- *Corresponding Author:
- M. Y. NASSAR
Chemistry Department, Faculty of Science, Benha University, Benha 13815
E-mail: m_y_nassar@yahoo.com
| Date of Submission | 03 July 2016 |
| Date of Revision | 05 January 2017 |
| Date of Acceptance | 24 April 2017 |
| Indian J Pharm Sci 2017;79(3):420-430 |
Abstract
Two fairly sensitive, simple and accurate methods have been developed and validated for the assay of omeprazole and lansoprazole in pure and dosage forms. The proposed spectrophotometric method was based on the oxidation of the title drugs with acidic potassium iodate solution resulted in liberation of iodine, which was then extracted and measured at λ 520 nm under the optimized experimental conditions. The method was proved to be accurate and precise and the linearity was found to be in the concentration range of 5-200 and 15-200 µg/ml, for omeprazole and lansoprazole, respectively, with apparent molar absorptivities of 2.42×10-4 and 2.01×10-4 lmol-1cm-1, and with the corresponding Sandell sensitivity value of 0.0281 and 0.0473 mgcm-2 for the afore mentioned drugs, respectively. Moreover, the kinetics of these reactions was investigated. On the other hand, the potentiometric method was based on the direct titration of the drugs with acidic N-bromosuccinimide solution with determination of the end point potentiometrically using a platinum indicator electrode under the optimum conditions. The concentration ranges were found to be 25-100 and 15-100 µg/ml with standard deviation of 0.007-0.042 and 0.005-0.034, and with relative standard deviation of 0.79-2.4 and 1.4-2.9 for omeprazole and lansoprazole, respectively. Additionally, the proposed methods could successfully be applied for the determination of the cited drugs in pharmaceutical dosage forms. The relative standard deviations for the results did not exceed 1%, confirming the high precision of the method and reproducibility of the results.
Keywords
Omeprazole, lansoprazole, potassium iodate, N-bromosuccinimide, spectophotometry, potentiometry and kinetics
Introduction
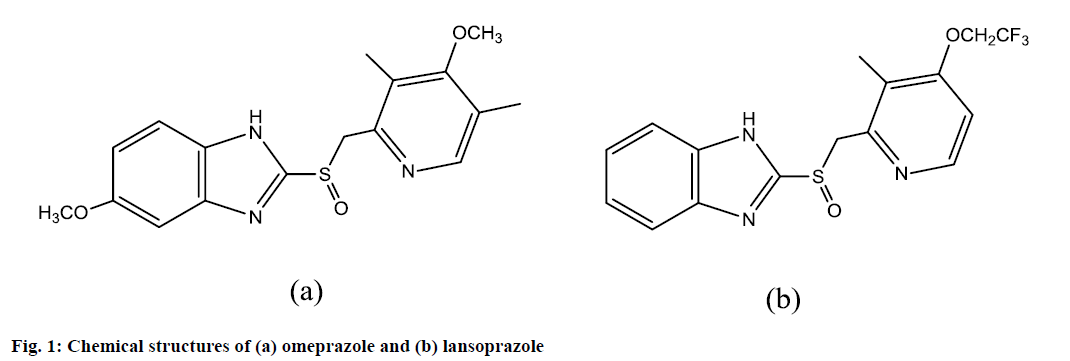
Omeprazole (Prilosec), 5-methoxy-2-[[(4-methoxy- 3,5-dimethyl-2-pyridinyl)methyl]sulphinyl]-1Hbenzimidazole[1] (figure 1a), is the first-in-class of the proton pump inhibitors (PPIs) that is widely used for the prophylaxis and treatment of both gastroduodenal ulcers and symptomatic gastro-esophageal reflux[2]. Consequently, this drug can increase the bioavailability of oral digoxin by suppressing acid production in the stomach and raising gastric permeability to digoxin[3,4]. Also, it is highly effective in the treatment of Zollinger-Ellison syndrome[5]. Its empirical formula is C17H19N3O3S, with a molecular weight of 345.42.
On the other hand, lansoprazole is effective in the treatment of various peptic diseases, including gastric and duodenal ulcer, reflux esophagitis and Zollinger- Ellison syndrome[1]. The PPIs are unstable at a low pH, and therefore, the oral dosage forms are supplied as enteric-coated granules encapsulated in a gelatin shell. The PPIs are also considered to be weak base pro-drugs that easily penetrate cell membranes and concentrate in acidic compartments, where they are converted into sulphonamide forms, representing the active inhibitors[6]. Additionally, PPIs are chemo sensitizing cytotoxic drugs, and active against various human tumor cells[7-9]. The active ingredient in Prevacid delayed-release capsules and Prevacid delayed-release orally disintegrating tablets is lansoprazole, a substituted benzimidazole, 2-[3-methyl-4-(2,2,2-trifluoroethoxy)- 2-pyridyl]methyl]sulfinylbenzimidazole. Its empirical formula is C16H14F3N3O2S with a molecular weight of 369.37 (figure 1b).
Several analytical methods have been developed for quantitative estimation of omeprazole and lansoprazole[1,9]. However, some of these methods have low limits of detection and quantitation, and others exhibit several shortcomings. For example, spectrophotometry[10-21], electrochemical methods[22], high-performance liquid chromatography (HPLC)[23-28], liquid chromatographyelectrospray ionization tandem mass spectrometry (LC/ESI-MS/MS)[29] and electrophoresis[30,31] have been used for determination of omeprazole. While for lansoprazole, spectrophotometry[32], HPLC[33-35], high performance thin layer chromatography (HPTLC)[36,37], capillary electrophoresis[38], and electro analytical methods[39] have been developed for its quantitative determination.
Organic sulfide compounds can readily be oxidized to sulfoxide forms (R2SO) and/or to sulfone forms (R2SO2), or even can be brominated using N-bromosuccinimide (NBS)[40-49]. Consequently, these are the ideas, which our techniques are based on for the determination of omeprazole and lansoprazole. In the present work, two simple, sensitive and accurate methods are described for the determination of omeprazole and lansoprazole. One is spectrophotometric assay based on oxidation of the drugs by potassium iodate in an acidic medium where an equivalent amount of iodine is liberated. The iodine is subsequently quantitatively extracted into cyclohexane and measured spectrophotometrically at 520 nm. The other method is based on the direct titration of the drug with NBS in an acidic medium and then detection the end point of the oxidation process potentiometrically using Pt electrode. Different factors affecting the two proposed methods have been investigated and their reaction mechanisms have been proposed, as well. It is noteworthy that both validation methods are original and had not previously been used for the determination of both drugs in pharmaceutical dosage forms.
Materials and Methods
All the absorption spectral measurements were made using double beam Shimadzu UV/Vis spectrophotometer (model 1700, Japan) equipped with 10 mm matched quartz cells. The potentiometric determination was done by using a pH meter (Jenway) with platinum electrode and magnetic stirrer, which was calibrated before and after each series of measurements.
All solvents and reagents were of analytical reagent grade. Omeprazole and lansoprazole reference standards and bulk powders were kindly supplied from El Arabeya Company for Pharmaceutical and Chemical Industries, Egypt. Their purities were found to be 99.77% and 99.78%, respectively, according to the manufacturers' method. The commercial formulations used included Omepak capsules labeled to contain (20 mg/capsule, Sedico Company, Egypt) and Losec capsules labeled to contain (30 mg/capsule, AstraZeneca, Egypt).
Standard solutions:
For the spectrophotometric method (method I) a standard stock solution of the analyte containing 1 μg/ml was prepared from a stock solution (1 mg/ml) by making the required dilution. The stock solution (1 mg/ml) was prepared by dissolving 100 mg of pure drug in 20 ml methanol and further diluted to 100 ml in a calibrated flask with the same solvent. The standard solution was kept in a refrigerator and found to be stable for at least one month if stored in a cool (<25°) and dark place. For the potentiometric method (method II) a standard stock solution of the analyte of concentration 1×10-3 M was prepared by dissolving an accurately weight of 34 or 36 mg for omeprazole or lansoprazole, respectively in 20 ml methanol and then further diluted in 100 ml calibrated flask to the mark.
Solutions of potassium iodate KIO3 (1% w/v) in water, and 30% (v/v) H2SO4 were prepared for method I. However, for working up using method II, a solution of NBS (1×10-3 M) was prepared by dissolving 17.8 mg of NBS in 20 ml water then completed to the mark in a 100 ml calibrated flask, and finally the obtained solution was standardized before use.
Spectrophotometric procedure (method I):
Different portions of omeprazole or lansoprazole (containing amounts in the range 0.0500-2.00 or 0.150- 2.00 mg, respectively) were transferred into 50 ml stoppered conical flasks. Then to each drug solution, 2 ml of potassium iodate solution was added followed by 10 ml cyclohexane and 2 or 2.5 ml sulphuric acid solution for omeprazole or lansoprazole, respectively. The obtained solution was mixed well upon shaking. The reaction mixture was then quantitatively transferred into separating funnel. The liberated iodine was extracted using cyclohexane. The extraction of the liberated iodine was repeated with cyclohexane (2×5 ml), the extracts were combined and then diluted to the mark if necessary with cyclohexane in 50 ml measuring flask. The absorbance was finally recorded at 520 nm against reagent blank.
Potentiometric procedure (method II):
Different volumes, 20-120 μg/ml of 1×10-3 M omeprazole or 10-140 μg/ml of 1×10-3 M lansoprazole, were transferred into 50 ml beakers. Subsequently, 3 ml of sulphuric acid solution was added to each solution and diluted with distilled water to 50 ml. Using Pt electrode as an indicator electrode, each drug solution was potentiometrically titrated with 1×10-3 M NBS solution by drop wise addition and constant stirring. The potential difference (E) was plotted against the added volume of the NBS titrant, (v).
Application to capsules:
The contents of twenty capsules were removed and finely powdered using an agate mortar. The combined contents were mixed and weighed accurately. A portion of the powder equivalent to 100 mg of omeprazole or lanzoprazole was accurately weighed and exactly 30 ml of methanol was added, sonicated for about 10 min, left for some time in a fridge, and then filtered into a 100 ml volumetric flask to remove any insoluble matter. The solution was then completed to the mark with the same solvent. The nominal contents of the capsules were determined either from the calibration graph or using the corresponding regression equation.
Results and Discussion
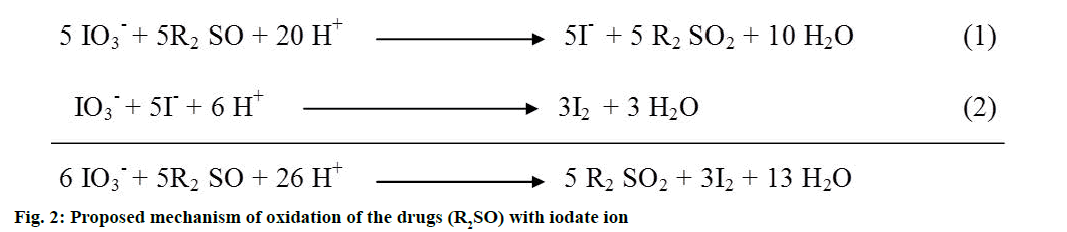
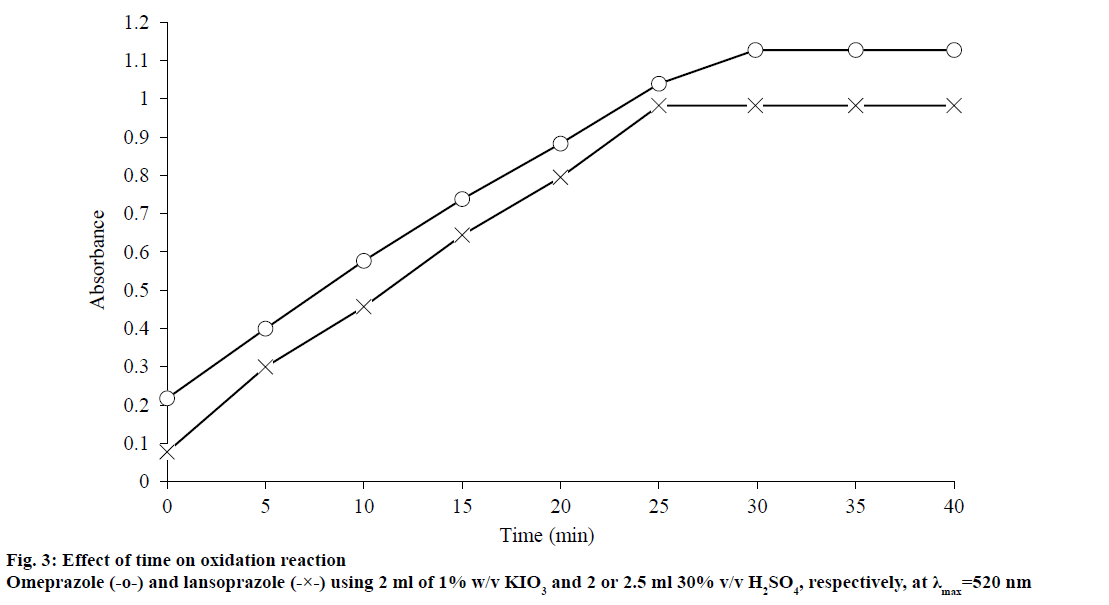
This work was conducted to establish two simple and accurate methods for the determination of omeprazole and lanzoprazole in pure and pharmaceutical dosage forms. Method I was based on the oxidation-reduction reaction between the drug and an excess of iodate ions in an acidic medium[43,44,52] resulting in liberation of iodine, which was then spectrophotometrically determined by measuring the absorbance at 520 nm, (figure 2). This oxidation reaction was a 2-electron oxidation reaction, in which one molecule of iodate oxidized one molecule of the drug resulting in the formation of a sulfone from the sulfoxide group. If the two drugs were labelled as R2SO then it was obvious from figure 2 that the drugs would be oxidized with IO3- to give sulfone derivatives (R2SO2) and iodide ions (Eqn. 1; figure 2), which in turn the latter would react with the excess acidified oxidant (IO3 -) to generate iodine according to the Dushman reaction, Eqn. 2[43]. However, this redox reaction was a stoichiometric one so that the absorbance of the released iodine was found to be linearity dependent on the concentration of the drugs, which served as the basis of the assay. The factors such as reaction time, volume of sulphuric acid solution, volume of potassium iodate solution, quantity of the used drugs, which could influence the oxidation of the drugs of interest, were carefully studied. In order to study the effect of time, samples were assayed and the absorbance was determined after varying the time intervals at the room temperature (25±1°) and the obtained data were depicted in figure 3. The data exhibited that 30 min were enough to complete the oxidation reaction and given the best results for omeprazole drug and 25 min are enough for the similar reaction for lansoprazole drug.
The optimum volume of 30% v/v sulphuric acid solution used for production of maximum colour intensity was 2 and 2.5 ml, for omeprazole and lansoprazole, respectively; however, larger volumes caused the colour to decrease. The influence of potassium iodate concentration on the oxidation of the drugs was studied and it was found that 2 ml of 1% w/v KIO3 were enough for complete oxidation of the omeprazole and lansoprazole drugs; however, higher concentration of potassium iodate had no effect on the absorbance and so the absorbance was nearly constant.
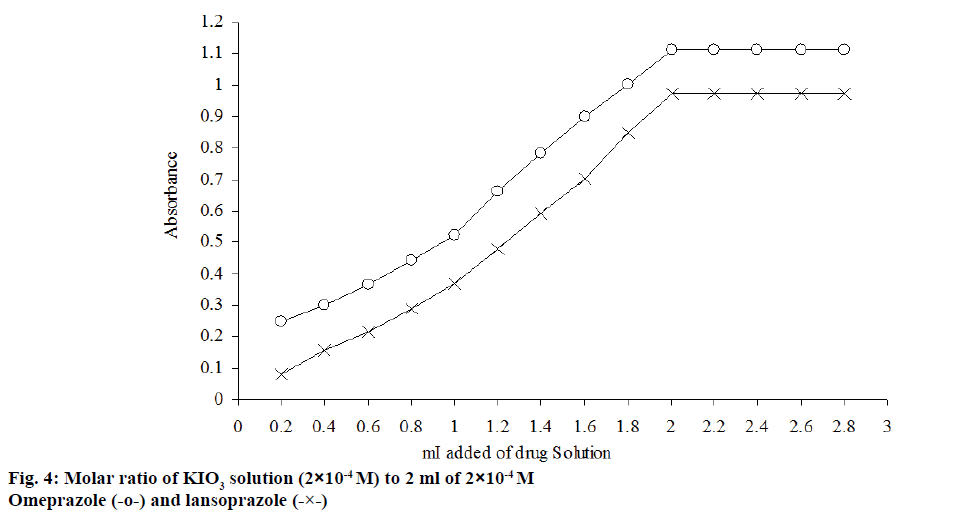
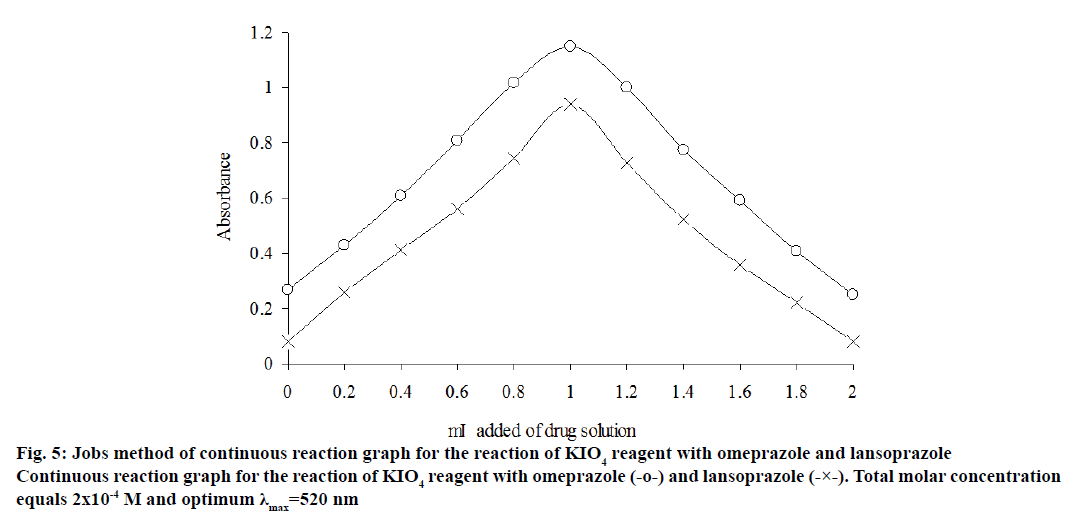
The stoichiometry of the reaction between the two drugs and potassium iodate was investigated by the continuous variation and molar ratio method[49] under the selected optimum conditions. The experimental results showed that the molar ratio of drug:iodate is 1:1 for the title drugs, as shown in figure`s 4 and 5.
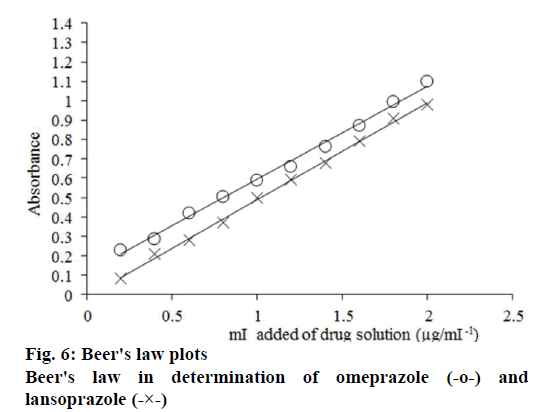
Under the optimum reaction conditions previously described, the calibration curves for both drugs were constructed. The regression equations for the results were derived using the least squares method. A linear regression analysis using absorbance data versus concentration of the drug was carried out. The slope and intercept data obtained from linear regression analysis of the calibration graph were used to calculate the concentration of an unknown sample, using the Eqn., absorbance = intercept+[(slope)×(concentration)].
Consequently, unknown concentration of the drug of interest may be directly obtained using this Eqn. It is noteworthy that, in all cases, Beer’s law plots, as shown in figure 6, were linear with very small intercepts and good correlation coefficients in the concentration range 5-200 and 15-200 μg/ml for omeprazole and lansoprazole, respectively. The apparent molar absorptivity[49] was found to be 2.42×10-4 and 2.01×10-4 mol-1cm-1, whereas Sandell sensitivity[50] was found to be 0.0281 and 0.0473 μgcm-2 for omeprazole and lansoprazole respectively, as presented in Table 1.
| Parameters | Omeprazole | Lansoprazole |
|---|---|---|
| λmax(nm) | 520 | 520 |
| Beer’s law limits (µg/ml) | 5 – 200 | 15 – 200 |
| Molar absorptivity (1 mo-1cm-1) | 2.42x10-4 | 2.01x10-4 |
| Sandell sensitivity (µgcm-1) | 0.0281 | 0.0473 |
| Slope | 0.184 | 0.234 |
| Intercept | 0.002 | 0.006 |
| Correlation coefficient | 0.9999 | 0.9998 |
| Standard error (r) | 0.075 | 0.053 |
| relative standard deviation (RSD) | 0.069 | 0.198 |
| F. test | 2.22 | 2.43 |
| T. test | 1.67 | 2.42 |
Table 1: Quantitative Parameters for the Determination of Omeprazole and Lansoprazole With Kio3 Using Method I
The reaction between omeprazole or lansoprazole and an acidic KIO3 solution increases gradually reaching its maximum after 25 and 30 min for omeprazole and lansoprazole, respectively. It was useful to elaborate a kinetically-based method for the determination of omeprazole and lansoprazole, hence, the reaction was investigated under various conditions. The reaction rate and maximum absorbance increased with increase KIO3 concentration and it is noteworthy that 2 ml of 1×10-3 M KIO3 was adequate for the maximum. The rate of reaction was followed at room temperature with various concentration of the drug in the range 0.2×10-4-1×10-4 M for omeprazole and lansoprazole, while KIO3 and H2SO4 concentration was kept constant, and it was found that the rate of reaction was concentration-dependent. The reaction rate was found to obey the following Eqn. 1, Rate = K' [drug]n, where, K' is the pseudo-order rate constant and n is the order of the reaction. The rate of the reaction may be studied by the variable time method and measured as ΔA/Δt, where, A is the absorbance and t is the time in second[50,51]. By taking logarithms of rates and concentration of drug, Eqn. 1 will be in the following form Eqn. 2, log(rate) = log[ΔA/Δt] = log K'+nlog[drug]. Regression of log(rate) vs. log[omeprazole] or log[lansoprazole] by the least squares method gave the regression Eqns. 3 and 4, Log [ΔA/Δt] = 1.021+0.98 log[omeprazole] (r=0.9890) and Log [ΔA/Δt] = 1.21+0.96 log[lansoprazole] (r=0.9910).
The quantitative estimation of omeprazole and lansoprazole under the previously optimized experimental conditions would result in a pseudo-first order with respect to their concentrations, where KIO3 concentration is kept constant. However, the rates will be directly proportional to omeprazole and lansoprazole concentration in a pseudo-first order rate equation as follows: rate = K' [drug], where K' is the pseudofirst order rate constant equation was the basis for several experimental, which were carried out to obtain omeprazole and lansoprazole concentration. Various analytical methods such as initial-rate, rate-constant, fixed-absorbance and fixed-time[50,51] have been tried and the most suitable analytical method was selected taking into account the sensitivity, applicability, the correlation coefficient (r), the slope of the calibration graphs, and the intercept.
In initial rate method, graphs of the rate (at the beginning of the reaction) versus drug concentration was not easy to obtain, because the first step of the reaction was too fast to follow, so tangents of the curves were not easy to draw and hence this method was therefore abandoned. In rate-constant method, values of log[absorbance] versus time for drug concentrations in the range 0.2×10-4-1×10-4 M for the two drugs were groped and plotted in and all the plots appeared to be rectilinear.
Pseudo-first order rate constant (K') corresponding to different drug concentration [C] were calculated from the slopes (log[A] vs. t) multiplied by – 2.303. Plus, regression of [C] versus K' produced the following Eqns, K' = 0.0003+12.24[C] (r = 0.989) for omeprazole and K' = 0.0002+39.28[C] (r = 0.899) for lansoprazole.
It is obvious that the values of r show poor linearity, which may be due to inconsistency of K' values. In fixed absorbance method, rates of the reactions were calculated for different drug concentrations in the range of 0.2×10-3-1.0×10-3 M at λ=520 nm. A pre-selected value of the absorbance; 0.6 for omeprazole and 0.3 for lansoprazole, was fixed and the time was detected in seconds. The reciprocal of time (1/t) was plotted against the initial concentration of the drug and hence the following equations of the calibration graphs were obtained by linear regression: 1/t = 0.0015+2.00002 (r = 0.997) for omeprazole and 1/t = 0.0013+3.00006 (r = 0.998) for lansoprazole. However, it is worthy to mention that the ranges of concentration, which give the most acceptable calibration plots using the above equations are limited, which can be considered a disadvantage.
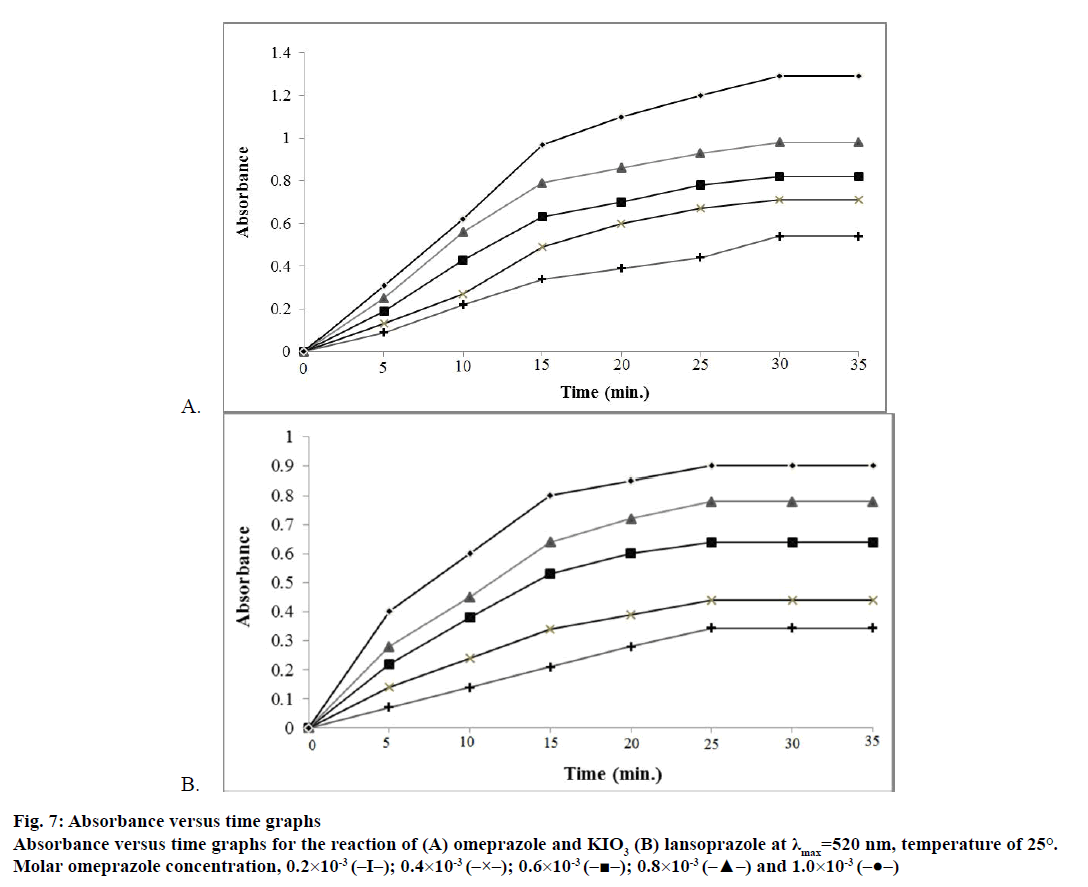
In the fixed-time method, the rates of reactions of interest were calculated for different concentrations of omeprazole and lansoprazole drugs. At a preselected fixed and determined time, the absorbance was recorded. Calibration plots of absorbance against initial concentrations of omeprazole and lansoprazole drugs, shown in figure 7A and B, were obtained at fixed times of 5, 10, 15, 20, 25, 30 and 35 min for omeprazole and lansoprazole and their calibration equations are presented in Table 2. It is obvious that the slopes of the calibration graphs increase with time and the most suitable values of the correlation coefficient (r)[50,51] and the intercepts are obtained at a fixed-time of 30 and 25 min for omeprazole and lansoprazole, respectively, which are therefore chosen as the most suitable time intervals for measurements for the respective drug.
| Drug | Time (min) | Regression equation | Correlation coefficient |
|---|---|---|---|
| Omeprazole | 5 | A = 0.002+2.5x104C | 0.9933 |
| 10 | A = 0.003+3.0x104C | 0.9934 | |
| 15 | A = 0.003+5.0x104C | 0.9935 | |
| 20 | A = 0.004+4.0x104C | 0.9992 | |
| 25 | A = 0.004+5. 2x104C | 0.9996 | |
| 30 | A = 0.005+6.6x104C | 0.9998 | |
| Lansoprazole | 5 | A = 0.004+7.0x104C | 0.9994 |
| 10 | A = 0.006+6.2x104C | 0.9997 | |
| 15 | A = 0.009+8.4x104C | 0.9996 | |
| 20 | A = 0.007+6.2x104C | 0.9997 | |
| 25 | A = 0.008+6.2x104 C | 0.9999 | |
| 30 | A = 0.007+5.5x104C | 0.9998 |
Table 2: Calibration Equations at Different Fixed Times for Omeprazole and Lansoprazole
The above developed method was successfully applied to omeprazole and lansoprazole in bulk powder and dosage pharmaceutical. Five replicate measurements were performed at five different concentrations and the relative standard deviation (RSD) were found to be 0.12 and 0.19 for omeprazole and lansoprazole, respectively in bulk powder and dosage pharmaceutical as shown in Tables 3 and 4. The performance of the proposed method was assessed by calculation of the t-value (for accuracy) and F-test (for precision). The results showed that the calculated values (Table 4) did not exceed the theoretical ones. Hence, there is no a remarkable difference between the proposed and the reference method, indicating that the proposed method is an accurate and precise as the reference one[26] and the certified values of the samples. Moreover, the results indicate a good agreement with the official method and the proposed method can be recommended for routine analysis in the majority of drug quality control laboratories. Plus, another favourable characteristic of the method is that the absorbance of the colored products formed is stable for at least 24 h.
| Taken (µg/ml) | Found (µg/ml) | Recovery % | SD | RSD | |
|---|---|---|---|---|---|
| Omeprazole | 20 | 20.09 | 100.45 | 0.089 | 0.24 |
| 40 | 39.82 | 99.55 | 0.075 | 0.04 | |
| 60 | 60.08 | 100.10 | 0.049 | 0.14 | |
| 80 | 79.99 | 99.98 | 0.043 | 0.05 | |
| 140 | 139.92 | 99.93 | 0.093 | 0.05 | |
| 180 | 180.03 | 100.01 | 0.650 | 0.09 | |
| 200 | 200.02 | 100.01 | 0.540 | 0.16 | |
| Lansoprazole | 20 | 20.01 | 100.01 | 0.056 | 0.16 |
| 60 | 59.92 | 99.86 | 0.046 | 0.14 | |
| 80 | 80.04 | 100.05 | 0.093 | 0.19 | |
| 140 | 139.98 | 99.92 | 0.023 | 0.22 | |
| 180 | 180.06 | 100.03 | 0.056 | 0.15 | |
| 200 | 199.11 | 99.55 | 0.029 | 0.19 | |
| 220 | 219.93 | 99.968 | 0.078 | 0.18 |
Table 3: Determination of Omeprazole and Lansoprazole In Bulk Powder Using an Acidic Kio3 Solution, Method I
| Drug | Recovery (%)±SD | T. test | F. test | |
| Proposed method | Official method | |||
| Omeprazole (10 mg/tab) | 101±0.15 | 99.39 | 1.67 | 2.22 |
| Lansoprazole (30mg/tab) | 100.63 | 100.10 | 2.42 | 2.43 |
Table 4: Determination of Omeprazole and Lansoprazole In Pharmaceutical Dosage form Using an Acidic Kio3 Solution, Method I
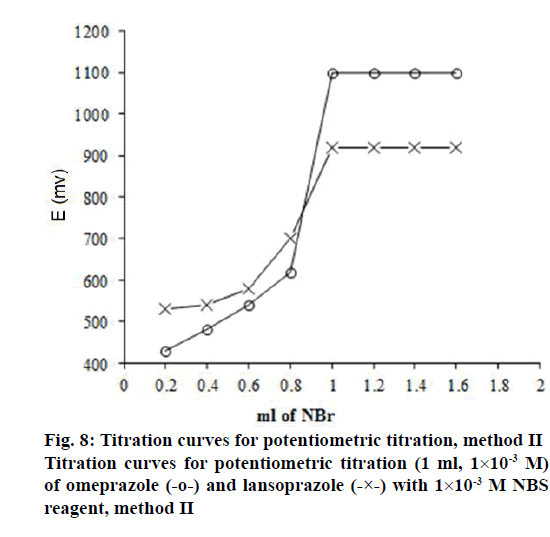
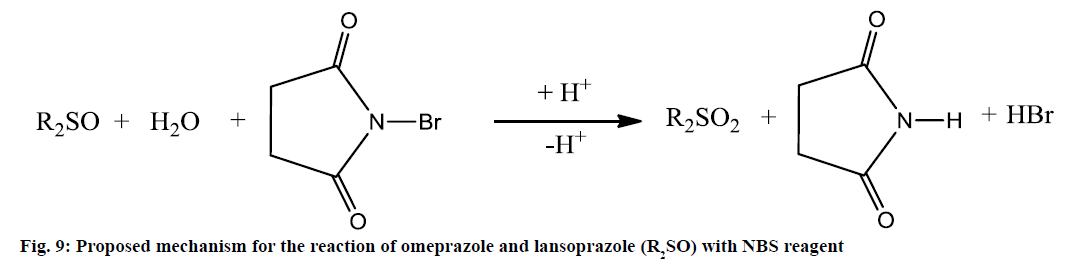
Method II is a potentiometric procedure in which NBS[52] is found to react quantitatively with omeprazole and lansoprazole in sulphuric acid medium. Omeprazole and lansoprazole are directly potentiometrically titrated in H2SO4 medium with 1×10-3 M NBS as titrant using Pt electrode. The titration curves of omeprazole and lansoprazole are shown in figure 8 and they exhibit well-defined s shaped titration curves. However, it is worthy to mention that in this technique, omeprazole and lansoprazole drugs (R2SO) undergo oxidation to give probably sulfone derivatives (R2SO2) and this may be represented by figure 9. In this case, NBS as an oxidant in an acidic media maybe behave like chloramine-T in oxidation of drug sulphide or sulfoxide compounds to sulfone forms (R2SO2)[48]. Moreover, as a confirmation to our proposed mechanism, it was also reported that NBS is able also to oxidize the sulphide form drugs into sulfone forms[53]. The stoichiometry of the reaction between the two drugs under investigation and NBS was studied. The experimental data revealed that the molar ratio of drug:NBS is 1:1 for both drugs.
The proposed method was successfully applied for determination of omeprazole and lansoprazole in bulk powder and dosage pharmaceutical potentiometrically. Omeprazole and lansoprazole can quantitatively be determined in the concentration range of 25-100 and 15-100 μg/ml for omeprazole and lansoprazole, respectively. The standard deviation values are found to be in the range 0.007-0.042 and 0.005-0.034 and the RSD values are found to be in the range 0.79-2.4 and 1.4-2.5 for omeprazole and lansoprazole, respectively, as presented in Table 5.
| Drug | Taken (ml) | Found (ml) | Recovery % | SD | RSD |
|---|---|---|---|---|---|
| Omeprazole | 0.2 | 0.201 | 100.5 | 0.007 | 2.40 |
| 0.4 | 0.399 | 99.7 | 0.010 | 1.50 | |
| 0.6 | 0.598 | 99.6 | 0.022 | 0.79 | |
| 1.0 | 1.032 | 100.3 | 0.042 | 1.60 | |
| Lansoprazole | 0.3 | 0.290 | 96.66 | 0.007 | 1.40 |
| 0.5 | 0.480 | 96.00 | 0.020 | 2.30 | |
| 0.7 | 0.709 | 101.20 | 0.034 | 2.50 | |
| 1.0 | 99.83 | 99.83 | 0.005 | 1.79 |
Table 5: Determination of Omeprazole and Lansoprazole In Bulk Powder Using 1×10-3 M Nbs Reagent, Method II
The amounts of lansoprazole and omeprazole assayed by the proposed method can be calculated using the Eqns., 1 ml of 1×10-3 M NBS=36 mg of omeprazole and 1 ml of 1×10-3 M NBS=34 mg of lansoprazole. In order to investigate the validity and the applicability of the proposed methods and the reproducibility of the obtained results in bulk powder (i.e., pure drugs), five replicate experiments at different concentrations of the drugs under investigation were carried out and the obtained results are presented in Table 5. From the obtained standard deviation and RSD values for each drug, it can be concluded that the proposed method is valid, applicable and highly reproducible. Plus, the validity and applicability of the proposed methods were also proved for application of the proposed method for determination of the drugs under investigation, omeprazole and lansoprazole, in capsules. The obtained results are given in Table 6. The fact that the mv values before the end point in the titration curves of pure of omeprazole and lansoprazole and their corresponding products are almost identical provide evidence that the other excipients that might be present in the product do not affect the titration curves. The results obtained for omeprazole and lansoprazole in their corresponding products show that the recoveries are in good agreement with the official method and RSD values are <2.5%. Thus, the reproducibility and accuracy is very satisfactory for the drug products as well as the drug substances. These results indicate that the content of each drug in the product can be safely determined using this method without interference from other substances in the preparations.
| Drug | Recovery (%) ± SD | T. test | F. test | |
| NBS method | Official method | |||
| Omeprazole (cap/ 20 mg/tab) 10 mg cap. | 102 | 99.97 | 2.2 | 2.46 |
| Lansoprazole (cap/ 30 mg/tab) 30 mg cap. | 100.53 | 100.20 | 1.82 | 2.81 |
Table 6: Determination of Omeprazole and Lansoprazole In Pharmaceutical Dosage Form Using 1×10-3m Nbs Reagent, Method II
In conclusion, the proposed spectrophotometric and potentiometric method could be utilized for routine analysis of pharmaceuticals since they offer simple methodology coupled with short analytical time, good reproducibility and accuracy. On comparing the results obtained by the proposed spectrophotometric method using KIO3 and potentiometric method using NBS titrant, it is obvious that the iodate method is sensitive to high concentration while the NBS method is sensitive to low concentrations as given before. The kinetically-based method proposed in this work for the quantitative analysis of omeprazole and lansoprazole is direct method and more sensitive than the previously methods.
Conflict of interest:
All authors declare no conflict of interests.
Financial support:
Nil.
References
- The British pharmacopoeia I and II. BP commission 7th ed. Great Britain: Majesty’s stationery Office, Dublin; 2014.
- Yanagihara GR, de Paiva AG, Neto MP, Torres LH, Shimano AC, Louzada MJQ, et al. Effects of long-term administration of omeprazole on bone mineral density and the mechanical properties of the bone. Rev Bras Ortop 2015;50:232-8.
- Souza F, Baptista T, Marques E, Barros R, Christianne B, Scaramello V. Omeprazole does not modulate pharmacokinetic of digoxin in patients with heart failure. Int J Cardio 2015;179:343-4.
- Gabello M, Valenzano M, Barr M, Zurbach P, Mullin J. Omeprazole induces gastric permeability to digoxin. Dig Dis Sci 2010;55:1255-63.
- Souney PF, Matthews SJ. Comprehensive Pharmacy Review. 2nd ed. Pennsylvania: Harwal Publishing Co.; 1994. p. 765-77.
- Ferrari S, Perut F, Fagioli F, Brach Del Prever A, Meazza C, Parafioriti A, et al. Proton pump inhibitor chemosensitization in human osteosarcoma: from the bench to the patients' bed. J Transl Med 2013;11:268.
- De Milito A, Canese R, Marino ML, Borghi M, Iero M, Villa A, et al. pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int J Cancer 2010;127:207-19.
- Marino ML, Fais S, Djavaheri-Mergny M, Villa A, Meschini S, Lozupone F, et al. Proton pump inhibition induces autophagy as a survival mechanism following oxidative stress in human melanoma cells. Cell Death Dis 2010;1:87.
- Azzarito T, Venturi G, Cesolini A, Fais S. Lansoprazole induces sensitivity to suboptimal. Doses of paclitaxel in human melanoma. Cancer Lett 2015;28:697-703.
- Mahmoud AM. New sensitive kinetic spectrophotometric methods for determination of omeprazole in dosage forms. Int J Anal Chem 2009;37-45.
- Bhandage A, Bhosale A, Kasture A, Godse VP. Extractive spectrophotometric determination of omeprazole in pharmaceutical preparations. Trop J Pharm Res 2009;8:449-54.
- Wahbi AM, Abdel-Razak O, Gazy AA, Mahgoub H, Moneeb MS. Spectrophotometric determination of omeprazole, lansoprazole and pantoprazole in pharmaceutical formulations. J Pharm DevTechnol 2009;14:516-23.
- Dhumal SN, Dikshit PM, Ubharay II, Mascarenhas BM, Gaitonde CD. Individual UV-spectrophotometric assays of trazodone hydrochloride and omeprazole from separate pharmacetical dosages. Indian Drugs 1991;28:565-7.
- Ozaltin N, Koçer A.Determination of omeprazole in pharmaceuticals by derivative spectroscopy. J Pharm Biomed Anal 1997;16:337-42.
- Castro D, Moreno MA, Torrado S, Lastres JL. Comparison of derivative spectrophotometric and liquid chromatographic methods for the determination of omeprazole in aqueous solutions during stability studies. J Pharm Biomed Anal 1999;21:291-8.
- El-Kousy NM, Bebawy LI. Stability-indicating methods for determining omeprazole and octylonium bromide in the presence of their degradation products. J AOAC Int 1999;82:599-606.
- Karljikovic-Rajic K, Novovic D, Marinkovic V, Agbaba D. First-order UV-derivative spectrophotometry in the analysis of omeprazole and pantoprazole sodium salt and corresponding impurities. J Pharm Biomed Anal 2003;32:1019-27.
- Salama F, El-Abasawy N, Abdel Razeq SA, Ismail MM, Fouad MM. Validation of the spectrophotometric determination of omeprazole and pantoprazole sodium via their metal chelates. J Pharm Biomed Anal 2003;33:411-21.
- Wahbi A, Abdel-Razak O, Gazy A, Mahgoub H, Moneeb M. Spectrophotometric determination of omeprazole, lansoprazole and pantoprazole in pharmaceutical formulations. J Pharm Biomed Anal 2002;30:1133-42.
- Li HK, Zhou WB, Li GSY. Spectrophotometric Determination of Omeprazole Based on the Charge Transfer Reaction between Omeprazole and Chloranilic Acid. Chin J Spectroscop Lab 2004;21:646-9.
- Qaisi AM, Tutunji MF, Tutunji LF. Acid decomposition of omeprazole in the absence of thiol: a differential pulse polarographic study at the static mercury drop electrode (SMDE). J Pharm Sci 2006;95:384-91.
- Rezk NL, Brown KC, Kashuba ADM. A Simple and sensitive bioanalytical assay for simultaneous determination of omeprazole and its three major metabolites in human blood plasma using RP-HPLC after a simple liquid-liquid extraction procedure. J Chromatogr B 2006;844:314-21.
- Hofmann U, Schwab M, Treiber G, Klotz U. Sensitive quantification of omeprazole and its metabolites in human plasma by liquid chromatography-mass spectrometry. J Chromatogr B AnalytTechnol Biomed Life Sci 2006;831:85-90.
- Linden R, ZiulkoskiI AL, Wingert M, Tonello P, Souto AA. Simultaneous determination of omeprazole, hydroxyl omeprazole and omeprazole sulphone in human plasma by isocratic HPLC-DAD: application to the phenotyping of CYP2C19 and CYP3A4 in Brazilian volunteers. J BrazChemSoc 2007;18:733-40.
- Sivasubramanian L, Anilkumar V. Simultaneous HPLC estimation of omeprazole and domperidone from tablets. Indian J Pharm Sci 2007;69:674-6.
- Salem H, Riad SM, Rezk MR, Ahmed K. Simultaneous determination of omeprazole, tinidazole and clarithromycin in bulk powders and Helicure tablets by TLC densitometric technique. J Pharm Educ Res 2013;4:34.
- Vittal S, Ganneboina R, Layek B, Trivedi RK, Hotha K, Bharathi DV. J Biomed Chromatogr 2009;23:390-6.
- Bendas ER, Abdelbary AA. Instantaneous enteric nano-encapsulation of omeprazole: pharmaceutical and pharmacological evaluation. Int J Pharm 2014;468:97-104.
- Perez-Ruiz T, Martínez-Lozano C, Sanz, E Bravo A, Galera R. Determination of omeprazole, hydroxyl omeprazole and omeprazole sulfone using automated solid phase extraction and micellarelectrokinetic capillary chromatography. J Pharm Biomed Anal 2006;42:100-6.
- Avgerinos A, KaridasTh, Potsides C, Axarlis S. Determination of lansoprazole in biological fluids and pharmaceutical dosage by HPLC. Eur J Drug MetabPharmacokinet 1998;23:329-32.
- Nevado JJB, Peñalvo GC, Dorado RMR, Robledo VR. Simultaneous determination of omeprazole and their main metabolites in human urine samples by capillary electrophoresis using electrospray ionization-mass spectrometry detection. J Pharm Biomed Anal 2014;92:211-9.
- Rahman N, Bano Z, Najmul S, Azmi H, Kashif M. A kinetic spectrophotometric method for the determination of lansoprazole in pharmaceutical formulations. J Serb ChemSoc 2006;71:1107-20.
- Pandya KK, Mody VD, Satia MC, Modi IA, Modi RI, Chakravarthy BK, et al. High-performance thin-layer chromatographic method for the detection and determination of lansoprazole in human plasma and its use in pharmacokinetic studies. J Chromatogr B Biomed SciAppl 1997;693:199-204.
- Dogrukol-Ak D, Tuncel M, Aboul-Enien HY. The determination of lansoprazole in pharmaceutical preparation by capillary electrophoresis. Chromatographia 2001;54:527-30.
- Yeniceli D, Dogrukol-Ak D, Tuncel M. Determination of lansoprazole in pharmaceutical capsules by flow injection analysis using UV-detection. J Pharm Biomed Anal 2004;36:145-8.
- Yardimc C, Özaltin N. Electrochemical studies and differential pulse polarographic analysis of lansoprazole in pharmaceuticals. Analyst 2001;126:361-6.
- Radi A. Anodic voltammetric assay of lansoprazole and omeprazole on a carbon paste electrode. J Pharm Biomed Anal 2003;31:1007-12.
- Özaltin N. Determination of lansoprazole in pharmaceutical dosage forms by two different spectroscopic methods.J Pharm Biomed Anal 1999;20:599-606.
- Narang, BD, Ayres GH. Spectrophotometric determination of palladium (II) with 5(p-imethylaminobenzylidene) rhodanine. AnalyticaChimicaActa 1961;24:241-9.
- Mathur NK, Narang CK. The Determination of Organic compounds with N-Bromosuccinimide and Allied Reagents. London: Academic Press; 1975.
- Kowalski P, Mitka K, Ossowska K, Kolarska Z. Oxidation of sulfides to sulfoxides. Part 1: oxidation using halogen derivatives. Tetrahedron 2005;61:1933-53.
- Kaczorowska, K., Kolarska, Z, Mitka K, Kowalski P. Oxidation of sulfides to sulfoxides. Part 2: Oxidation by hydrogen peroxide. Tetrahedron 2005; 61:8315-27.
- Chikwana, E, Davis B, Morakinyo MK, Simoyi RH. Oxyhalogen-sulfur chemistry-Kinetics and mechanism of oxidation of methionine by aqueous iodine and acidic iodate. Can J Chem 2009;87:689-97.
- Wang X, Xu D, Miao C, Zhanga Q, Sun W. N-Bromosuccinimide as an oxidant for the transition-metal-free synthesis of 2-aminobenzoxazoles from benzoxazoles and secondary amines. Org BiomolChem 2014;12:3108-13.
- Ghaemi A, Rayati S, Zakavi S, Safari N. Highly efficient oxidation of sulfides to sulfones with tetra-n-butylammonium hydrogen monopersulfatecatalyzed by ß-tri-and tetra-brominated meso-tetraphenylporphyrinatomanganese (III) acetate. ApplCatal A 2009;353:154-9.
- Basavaiah K, Prameela HC. Two simple methods for the estimation of albendazole and its dosage forms using chloramine-T.Farmaco 2003;58:527-34.
- Mohamed A. Utility of diphenylamine and N-bromosuccinimide for colorimetric determination of certain phenothiazine drugs. Talanta 1997;44:1173-82.
- Mohana K, Bhandarkar P. Oxidation of 2-phenylethylamine with N-Bromosuccinimide in acid and alkaline media: a kinetic and mechanistic study. J Chin ChemSoc 2007;54:1223-32.
- Yatsimirskii K. Kinetic Methods of Analysis. Oxford: Pergamon Press; 1966.
- Sandell E. Colorimetric Determination of Traces of Metals, 3rd ed. New York: Interscience; 1959.
- Laitinen I, Harris W. Chemical Analysis. 2nd ed. New York: McGraw-Hill; 1975.
- El-Yazbi F, Blaih S. Spectrophotometric and titrimetric determination of clindamycin hydrochloride in pharmaceutical preparations. Analyst 1993;118:577-9.
- Basavaiah K, Nagegowda P, Chandrashekar U. Determination of tinidazole by potentiometry, spectrophotometry and high performance liquid chromatography. Indian J Chem Tech 2005;12:273-80.