography (MEEKC). Forensic SciInt 2012;220:279-83.
- Hubert S, Briancon S, Hedoux A, Guinet Y, Paccou L, Fessi H, et al. Process induced transformations during tablet manufacturing: phase transition analysis of caffeine using DSC and low frequency micro-Raman spectroscopy. Int J Pharm 2011;420:76-83.
- https://books.google.co.in/books/about/USP_38_NF_33_The_United_States_Pharmacop.html?id=RKTRoQEACAAJ&redir_esc=y.
- https://books.google.co.in/books/about/British_Pharmacopoeia_2014.html?id=HW0angEACAAJ&redir_esc=y.
- Sullivan C, Sherma J. Development and validation of an HPTLC‐densitometry method for assay of caffeine and acetaminophen in multicomponent extra strength analgesic tablets. J LiqChromatogrRelatTechnol 2003;26:3453-62.
- Alves JCL, Poppi RJ. Simultaneous determination of acetylsalicylic acid, paracetamol and caffeine using solid-phase molecular fluorescence and parallel factor analysis. AnalyticaChimicaActa 2009;642:212-6.
- Dinç E, Baleanu D. Two new spectrophotometric approaches to the multicomponent analysis of the acetaminophen and caffeine in tablets by classical least-squares and principal component regression techniques. Il Farmaco 2002;57:33-7.
- Blanco M, Alcalá M. Simultaneous quantitation of five active principles in a pharmaceutical preparation: Development and validation of a near infrared spectroscopic method. Eur J Pharm Sci 2006;27:280-6.
- Dinc E, Ozdemir A, Baleanu D. An application of derivative and continuous wavelet transforms to the overlapping ratio spectra for the quantitative multi-resolution of a ternary mixture of paracetamol, acetylsalicylic acid and caffeine in tablets. Talanta 2005;65:36-47.
- Sena MM, Poppi RJ. N-way PLS applied to simultaneous spectrophotometric determination of acetylsalicylic acid, paracetamol and caffeine. J Pharm Biomed Anal 2004;34:27-34.
- Ito M, Suzuki T, Yada S, Nakagami H, Teramoto H, Yonemochi E, et al. Development of a method for nondestructive NIR transmittance spectroscopic analysis of acetaminophen and caffeine anhydrate in intact bilayer tablets. J Pharm Biomed Anal 2010;53:396-402.
- Lourenção BC, Medeiros RA, Rocha-Filho RC, Mazo LH, Fatibello-Filho O. Simultaneous voltammetric determination of paracetamol and caffeine in pharmaceutical formulations using a boron-doped diamond electrode. Talanta 2009;78:748-52.
- Sanghavi BJ, Srivastava AK. Simultaneous voltammetric determination of acetaminophen, aspirin and caffeine using an in situ surfactant-modified multi-walled carbon nanotube paste electrode. ElectrochimicaActa 2010;55:8638-48.
- Emre D, Ozaltin N. Simultaneous determination of paracetamol, caffeine and propyphenazone in ternary mixtures by micellarelectrokinetic capillary chromatography. J Chromatogr B AnalytTechnol Biomed Life Sci 2007;847:126-32.
- Amiri-Aref M, Raoof JB, Ojani R. A highly sensitive electrochemical sensor for simultaneous voltammetric determination of noradrenaline, acetaminophen, xanthine and caffeine based on a flavonoid nanostructured modified glassy carbon electrode. Sens Actuators B Chem 2014;192:634.
- https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf.
- https://www.iso.org/standard/29366.html.
- https://www.iso.org/standard/35664.html.
- https://www.iso.org/standard/39883.html.
- https://www.iso.org/standard/50461.html.