- *Corresponding Author:
- H.H. Zhang
Department of Pharmacy, Hebei North University, Zhangjiakou, Hebei-075 000, People's Republic of China
E-mail: zhanghaihongzhh@163.com
| Date of Submission | 29 May 2016 |
| Date of Revision | 11 September 2016 |
| Date of Acceptance | 23 September 2016 |
| Indian J Pharm Sci 2016;78(5):608-614 |
This is an open access article distributed under terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Vigna angularis, also called adzuki bean, a member of the family Fabaceae, has long been cultivated in Asian countries. This legume has received considerable attentionowing to the presence ofpolyphenols. The optimal extraction conditions for total polyphenols from V. angularis and their antioxidant activities were investigated in this study. Ultrasound-assisted extraction was used to optimize the extraction of total polyphenols from V. angularis. Factors that influenced the extraction efficiency, such as ethanol concentration, liquid-solid ratio, and ultrasonic time were investigated by Box-Behnken design. Folin-ciocalteu colorimetry was adopted to determine the total polyphenol content by using gallic acid as a reference. Antioxidant activities of the total polyphenols were evaluated by 1,1-diphenyl-2-picrylhydrazyl, hydroxyl and superoxide radical-scavenging activity assays. The optimized extraction conditions were as follows: ethanol concentration of 44.3%; liquid-solid ratio of 21.6:1 and ultrasonic extraction time of 30 min. Under these optimal conditions, the model’s predicted extraction efficiency was 4.19 mg/g. The mathematical model had a high correlation (P<0.05) and could be employed to extract total polyphenols from V. angularis. In vitro antioxidant activity results showed that total polyphenols had strong antioxidant capacity and could be used as potential antioxidant agents. This study provided a reference for the exploration of new natural antioxidants.
Keywords
Vigna angularis (Adzuki bean), total polyphenols, extraction, Box-Behnken design, response surface methodology, antioxidant activity
The extraction of phytochemicals from food plants using solvents is an important step in separation and bioactivity research. Thus, efficient extraction techniques are required to harvest the natural products. Ultrasound-assisted extraction (UAE) is an efficient extraction technique that can help solutes more rapidly diffuse from the solid material into the solvent [1]. UAE has been widely used owing to its high efficiency, shorter processing time and reduced solvent consumption.
Vigna angularis, also called adzuki beans, a member of the family Fabaceae, is an important food crop and used as folk medicines in Asian countries [2]. V. angularis have relatively small beans (about 5 mm). Most of the adzuki beans cultivated in Eastern Asian countries are red in color. This legume is rich in dietary fibers, proteins, minerals, vitamin A, vitamin B9 and folate [3]. Due to its biochemical components, V. angularis has been evaluated as a new treatment option. In addition, it has received considerable attention owing to the presence of polyphenols, such as proanthocyanidins and quercetin [4]. Numerous studies have demonstrated that polyphenols possess antioxidant [5,6], anticarcinogenic [7,8], antiglycation [9] and neuroprotective [10] properties. However, there are no data relating to optimized extraction conditions of total polyphenols (TPs) from V. angularis using response surface methodology (RSM), and there is little information about their antioxidant activities. RSM is a statistical technique, which includes the design of experiment, modeling, identifying optimal conditions, predicting the maximum response and an evaluation of interactions. It has been previously employed to optimize the extraction of phenolic compounds [11,12].
In this study, process parameters that affect UAE such as ethanol concentration, liquid-solid ratio, ultrasonciation time were optimized using RSM, by employing a Box-Behnken design (BBD), to maximize the yield of TPs from V. angularis. The antioxidant activities of the TPs were investigated by measuring 1,1-diphenyl-2- picrylhydrazyl (DPPH) radical, hydroxyl radical and superoxide radical scavenging.
Materials and Methods
Dried adzuki beans were purchased from Zhang Jiakou herbal material company, Hebei Province, China. A specimen is deposited at Department of Pharmacy, Hebei North University, Zhang Jiakou, China. Gallic acid was supplied by the National Institutes for Food and Drug Control, China (Batch number: 110831- 201204). Folin-Ciocalteu reagent, 1,1-diphenyl-2- picrylhydrazyl (DPPH*), nitrobluetetrazolium (NBT), nicotinamide adenine dinucleotide hydrogen (NADH) and phenazine methosulfate (PMS) were purchased from Sigma Chemical Co., Ltd. All the other reagents used were of analytical grade.
Extraction process
Dried adzuki beans were ground and sifted through a 0.75 mm sieve. An ultrasonic cleaner (KQ-250V, Kunshan Ultrasonic Instruments Co., Ltd, Kunshan, China) was used for the preparation of TPs. V. angularis powder (6.0 g) was mixed with solvent in a conical flask and sonicated (frequency 50 kHz, power 100 W and temperature of 25°) under different extraction conditions. Changes to only a single-factor were performed in a series of experiments, where the ethanol concentration ranged from 20-80%,liquidsolid ratio ranged from 5:1 to 40:1, the ultrasonic time ranged from 20-60 min and the number of extractions ranged from 1 to 5. In the optimization experiments, the experimental variable settings were based on the BBD results with a three-factor, three-level system, which resulted in an experimental design of 15 experimental points, including three central points. The three independent variables were coded at three levels (-1, 0, +1), 0 was central point, and the high and low levels of the factors are coded as +1 and -1, respectively. At the end of the ultrasonic time, the extract solution was centrifuged (10 min, 3000 rpm), filtered and analyzed for TPs content by UV/Vis spectrophotometry (model 2100, Labtech, USA).
Determination of TP content
The TP content in the extracts was determined using Folin-Ciocalteu colorimetry as described previously [13]. In each replicate, 1 ml of the diluted extract solution, 1 ml of Folin-Ciocalteu reagent and 20 ml of 20% Na2CO3 were mixed together and vortexed. The mixture was diluted to 50 ml with distilled water and then incubated for 30 min in the dark at room temperature. After incubation the absorbance of the mixture against the blank solution was measured at 760 nm. Gallic acid solutions in methanol (20, 40, 60, 80, 100, 200 μg/ml) were used as standards, and were treated in a similar manner to the samples. A standard curve was conducted, resulting in the following regression Eqn., A=0.0036C+0.0084 (r=0.9992), with good linearity in the range of 20-200 μg/ml. The TP content of the sample was expressed as gallic acid equivalent (mg/g dry weight).
DPPH radical scavenging activity assay
TPs were prepared on the basis of the optimal level and were diluted to different concentrations (0.15, 0.2, 0.4, 0.6, 0.8 and 1 mg/ml). DPPH radical scavenging activity was tested following a previously published protocol [14,15]. Briefly, 2 ml of 0.2 mM DPPH was mixed with 2 ml of extract solution at different concentrations. The reaction mixture was vortexed thoroughly and incubated in the dark at room temperature for 30 min. The absorbance of the mixture was measured spectrophotometrically at 517 nm. Ascorbic acid was used as positive control. percent DPPH radical scavenging activity was calculated with the following Eqn., DPPH radical scavenging activity (%)= [(A0 - A1)/A0]×100, where A0 is the absorbance of the control, and A1 is the absorbance of sample.
Hydroxyl radical scavenging activity assay
The hydroxyl radical scavenging activity was carried out as described previously, with slight modifications [16]. The reaction contained 1 ml of EDTA-Fe (II) (0.945 mM), 1 ml of H2O2 (3%, V/V), 1 ml of safranin (40 μg/ml), 1.5 ml of extract solution (0.15-1 mg/ml), and 1 ml of phosphate buffered saline (0.15 M, pH 7.4). The mixture was then incubated at 37° for 1 h. The absorbance of the mixture was measured at 510 nm using ascorbic acid as a positive control. Percent hydroxyl radical scavenging activity was calculated using the following equation, hydroxy radical scavenging activity (%)= [(A0-A1)/A0]×100, where A0 is absorbance of blank and A1 is absorbance of sample.
Superoxide radical scavenging activity assay
Sample solutions were prepared as per section 1.4. Superoxide radical scavenging activity was determined according to the NBT reduction method [17]. Extract samples (1.5 ml) of different concentrations were mixed with 1.5 ml of reaction solution containing 0.5 ml of NBT solution (300 μmol/l), 0.5 ml of NADH solution (468 μmol/l) and 0.5 ml of PMS solution (60 μmol/l). The mixture was incubated at RT for 5 min, and the absorbance was read at 560 nm. The Percentage superoxide radical scavenging activity was calculated using the following equation, Superoxide radical scavenging activity (%)= [1-(A1-A2)/A0]×100, where A0 is the absorbance of the control group, A1 is the absorbance of sample and A2 is the absorbance of blank.
Statistical analysis
All the experiments were performed in triplicate, and the data were expressed as the mean±SD (standard deviation). Optimal extraction conditions were determined by RSM, which was performed using the Design-Expert Version 9.0.2 software (Stat-Ease, Inc., Minneapolis, MN). Experimental data were analyzed using variance (ANOVA) and the adequacy of the model was determined by evaluating the lack of fit, coefficient of regression (R2) and the Fisher test value (F-value). The level of significance of the model and model variables was set at P<0.05. The contour plots and response surfaces were drawn using Design-Expert 9.0.2. Then, the maximum was predicted by analysis for the response surfaces.
Results and Discussion
Single-factor experiments are defined as experiments where there is a change in only one factor at a time, while the other factors are fixed when performing an experiment. These experiments can help to determine reasonable factors and levels in the design of future experiments. In this study, the effects on the extraction of TPs were investigated.
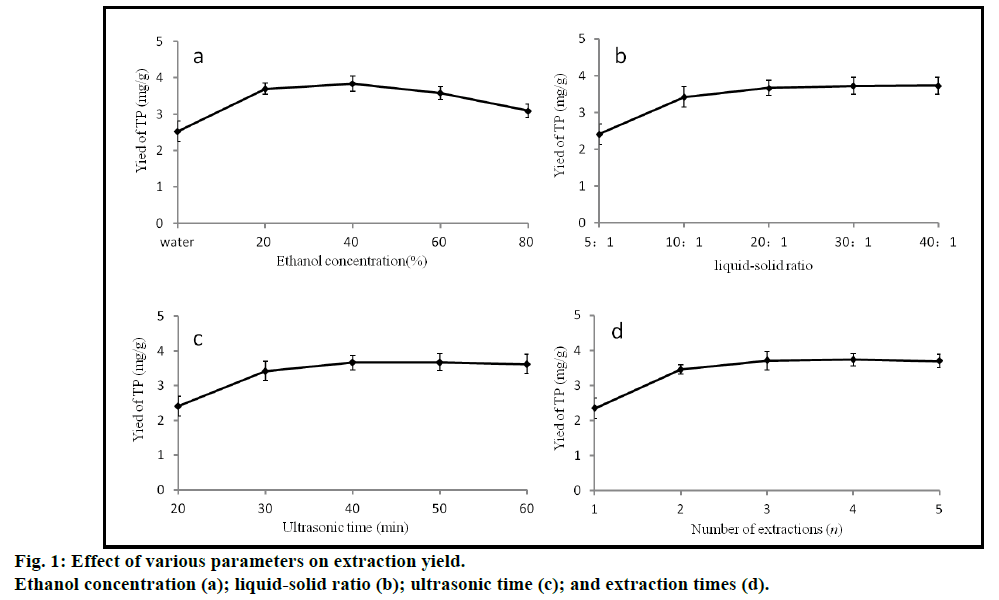
Fig. 1 illustrated the effect of ethanol concentration, liquid-solid ratio, ultrasonic time and extraction times on the yield of TPs. TPs were extracted with water and different concentrations of ethanol (20, 40, 60 and 80%). The optimal ethanol concentration for obtaining extracts was 40% in all the samples tested, therefore, ethanol concentrations between 20 and 60% were investigated in RSM. In fact, ethanol concentration was found to be the dominant factor in maximizing TP extraction. Solvent volume also had a significant effect on the extraction efficiency [18,19]. The ratio of liquid to solid was changed from 5:1 to 40:1 during the experiments and it was found that the extraction yield of TPs rose as the ratio of liquid to solid was increased, reaching a maximum (3.66 mg/g) when the ratio was 20:1. However, there were few changes when the ratio increased further. For this reason, ratios of 10:1, 20:1, 30:1 were all found to give high yields of TPs. Different ultrasonic treatment time (20, 30, 40, 50, 60 min) was also used in the experiment. The TP extraction yield increased gradually up to 40 min of treatment, but after 40 min, there was no difference in yield. These results were expected since an increase in ultrasonic time enhances the solubility of phenolic compounds, but longer ultrasonic times might also have increased the resolution ratio of the compounds resulting in a stable yield [20]. Therefore, in the optimization process, 30, 40, 50 min were chosen as the level of extraction time. The influence of multiple extraction rounds (1, 2, 3, 4 and 5) on the yield of TPs was also tested. The extraction yield of TPs increased gradually as the number of extraction rounds increased, with TPs yields of 3.71 mg/g after the third extraction, 3.73 mg/g after the fourth extraction and 3.69 mg/g after the fifth extraction. Therefore, three rounds of extraction were chosen as the best options as it took into account the costs and benefits. The number of extraction rounds was a discontinuous value and was not an examined factor in RSM, so was fixed at 3 rounds in the response surface experiments.
BBD was developed based on the results of the singlefactor experiments. The code and levels in the BBD and the responses were listed in Table 1. Among them, the highest yield of TPs was in the combination of 40% ethanol, liquid-solid ratio of 20:1 and ultrasonication time of 40 min. The ANOVA for the response surface of the quadratic model was listed in Table 2. The model F-value of 44.96 implied the model was significant (P<0.05). X1, X2, X1X3, X12 and X22 were significant model terms with low P-values (P<0.05). Other terms (X3, X1X2, X2X3 and X32) were of no significance since they had larger P-values (P>0.05). The results of the multiple regression analysis for response variables were as follows (the equation is in terms of coded factors, response Y is the yield of TPs): Y=4.136+0.150X1+0.189X2+0.019X3-0.045X1X2- 0.255X1X3-0.023X2X3-0.933X12-0.630X22+0.015X32
A lack of fit with P>0.05 (P=0.0804) was not statistically significant, which showed that the model fitted well with the experimental data. The R2 of 0.8138 is in reasonable agreement with the Adj R2 of 0.9658 i.e. the difference is less than 0.2. Therefore, this experimental model was adequate and reproducible for predicting the TPs yield. Contour plots and response surfaces were shown in fig. 2. TPs content increased with increased ethanol concentration and the ultrasonication time, and the interaction between the two factors was significant (P<0.05). However, interactions between the other factors were not significant, and this was in agreement with the ANOVA results. The RSM guided optimization demonstrated that the optimum extraction conditions for maximizing TPs yield were 44.3% (ethanol concentration), 21.6:1 (liquid-solid ratio) and 30 min (ultrasonication time). A verification test was performed on the basis of this optimal level, and the observed responses Y=4.16 (mg/g) agreed well with the predicted response (4.19 mg/g), and the relative standard deviation (RSD) of Y was 3.5%. The predicted values and test data of the optimized formulation showed a good correlation.
Free radicals are responsible for many diseases, including degenerative diseases, cancer, cardiovascular diseases and so on [21]. The assay of radical scavenging activity is a widely used method to evaluate the antioxidant activity of various plant extracts. Fig. 3 showed the radical scavenging activity of TPs from V. angularis on DPPH radicals, hydroxyl radicals and superoxide radicals. Antioxidant capacity of the TPs was determined by diminishing the absorbance of DPPH radicals. Within the experimental concentration range of 0.15~1 mg/ml, DPPH radical scavenging activity increased from 21.0±0.68% to 52.5±2.14%. It was evident from the data that TPs showed strong scavenging activity for DPPH radicals in a concentration-dependent manner.
Oxidative damage is usually caused by hydroxyl radicals [22]. Therefore, scavenging hydroxyl radicals is important to many diseases that are the result of oxidative stress injury. It was found that the TPs significantly decreased hydroxyl radical concentration in a dose-dependent manner at the test concentrations. Superoxide radicals cause oxidative damage to many biomolecules and are the most common free radicals produced in vivo by several oxidative enzymes [23]. The ability of the TPs to scavenge superoxide radicals was determined by the reduction of NBT. The scavenging effect was 12.5±2.51% at 0.15 mg/ml, 14.3±1.53% at 0.2 mg/ml, 21.7±1.53% at 0.4 mg/ml, 27.6±2.65% at 0.6 mg/ml, 36.5±1.53% at 0.8 mg/ml and 42.2±2% at 1 mg/ml. A significant concentration-dependent scavenging effect was shown against superoxide radicals. At a concentration of 1 mg/ml, radical scavenging activities were 52.5±2.14%, 44.9±3.06% and 42.2±2% for DPPH radicals, hydroxyl radicals and superoxide radicals, respectively. The strongest scavenging activity was shown for DPPH radicals, followed by hydroxyl radicals and superoxide radicals.
Considering the results, V. angularis extracts showed potent antioxidant activity. The antioxidant function of these extracts is associated with polyphenols. The radical scavenging properties of polyphenols are thought to be due to their hydroxyl groups that possess hydrogen donating ability [24]. Therefore, TPs of V. angularis can be considered as potential radical scavengers.
In this study, TPs have been successfully prepared from V. angularis using UAE, and the extraction conditions were optimized using a single-factor experiment approach coupled with BBD. The three factors investigated in this study could be ranked as follows in terms of influence on extraction performance: liquidsolid ratio>ethanol concentration>ultrasonic time. The results demonstrated that a combined treatment of 44.3% (ethanol concentration), 21.6:1 (liquidsolid ratio), 30 min (ultrasonic time) and three rounds of extraction were optimal for maximizing the TP yield. On the basis of these optimal conditions, the predicted response was 4.19 mg/g. The prediction models developed here showed good correlation with the experimental data at the 95% confidence level. Extraction is the first step for the production of polyphenols, and the results presented here are useful for the further utilization of V. angularis. This study has also provided a reference point for the extraction of polyphenols from other herbal medicines that are rich in phenolic compounds. TPs from in V. angularis showed strong antioxidant activity against DPPH radicals, hydroxyl radicals and superoxide radicals. V. angularis is a natural plant resource and could serve as a source of high-value antioxidants or nutraceuticals.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Vardanega R, Santos DT, Meireles MAA. Intensification of bioactive compounds extraction from medicinal plants using ultrasonic irradiation. PharmacognRev 2014;8:88-95.
- Yu T, Ahn HM, Shen T, Yoon K, Jang HJ, Lee YJ, et al. Antiinflammatory activity of ethanol extract derived from phaseolusangularis beans. J Ethnopharmacol 2011;137:1197-206.
- Baracho NC, Monteiro NF, Borges MG, Arguelho RR. Effect of aqueous extract of the Vigna angularis in rats subjected to an experimental model of moderate chronic kidney disease. Acta Cir Bras 2016;31:527-32.
- Mukai Y, Sato S. Polyphenol-containing azuki bean (Vigna angularis) seed coats attenuate vascular oxidative stress and inflammation in spontaneously hypertensive rats. J Nutr Biochem 2011;22:16-21.
- Oszmiański J, Nowicka P, Teleszko M, Wojdyło A, Cebulak T, Oklejewicz K. Analysis of phenolic compounds and antioxidant activity in wild blackberry fruits. Int J Mol Sci 2015;16:14540-53.
- Zhao Y, Hou Y, Tang G, Cai E, Liu S, Yang H, et al. Optimization of ultrasonic extraction of phenolic compounds from Epimedium brevicornum maxim using response surface methodology and evaluation of its antioxidant activities in vitro. J Anal Methods Chem 2014;2014:864654.
- Aravindan S, Delma CR, Thirugnanasambandan SS, Herman TS, Aravindan N. Antipancreatic cancer deliverables from sea: First-hand evidence on the efficacy, molecular targets and mode of action for multifarious polyphenols from five different brown-algae. PLoS ONE 2013;8:e61977.
- Ding Y, Yao H, Yao Y, Yenwong FL, Zhang Z. Protection of dietary polyphenols against oral cancer. Nutrients 2013;5:2173-91.
- Shakthi DA, Sathishkumar T, Kumaresan K, Rapheal VS. Extraction process optimization of polyphenols from Indian citrus sinensis – as novel antiglycative agents in the management of diabetes mellitus. J Diabetes Metab Disord 2014;13:11.
- Bhullar KS, Rupasinghe HPV. Polyphenols: multipotent therapeutic agents in neurodegenerative diseases. Oxid Med Cell Longev 2013;2013:891748.
- Maran JP, Priya B, Manikandan S. Modeling and optimization of supercritical fluid extraction of anthocyanin and phenolic compounds from Syzygiumcumini fruit pulp. J Food Sci Technol 2014;51:1938-46.
- Sousa JN, Pedroso NB, Borges LL, Oliveira GAR, Paula JR, Conceição EC. Optimization of ultrasound-assisted extraction of polyphenols, tannins and epigallocatechingallate from barks of Stryphnodendronadstringens (Mart.) Coville bark extracts. Pharmacogn Mag 2014;10:S318-23.
- Nguimbou RM, Boudjeko T, Njintang NY, Himeda M, Scher J, Mbofung CMF. Mucilage chemical profile and antioxidant properties of giant swamp taro tubers. J Food Sci Technol 2014;51:3559-67.
- Sánchez JC, García RF, Cors MTM. 1,1-diphenyl-2-picrylhydrazyl radical and superoxide anion scavenging activity of Rhizophora mangle (L.) bark. Pharmacogn Res 2010;2:279-84.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958;181:1199-1200.
- Winterbourn CC, Sutton HC. Hydroxyl radical production from hydrogen peroxide and enzymatically generated paraquat radicals: catalytic requirements and oxygen dependence. Arch Biochem Biophys 1984;235:116-26.
- Nishikimi M, Appaji Rao N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazinemethosulfate and molecular oxygen. Biochem Biophys Res Commun 1972;46:849-54.
- Dahmoune F, NayakB, Moussi K, Remini H, Madani K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. Leaves. Food Chem 2015;166:585-95.
- Lai J, Wang H, Wang D, Fang F, Wang F, Wu T. Ultrasonic extraction of antioxidants from Chinese sumac (Rhustyphina L.) fruit using response surface methodology and their characterization. Molecules 2014;19:9019-32.
- Liyana-Pathirana C, Shahidi F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem 2005;93:47-56.
- Fearon IM, Faux SP. Oxidative stress and cardiovascular disease: Novel tools give (free) radical insight. J Mol Cell Cardiol 2009;47:372-81.
- Athukorala Y, Kim KN, Jeon YJ. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem Toxicol 2006;44:1065-74.
- Kumar T, Jain V. Appraisal of total phenol, flavonoid contents, and antioxidant potential of folkloric Lannea coromandelica using in vitro and in vivo assays. Scientifica (Cairo) 2015;2015:203679.
- Iqbal J, Zaib S, Farooq U, Khan A, Bibi I, Suleman S. Antioxidant, antimicrobial, and free radical scavenging potential of aerial parts of Periploca aphylla and Ricinus communis. ISRN Pharmacology 2012;2012:563267.
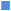 - DPPH radical, -□- hydroxyl radical, -
- DPPH radical, -□- hydroxyl radical, - - superoxide radical.
- superoxide radical.



