- *Corresponding Author:
- Panna Thapa
Department of Pharmacy, School of Science, Kathmandu University, Dhulikhel, Kavre, Nepal
E-mail: pannathapa@ku.edu.np
| Date of Received | 29 May 2023 |
| Date of Revision | 02 May 2024 |
| Date of Acceptance | 28 May 2025 |
| Indian J Pharm Sci 2025;87(3):94-101 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This work is focused on the development and optimization of ursolic acid liposomes to overcome the solubility issue of ursolic acid using a response surface diagram. Ursolic acid-loaded liposomes were prepared by the thin-film hydration technique. The effect of two independent factors, the soya lecithin to cholesterol ratio and lipid to drug ratio are the characteristics of liposomes. Two responses, entrapment efficiency and particle size of formulated liposomes were examined. A quadratic polynomial model equation was generated to predict the relationship between independent factors and the responses where a positive correlation was found for both cholesterol and soya lecithin towards entrapment efficiency and particle size. The analysis of results from thirteen different formulations of liposomes showed zeta potential, an encapsulation efficiency, and a particle size of (-44.4±0.03 to -93.8±0.08) mV, (58.520±0.50 to 82.360±0.03) % and (48.2±0.1 to 6034±0.03) μm, respectively. Moreover, the optimized formulation was able to exhibit the desirability functional value of 1 with the highest entrapment efficiency % of 84.37 %.
Keywords
Liposome, ursolic acid, cholesterol, soya lecithin, entrapment efficiency, encapsulation
Ursolic acid is a ubiquitous pentacyclic triterpene compound, available in the plant kingdom in the form of free acids and a glycan[1]. The major sources of ursolic acid are the leaves of rosemary, lavender, thyme, rhododendron, fruit peels of apples, and flowers of Rhododendron arboretum[2,3]. Ursolic acid exhibits numerous beneficial effects such as antitumor, anti-inflammatory, anticancer, antioxidant, antimicrobial, hepatoprotective, antiwrinkle, and chemotherapies[1-5]. It also helps in the prevention of chronic diseases modulating several signalling pathways[3].
The Biological Drug Classification System (BCS) has categorized ursolic acid into class IV[6] due to its weak water solubility, low bioavailability, and poor pharmacokinetics[7,8]. These characteristics have, therefore restricted its use in pharmacodynamics and pharmacological applications. Under such conditions, vesicular drug delivery methods such as liposomes, have demonstrated a promising improvement in the solubility and bioavailability of poorly water-soluble drugs of ursolic acid. Because of the biocompatibility and capacity of liposomes to encapsulate hydrophobic compounds in the lipid domain[1], the liposomal delivery system has attracted the attention of researchers as a noteworthy method to improve the dissolution of poorly soluble drugs[9].
A liposome is a spherical vesicle made up of single or multiple concentric phospholipid bilayers, encapsulating an aqueous compartment[10]. These vesicular carriers can entrap hydrophobic, hydrophilic, and amphiphilic drugs in their liposomal interior and membrane, respectively[11]. They perform biocompatible and non-toxic properties where antioxidants with various solubility profiles can also be encapsulated[12]. They can easily penetrate the human skin, bear the ability of controlled drug release increase their targeted efficiency, and exhibit good stability in suspension[12-14]. Therefore, liposomes can be taken as a promising transdermal delivery system of ursolic acid.
In any pharmaceutical formulation, the design of experimental methodologies such as Central Composite Design (CCD) of Response Surface Methodology (RSM), is beneficial to overcome the issue that may arise in a huge amount of data interpretation, for improving the design, and synthesis of new products[15,16]. RSM is an effective method for optimizing drug delivery systems by rapidly examining the correlations between the elements under investigation and results with the least possible number of experimental trials[17]. RSM includes the generation of polynomial equations and the mapping of measured responses over the experimental domain to choose the best formulation[15,17].
Various literature and reported works have been previously carried out on the extraction, estimation, characterization, and utilization of ursolic acid as a natural antioxidant, anticancer, and various inhibitors of superoxide dismutase and antiwrinkle agents[2,5,18]. The present work is designed to optimize and entrap the less water-soluble ursolic acid in a liposome by response surface design.
Materials and Methods
Soya lecithin, cholesterol, stearic acid, and Tween 80 were purchased from Across (United States of America (USA)) and Merck (Germany), respectively. Minitab 16 software was used to generate and evaluate the experimental design and evaluate the effect of variables on responses. Ursolic acid isolated from Rhododendron arboreum was used as a drug for its encapsulation into the liposomal drug delivery system.
Compatibility studies by FTIR spectroscopy for drug and excipients:
Compatibility between ursolic acid and excipients was determined by Fourier Transform Infrared Spectroscopy (FTIR), Shimadzu, (Japan) adopting the Potassium bromide (KBr) pellet spectroscopic method in the wavelength range of 500-4000 cm-1. Interaction between the components was indicated either by producing additional peaks or by the absence of the characteristic peaks corresponding to the ursolic acid and excipients.
Calibration curve of ursolic acid:
Different concentrations of (100, 200, 400, 600, and 700 ppm) of ursolic acid were prepared using High-Performance Liquid Chromatography (HPLC)-grade methanol as solvent and its calibration curve was determined using HPLC (Agilent-1200). The HPLC conditions reported by Wang et al. and Taralkar et al.[8,19] included a Diamonsil-C18 column (5 μm 4.6×250 mm), mobile phase composed of acetonitrile and methanol (80:20), 0.5 ml/min flow rate, 205 nm detection wavelength, 10 μl injection volume, and 40° column temperature.
Preparation of liposome:
Liposomes of ursolic acid were prepared by the thin-film hydration technique due to its advantage of yielding liposomes with higher encapsulation efficiency, lower release rate, and higher stability[20]. At first, the hydrophobic components, soya lecithin, cholesterol, and ursolic acid (weight ratio of 50:6:5) were dissolved in a 20:10 ratio of methanol and chloroform containing 10 mg stearic acid and 0.1 %, v/v of tween 80 in a 250 ml round bottom flask[8]. The solvent was then evaporated to form a thin film of lipids on the wall of the flask. A milky white suspension was formed after the addition of 10 ml of the phosphate buffer (pH-7.4), which was then agitated on a shaker for 3 h at the speed of 180 rpm. The film thus formed was kept overnight and the suspension was centrifuged for 10 min at 5900 rpm[21]. The supernatant obtained was removed gently. Liposomes thus formed were subjected to the determination of zeta potential, Entrapment efficiency, and particle size. Zeta potential directly correlates with the stability of liposomes whereas particle size is an important parameter that affects the amount of drug loading. Entrapment efficiency estimates the efficacy of phospholipids to retain actives to deliver an adequate amount of drug.
Particle size and Zeta potential analysis:
Liposomal dispersion was characterized by the determination of zeta potential by dynamic light scattering technology using a Nano Particle Analyser (Horiba Scientific SZ-100V2, Japan).
Entrapment efficiency:
The liposomal formulation was subjected to centrifugation at 15 000 rpm for 15 min at 4° to separate the free drug at the wall of the centrifuged tube. The supernatant was collected and further centrifuged at 15 000 rpm at 4° for another 30 min. A clear supernatant and pellets of liposome were obtained. 1 ml of supernatant thus obtained was dissolved in 10 ml of methanol and sonicated for 20 min. The amount of ursolic acid present in the liposomal formulations was determined by HPLC (Agilent-1200). The HPLC conditions were the same as those used in the determination of the calibration curve of ursolic acid[8,19]. Entrapment efficiency was calculated using Eqn 1, % EE=Total drug-entrapped drug/total drug×100 (1).
Statistical analysis:
Based on the RSM, the runs were conducted in CCD to visualize the effects of independent factors (soya lecithin and cholesterol) on the response along with the experimental conditions in Minitab version 16 software. Depending upon the maximum and minimum limit of phospholipid (10-100) and cholesterol (1-10), 13 formulations were given by two-way factor CCD while keeping the concentration of ursolic acid, Tween 80, and stearic acid constant as presented in Table 1.
| Ingredients | Lecithin (mg) | Cholesterol (mg) | stearic (mg) | Ursolic acid (mg) | CH3OH and CH3CL (ml) | PBS (7.4 pH ml) | Tween 80 (1 % v/v mg) |
|---|---|---|---|---|---|---|---|
| LF1 | 0 | 10 | 10 | 5 | 20:10 | 10 | 0.01 |
| LF2 | 50 | 5 | 10 | 5 | 20:10 | 10 | 0.01 |
| LF3 | 50 | 5 | 10 | 5 | 20:10 | 10 | 0.01 |
| LF4 | 0 | 5 | 10 | 5 | 20:10 | 10 | 0.01 |
| LF5 | 50 | 5 | 10 | 5 | 20:10 | 10 | 0.01 |
| LF6 | 100 | 0 | 10 | 5 | 20:10 | 10 | 0.01 |
| LF7 | 50 | 5 | 10 | 5 | 20:10 | 10 | 0.01 |
| LF8 | 120.71 | 5 | 10 | 5 | 20:10 | 10 | 0.01 |
| LF9 | 0 | 0 | 10 | 5 | 20:10 | 10 | 0.01 |
| LF10 | 50 | 5 | 10 | 5 | 20:10 | 10 | 0.01 |
| LF11 | 100 | 10 | 10 | 5 | 20:10 | 10 | 0.01 |
| LF12 | 50 | 12.07 | 10 | 5 | 20:10 | 10 | 0.01 |
| LF13 | 50 | 0 | 10 | 5 | 20:10 | 10 | 0.01 |
Table 1: Formulation Chart for Liposome Containing Ursolic Acid
Based on the conditions of the responses in achieving the highest entrapment efficiency, the optimum concentration of the independent variables for liposome formulation was selected. Using polynomial Eqn 2 and the generalized response surface model as presented, the behaviour of the response surface was examined for the response function (Y) as, Y=β0+β1X1+β2X2+β11X12+β22X22+β12X1X2 (2),
Where, Y is the predicted response; β0 is a constant; β1 and β2 are the linear, quadratic, and interaction coefficients, X1 and X2 are independent variables (related to cholesterol and Lecithin, respectively).
The significance of the variation between the independent variables was ascertained using analysis of variance. The simplified model comprised all significant independent variable effects (p<0.05). Three-dimensional response surface plots were created to visualize the interaction effect of the variables on the responses[17].
Results and Discussion
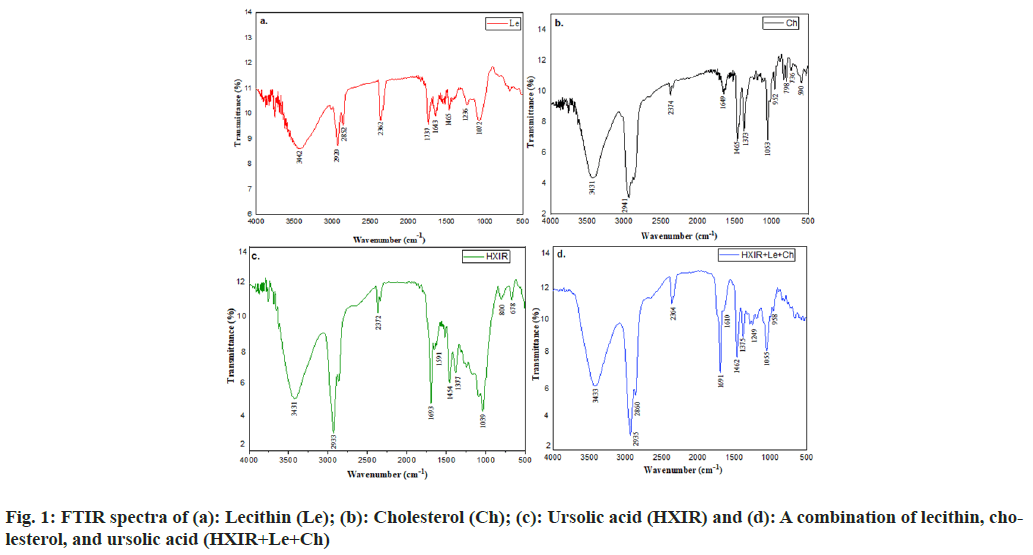
In the present study, the possible interaction between the ursolic acid and excipients mixture (lecithin and cholesterol) was carried out. The results revealed no considerable changes in FTIR spectra of ursolic acid when mixed with excipients in comparison to pure ursolic acid, lecithin, and cholesterol. FTIR spectra of lecithin, cholesterol, and ursolic acid with these excipients and their significance are presented in fig. 1 and Table 2, respectively. Similar results of FTIR spectra of lecithin, cholesterol, and ursolic acid are reported by Kuligowski et al., Vyas and Joshi et al. and Naika et al. respectively[22-24].
| Wavenumber (cm-1) HXIR | Significance | Wavenumber related to the mixture (HXIR, Le, Ch) cm-1 |
|---|---|---|
| 3431 | OH group stretching | 3433 |
| 2933 | C-H stretching in CH3 and CH2 | 2933 |
| 1693 | C=O stretching of COOH group | 1691 |
| 1591 | C=C structure | 1610 |
| 1317 | C-H deformation in gem dimethyl | 1375 |
| 1059 | C-O functional group | 1055 |
Table 2: Comparison Between Peaks Obtained in Drug and Mixture
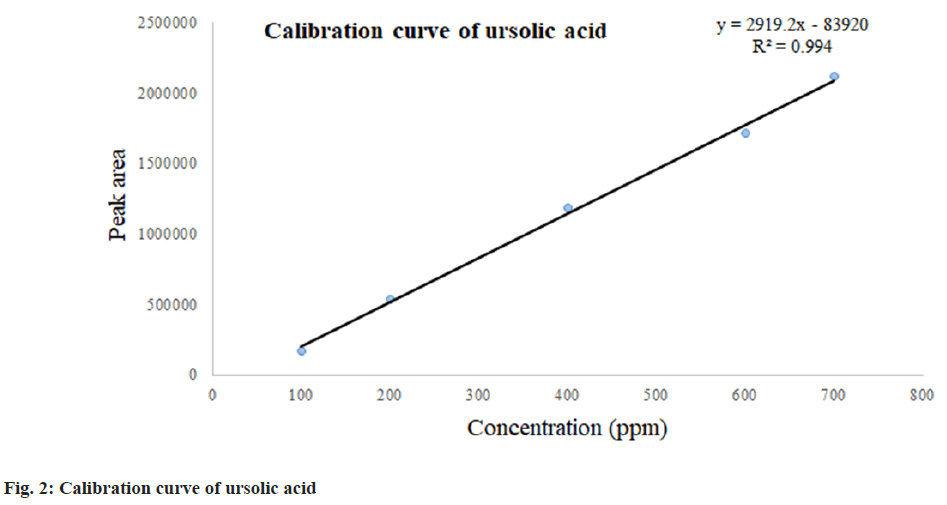
The isolated ursolic acid showed its characteristic peak at the retention time of 12.69 min as reported by Taralkar and Chattopadhyay et al.[19]. The calibration curve obtained from the HPLC showed a linear equation of y=2919.2x-83920 with a regression line coefficient of R2 (0.994). The calibration curve for ursolic acid is presented in fig. 2.
Zeta potential determination is an important parameter in liposome formulation. The value of the zeta potential of a liposome preparation helps to predict the fate of liposomes in vivo[25]. Particles that are small enough with high zeta potential will confer stability by resisting their aggregation[26]. In this study, 13 different liposome dispersions with varying concentrations of cholesterol and soya lecithin were prepared depending on the higher and lower limit of variables. The Zeta potential of formulations was found in the range of -93.8 and -44.4 mV (Table 3), signifying sufficient charge available in the prepared formulations avoiding the aggregation of vesicles. The result of zeta potential thus obtained is due to the presence of stearic acid that is used during formulation. The range of zeta potential above 60 and that between 40 and 60 represent the excellent and good stability behaviour of colloidal solutions, respectively[27].
| Independent variables | Constraints | ||
|---|---|---|---|
| Low | High | Goal | |
| Particle size (Y1) | 48.2 | 6034 | |
| Entrapment (Y2) | 59.25 | 83.56 | Maximize |
Table 3: Higher and Lower Limits of Particle Size and Entrapment Efficiency
The polynomial equation obtained for particle size and entrapment efficiency was subjected to multiple regression analysis and the data obtained were fitted to the Eqn 2.
X1 and X2 represent the average result of changing one factor at a time from low to high value. The interaction term X1 and X2 shows how the response changes when 2 factors are simultaneously changed. Furthermore, depending on the results of particle size and entrapment efficiency, an objective was set to maximize the entrapment efficiency.
The average particle sizes±Standard Deviation (SD) were found in the range of 48.28±0.10 μm to 6034±0.13 μm. From several studies, it was observed that the size of liposomes determines their in vivo and ex vivo performances. Some reports have shown the effect of liposomal size on drug release as well as drug deposition in the skin. Thus, the selected method should be in the optimum range for effective delivery. Formulation LF1, LF4, and LF9 show a particle size of less than 100 μm which signifies that these formulations couldn’t form the vesicles. It was justified by its negligible entrapment values. However, formulation LF6 shows the highest particle size of 6034 μm where the phospholipids form vesicles in contact with water which was indicated by its high entrapment value. In order to understand the effect of lipid concentration on the vesicle size, the polynomial equation was derived. The polynomial equation given for particle size is shown in Eqn 3, Particle size (Y1)=568+78.7*X1+28*X2-0.196*X12+33.7 *X22-7.11 *X1 * X2 (3).
A positive correlation was observed for both of the variables lecithin and cholesterol with R2 value of 0.9833. Thus, with an increase in the concentration of lecithin and cholesterol, vesicle size was increased. The coefficient of both variable results revealed that the effect of soya lecithin was more prominent than that of cholesterol. A similar type of result on the direct effect of cholesterol on liposomal particle size is also shown in the study carried out by Shaker et al.[28] on the factors affecting liposomal particle size while the prominent effect of soya lecithin analogous to our study is reported by Narsaiah et al.[29].
Cholesterol is widely employed in the synthesis of liposomes to improve the physical stability and rigidity of the phospholipid bilayer. As the concentration of cholesterol in the liposomes increases, the permeability of the liposome membrane to solutes decreases[28]. The mean liposome diameter will increase as the concentration of cholesterol rises because more cholesterol molecules will be scattered in the phospholipid bilayer[28]. It is reported that greater cholesterol levels improve membrane fluidity, which promotes the distribution of the aqueous phase inside liposomal vesicles and prevents the phospholipid bilayer from tight packing[30].
Determination of entrapment efficiency is an important parameter in the study of liposomes as it affects drug release and skin deposition. It is expressed as the fraction of drug incorporated into liposomes relative to the total amount of drug used. Depending upon the linear equation, the entrapment efficiency of 13 different formulations was calculated at the same wavelength (205 nm). Entrapment efficiency of all batches was in the range of 58.52 % to 83.56 %. In order to understand the effect of lipid concentration on entrapment efficiency, the coefficient observed for entrapment efficiency is fitted to Eqn 4, Entrapment (Y2)=1.21+1.889*X1+9.08*X2-0.01109*X12-0.245*X22-0.088*X1*X2 (4). A positive correlation was observed for both variables (lecithin and cholesterol) with an R2 value of 0.9966. Thus, with an increase in the concentration of lecithin and cholesterol, entrapment efficiency was found to increase. The analogous result of an increase in entrapment efficiency with an increase in cholesterol is compared to that reported by Rushmi et al.[31]. It is mentioned that increased amounts of cholesterol fill up the free spaces within the lipid chains which reduces their flexibility and attenuates the molecular mobility.
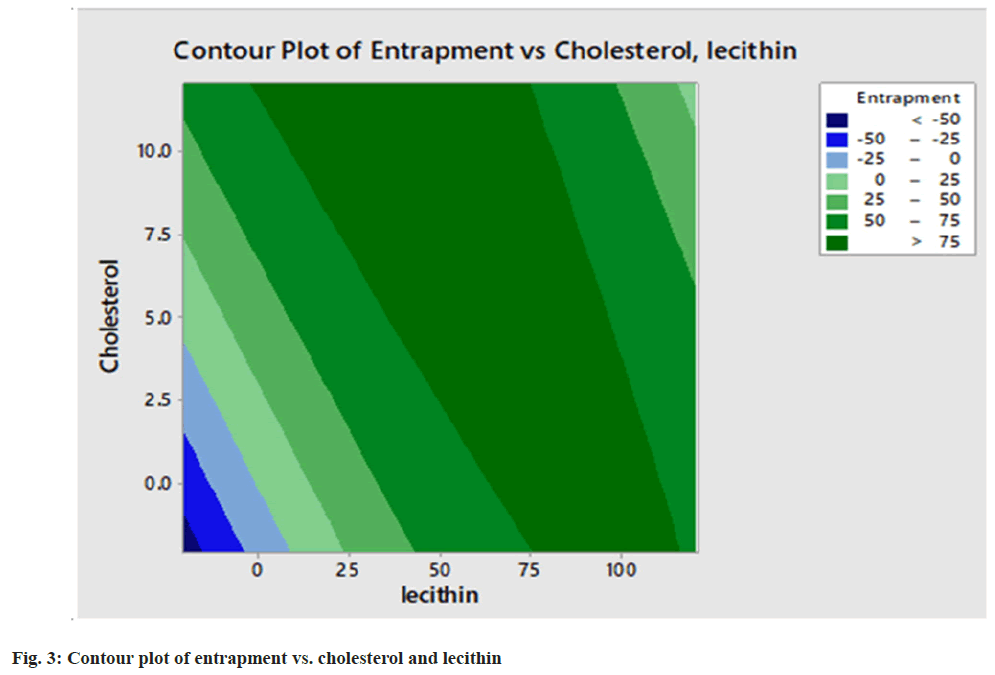
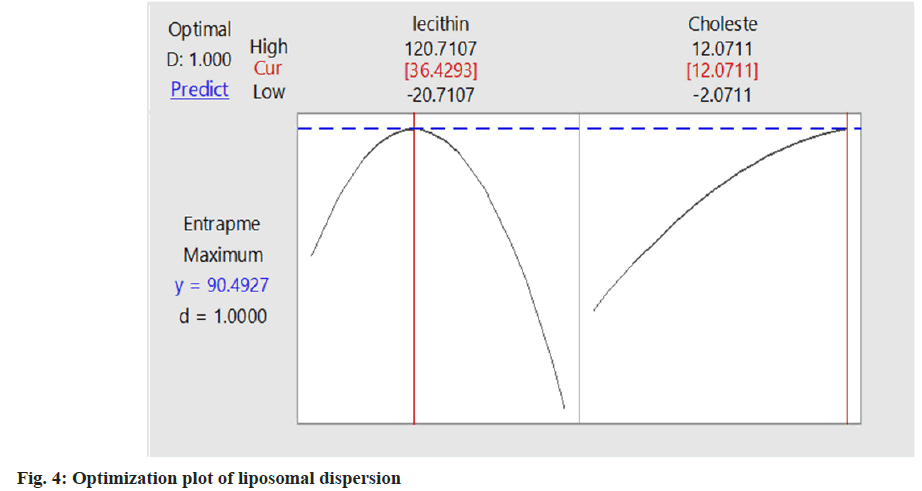
Varying the concentration of two independent variables (soya lecithin and cholesterol), all the batches of liposomal dispersion (2941.9±0.05) having a minimum vesicle size and intermediate entrapment efficiency were selected as the best formulation. Based on desirability function value optimization was carried out as shown in fig. 3. The optimum value of lecithin (120.71 mg) and cholesterol (12.07 mg) gives the highest desirability function value of 1 with entrapment efficiency % 90.49 which is the optimized batch of liposomal dispersion. The desirability function was used for the optimization process to obtain the levels of lecithin (X1) and cholesterol (X2). The contour plot of entrapment against cholesterol and lecithin is shown in fig. 4. Though the entrapment efficiency of 90.49 % was shown by the software using the optimized formulation only the entrapment of 84.37 % of the drug was found in the result as in Table 4. The coefficients B1, B2, B11, and B22 that are obtained for the entrapment efficiency were found significant at p<0.05 with an R2 value of 0.9966 (Table 5 and Table 6).
| Formulations | Zeta potential (mV) | Particle size (µm) | Entrapment efficiency |
|---|---|---|---|
| LF1 | -46.9±0.12 | 82.9±0.02 | 9.884±0.03 |
| LF2 | -84.3±0.03 | 3239±0.22 | 81.730±0.41 |
| LF3 | -83.2±0.03 | 3196±0.03 | 80.340±0.32 |
| LF4 | -93.72±0.08 | 48.2±0.10 | 0.000 |
| LF5 | -80 5±0.12 | 3205±0.02 | 82360±0.03 |
| LF6 | -66.1±0.03 | 6034±0.13 | 69.900±10.10 |
| LF7 | -79.8±0.02 | 3205±0.10 | 81.67±0.34 |
| LF8 | -73.0±0.01 | 3315.3±0.03 | 59.250±012 |
| LF9 | -45.3±0.11 | 65.7±0.01 | 0.000 |
| LF10 | -81.3±0.21 | 3247.0±0.03 | 83.560±0.04 |
| LF11 | -63.2±0.11 | 4639.4±0.07 | 58.520±0.50 |
| LF12 | -57.2±0.03 | 2941.9±0.05 | 81.000±0.04 |
| LF13 | -44.4±0.03 | 5744.8±0.06 | 64.620±0.03 |
Table 4: Zeta Potential, Particle Size, and Entrapment Efficiency of Liposome Prepared
| Coefficients | B0 | B1 | B2 | B11 | B22 | B12 |
|---|---|---|---|---|---|---|
| Entrapment | 1.21 | 1.889 | 9.08 | -0.01109 | -0.245 | -0.0883 |
| p value | 0.00 | 0.000 | 0.003 | 0.000 | 0.05 | 0.000 |
Table 5: Table Showing P-Value of Entrapment Efficiency
| Formulations variables | Optimum values (mg) | Entrapment efficiency |
|---|---|---|
| Lecithin | 120.71 | 84.37% |
| Cholesterol | 12.07 |
Table 6: Optimum Values of Lecithin and Cholesterol for Maximum Entrapment Efficiency
In conclusion, less soluble ursolic acid was successfully entrapped within a liposome and optimized using RSM. Optimized liposomes containing ursolic acid showed the highest entrapment of 84.61±0.13 % with an R2 value of 0.996 which was found to be significant at p<0.05. In addition, the entrapment efficiency showed a positive correlation with the concentration of soya lecithin and cholesterol. Furthermore, it is recommended to carry out permeability and absorption studies of ursolic acid for both in vitro and in vivo models.
Acknowledgments:
The authors gratefully acknowledge the University Grants Commission (UGC) Nepal for providing a Ph.D. research fellowship (Grant No. PhD/74_75/ HS-2) to Bigyan Joshi.
Conflict of interests:
The authors declared no conflict of interests.
References
- Khan K, Aqil M, Imam SS, Ahad A, Moolakkadath T, Sultana Y, et al. Ursolic acid loaded intra nasal nano lipid vesicles for brain tumour: Formulation, optimization, in-vivo brain/plasma distribution study and histopathological assessment. Biomed Pharmacother 2018;106:1578-85.
[Crossref] [Google Scholar] [PubMed]
- Shilajan S, Swar G. Simultaneous estimation of three triterpenoids‑ursolic acid, b‑sitosterol and lupeol from flowers, leaves and formulations of Rhododendron arboreum smith. using validated HPTLC method. Int J Green Pharm 2013;7(3):206-10.
- Seo DY, Lee SR, Heo JW, No MH, Rhee BD, Ko KS, et al. Ursolic acid in health and disease. Korean J Physiol Pharmacol 2018;22(3):235-48.
[Crossref] [Google Scholar] [PubMed]
- Huaman MA, Quispe AL, Quispe RI, Flores CA, Caycho JR. A simple method to obtain ursolic acid. Results Chem 2021;3:1-10.
- Sultana N. Clinically useful anticancer, antitumor, and antiwrinkle agent, ursolic acid and related derivatives as medicinally important natural products. J Enzyme Inhib Med Chem 2011;26(5):616-42.
[Crossref] [Google Scholar] [PubMed]
- Yu D, Kan Z, Shan F, Zang J, Zhou J. Triple strategies to improve oral bioavailability by fabricating coamorphous forms of ursolic acid with piperine: Enhancing water-solubility, permeability, and inhibiting cytochrome p450 isozymes. Mol Pharm 2020;17(12):4443-62.
[Crossref] [Google Scholar] [PubMed]
- Liu J. Oleanolic acid and ursolic acid: Research perspectives. J Ethnopharmacol 2005;100(1-2):92-4.
[Crossref] [Google Scholar] [PubMed]
- Wang M, Zhao T, Liu Y, Wang Q, Xing S, Li L, et al. Ursolic acid liposomes with chitosan modification: Promising antitumor drug delivery and efficacy. Mater Sci Eng C 2017;71:1231-40.
[Crossref] [Google Scholar] [PubMed]
- Lee MK. Liposomes for enhanced bioavailability of water-insoluble drugs: In vivo evidence and recent approaches. Pharmaceutics 2020;12(3):1-6.
[Crossref] [Google Scholar] [PubMed]
- Shrestha S, Budhathoki U. Formulation, ex-vivo and in-vitro characterization of liposomal drug delivery system of Fexofenadine. Thai J Pharm Sci 2022;46(1):56-60.
- Zhao X, Liu J, Hu Y, Fan Y, Wang D, Yuan J, et al. Optimization on condition of glycyrrhetinic acid liposome by RSM and the research of its immunological activity. Int J Biol Macromol 2012;51(3):299-304.
[Crossref] [Google Scholar] [PubMed]
- Peralta MF, Guzmán ML, Pérez AP, Apezteguia GA, Fórmica ML, Romero EL, et al. Liposomes can both enhance or reduce drug penetration through the skin. Sci Rep 2018;8(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Bseiso E, Nasr M, Sammour O, Abd El Gawad N. Recent advances in topical formulation carriers of antifungal agents. Indian J Dermatol Venereol Leprol 2015;81(5):457-63.
[Crossref] [Google Scholar] [PubMed]
- Yadav D, Sandeep K, Pandey D, Dutta RK. Liposomes for drug delivery. J Biotechnol Biomater 2017;7(4):1-8.
- Aziz DE, Abdelbary AA, Elassasy AI. Implementing central composite design for developing transdermal diacerein-loaded niosomes: Ex vivo permeation and in vivo deposition. Curr Drug Deliv 2018;15(9):1330-42.
[Crossref] [Google Scholar] [PubMed]
- Ghelich R, Jahannama MR, Abdizadeh H, Torknik FS, Vaezi MR. Central Composite Design (CCD)-Response Surface Methodology (RSM) of effective electrospinning parameters on PVP-B-Hf hybrid nanofibrous composites for synthesis of HfB2-based composite nanofibers. Compos B Eng 2019;166:527-41.
- Hassan H, Adam SK, Alias E, Meor Mohd Affandi MM, Shamsuddin AF, Basir R. Central composite design for formulation and optimization of solid lipid nanoparticles to enhance oral bioavailability of acyclovir. Molecules 2021;26(18):5432-41.
[Crossref] [Google Scholar] [PubMed]
- Somantri AD, Kurnia D, Zainuddin A, Dharsono HD, Satari MH. Action mode of ursolic acid as a natural antioxidant and inhibitor of superoxide dismutase: In vitro and in silico study. J Adv Pharm Technol Res 2021;12(4):389-94.
[Crossref] [Google Scholar] [PubMed]
- Taralkar SV, Chattopadhyay S. A HPLC method for determination of ursolic acid and betulinic acids from their methanolic extracts of Vitex negundo Linn. J Anal Bioanal Tech 2012;3(3):1-6.
- Tagrida M, Benjakul S. Liposomes loaded with betel leaf (Piper betle L.) extract: Antibacterial activity and preservative effect in combination with hurdle technologies on tilapia slices. Food Control 2022;138:1-10.
- Moghimipour E, Salami A, Monjezi M. Formulation and evaluation of liposomes for transdermal delivery of celecoxib. Jundishapur J Nat Pharm Prod 2015;10(1):e1-11.
[Crossref] [Google Scholar] [PubMed]
- Kuligowski J, Quintas G, Garrigues S, Lendl B, de la Guardia M. Recent advances in on-line Liquid Chromatography-Infrared Spectrometry (LC-IR). Trends Anal Chem 2010;29(6):544-52.
- Vyas PM, Joshi M. Surface micro topographical and dielectric studies of cholesterol crystals. Adv Mater Res 2013;665:289-96.
- Naika HR, Bhavana S, Da Silva JA, Lingaraju K, Mohan VC, Krishna V. In silico and in vivo wound healing studies of ursolic acid isolated from Clematis gouriana against GSK-3 beta. Nus Biosci 2016;8(2):232-44.
- Panalytical M. Liposomes and the use of zeta potential measurements to study sterically stabilized liposomes. AZoNano 2005.
- Revathi S, Dhanaraju MD. Optimization and characterization Ezogabine-loaded nanosuspension for enhancement of bioavailability by “bottom-up” technology using 32 factorial design. J Drug Deliv Ther 2019;9(3):227-37.
- Kumar A, Dixit CK. Methods for characterization of nanoparticles. In Advances in nanomedicine for the delivery of therapeutic nucleic acids: Woodhead Publishing; 2017. p. 43-58.
- Shaker S, Gardouh AR, Ghorab MM. Factors affecting liposomes particle size prepared by ethanol injection method. Res Pharm Sci 2017;12(5):346-52.
[Crossref] [Google Scholar] [PubMed]
- Narsaiah K, Jha SN, Wilson RA, Mandge HM, Manikantan MR, Malik RK, Vij S. Pediocin-loaded nanoliposomes and hybrid alginate–nanoliposome delivery systems for slow release of pediocin. BioNanoScience 2013;3:37-42.
- Duangjit S, Pamornpathomkul B, Opanasopit P, Rojanarata T, Obata Y, Takayama K, et al. Role of the charge, carbon chain length, and content of surfactant on the skin penetration of meloxicam-loaded liposomes. Int J Nanomed 2014;9:2005-17.
[Crossref] [Google Scholar] [PubMed]
- Rushmi ZT, Akter N, Mow RJ, Afroz M, Kazi M, de Matas M, et al. The impact of formulation attributes and process parameters on black seed oil loaded liposomes and their performance in animal models of analgesia. Saudi Pharm J 2017;25(3):404-12.
[Crossref] [Google Scholar] [PubMed]