- Corresponding Author:
- V. Sankar
Department of Pharmaceutics, PSG College of Pharmacy, Coimbatore-641 004, India
E-mail: sansunv@yahoo.co.in
| Date of Submission | 18 January 2012 |
| Date of Revision | 22 December 2012 |
| Date of Acceptance | 25 December 2012 |
| Indian J Pharm Sci 2012, 74 (6): 556-563 |
Abstract
Stavudine oral disintegration tablets were formulated to minimize the bitter taste and to reduce the first-pass hepatic metabolism. The various precompression parameters like the angle of repose, bulk density, compressibility index and Hausner's ratio were determined for the powder blend. In this study, 14 formulations of stavudine oral disintegration tablet were prepared by direct compression method. The tablets were evaluated for weight variation, percentage friability, disintegration time, hardness, wetting time and water absorption ratio. The in vitro dissolution study results of the batch S1 (stavudine+crospovidone+sodium starch glycollate) are encouraging as highest dissolution rate (99.2% in 100 min) and lowest time of disintegration (56 s) was achieved. The in vivo drug release studies were carried out in rabbits and the relative bioavailability of formulation S1 was found to be 2.83 times greater than that of conventional tablets.
Keywords
Crospovidone and sodium starch glycollate, HIV, oral disintegration tablets, stavudine
For the past one decade, there has been an enhanced demand for more patient friendly and compliant dosage forms. As a result, the demand for developing new technologies has been increasing annually. Since the development cost of new drug molecules is very high, efforts are now being made by pharmaceutical companies to focus on the development of new drug dosage forms for existing drugs with improved safety and efficacy together with reduced dosing frequently, and the production of more cost effective dosage forms.
For most therapeutic agents used to produce systemic effects, the oral route is still preferred way of administration, owing to its several advantages and high patient compliance compared to many other routes. Tablets and hard gelatin capsules constitute a major portion of drug delivery systems that are currently available. However, many patient groups such as the elderly, children and patients who are mentally challenged, uncooperative, nauseated, or on reduced liquid intake or diets have difficulty in swallowing these dosage forms [1].
To fulfil these medical needs, pharmaceutical technologists have developed a novel oral dosage form known as orally disintegrating tablets (ODTs), which disintegrate rapidly in saliva, usually in seconds, without the need to take water. Drug dissolution and absorption as well as the onset of clinical effect and drug bioavailability may be significantly greater for ODTs when compared to conventional dosage forms.
Although chewable tablets have been on the market for some time, they are not the same as the new ODTs. Patients for whom chewing is difficult or painful can use these new tablets easily. ODTs can be used easily in children who have lost their primary teeth but do not have full use of their permanent teeth [2].
Recent market studies indicate that more than half of the patient population prefers ODTs to other dosage forms. The US Food and Drug Administration Center for Drug Evaluation and Research (CDER) defines in the ?Orange Book?, that an ODT as a ?solid dosage? form containing medicinal substances, which disintegrates rapidly, usually within a matter of seconds, when placed on the tongue. The significance of these dosage forms highlighted by adoption of term ?orodispersible tablet? by the European Pharmacopoeia which describe, it as a tablet that can be placed in oral cavity where it disperse rapidly before swallowing [3].
ODT products have been developed for numerous indicators ranging from migraines (for which rapid onset of action is important) to mental illness (for which patient compliance is important for treating chronic indications such as depression and schizophrenia). Different taste masking techniques such as polymer coating, complex formation, granulation, microencapsulation, ion exchange resins are used in pharmaceutical industries to overcome the bitter taste of drug. In this work, stavudine oral disintegration tablets were formulated to minimize the bitter taste and to reduce the first-pass hepatic metabolism [4-6]. The prepared tablets come under the category of fast dispersible, slow releasing tablets [7].
Materials and Methods
Stavudine was obtained as a gift sample from Strides Arcolab, Bangalore. Saccharin sodium, talc and polyvinyl pyrrolidine (PVP) were purchased from Loba Chem. Pvt. Ltd., Mumbai. Crospovidone was purchased from BASF Corporation, USA. Sodium starch glycollate and microcrystalline cellulose were purchased from Otto Kemi, Mumbai. Menthol and starch were purchased from Reachem Laboratory, Chennai. Eudragit RS 100 and Eudragit RL 100 were purchased from Ozone International, Mumbai.
Angle of repose (θ)
The angle of repose values of stavudine and the powder blends were determined by the funnel method (Reposogram). The accurately weighed powder blend was taken in a funnel. The height of the funnel was adjusted in such a way that the tip of the funnel just touches the apex of the heap of the powder. The powder was allowed to flow through the funnel freely onto the surface. The diameter of the powder cone was measured and angle of repose was calculated using the equation, q=tan-1 (h/r), where, h is the height of the powder cone and r is the radius of the powder cone.
Bulk density
Loose bulk density (LBD) and tapped bulk density (TBD) of stavudine and the powder blends were determined using bulk density apparatus (Electrolab, India). Stavudine was passed through 18# sieve to break the clumps, if any. Accurately weighed 5 g of the drug was placed in a 100 ml graduated measuring cylinder. Initial volume was observed. The cylinder was tapped initially 200 times from a distance of 14±2 mm. The tapped volume (Va) was measured to the nearest graduated unit. The tapping was repeated additional 200 times. Again, the tapped volume was measured to the nearest graduated unit. The same thing was done for powder blends [8]. The LBD and TBD were calculated in g/ml using the formulae, LBD=Weight of the powder/volume of the packing and TBD=Weight of the powder/tapped volume of the packing.
Compressibility index (Carr?s index)
The compressibility index of the powder blends was determined by Carr?s compressibility index. Carr?s index (%) can be calculated by using the formula, Carr'sindex (%)=((TBD-LBD)/TBD)×100.
Hausner ratio
Hausner ratio is an indirect method to determine the powder flow property. It is a very important parameter to be measured since it determine the mass of uniformity of the dose. Hausner ratio =TBD/LBD, where TBD is the tapped bulk density and LBD is the loose bulk density.
Formulation of tablets
Stavudine tablets (30 mg) were prepared using different ratios of superdisintegrants by direct compression. The superdisintegrants such as sodium starch glycolate and crospovidone were used in different proportions. All the ingredients were passed through 40# sieve and were subjected for drying to remove the moisture content at 40-45°. Weighed amount of drug and excipients except talc were mixed in a polybag for 20 min manually. The mixed blend of drug and the excipients was compressed on Rimek 10 station rotary punching machine using 8 mm diameter flat faced punches [9]. Ingredients for the stavudine tablet formulations S1-S14 are given in Table 1.
| Ingredients | Formula for one tablet (mg) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | |
| Stavudine | 30 | 20 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Crospovidone | 30 | 15 | 30 | - | 30 | - | - | 30 | 30 | 30 | - | 30 | 30 | 30 |
| Saccharine Sodium | 30 | 15 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Sodium Starch Glycollate | 30 | 15 | - | 30 | - | 30 | 30 | - | 30 | 30 | 30 | - | 30 | 30 |
| Menthol | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Talc | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Micro Crystalline Cellulose | 20 | 15 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Eudragit RS 100 | - | - | - | - | - | - | - | - | - | - | 30 | - | 30 | - |
| Eudragit RL 100 | - | - | - | - | - | - | - | - | - | - | - | 30 | - | 30 |
| Starch | - | - | - | - | 10 | 10 | - | - | 10 | - | - | - | - | - |
| PVP# | - | - | - | - | - | - | 10 | 10 | - | 10 | - | - | - | - |
| #PVP=Polyvinyl pyrrolidine | ||||||||||||||
#PVP=Polyvinyl pyrrolidine
Table 1: Composition of Stavudine Tablets
Weight variation test
Twenty tablets were weighed individually and all together. Average weight was calculated from the total weight of all tablets. The individual weights were compared with the average weight. The percentage difference in the weight variation should be within the permissible limits (±7.5%) [10]. The percentage deviation was calculated using the formula, Percentage deviation=((Individual weight-Average weight)/ Average weight)×100.
Dimensions
The thickness of tablets was measured using Vernier calipers. Six tablets from each batch were selected and evaluated. The extent to which the thickness of each tablet deviated from ±5% of the standard value was determined.
Friability testing
Friability is the loss of weight of tablet in the container/package, due to removal of fine particles from the surface. This in process quality control test is performed to ensure the ability of tablets to withstand the shocks during processing, handling, transportation and shipment. Roche friabilator (Electrolab, Mumbai) was used to measure the friability of the tablets. Ten tablets were weighed collectively and placed in the chamber of the friabilator. In the friabilator, the tablets were exposed to rolling, resulting free fall of tablets (6 inches within the chamber of the friabilator). It was rotated at a rate of 25 rpm. After 100 rotations (4 min), the tablets were taken out from the friabilator and intact tablets were again weighed collectively [11]. The percent friability was determined using the formula, %Friability =((W1-W2)/W1)×100, where, W1 is the weight of the tablet before test and W2 is the weight of the tablets after test.
Hardness test
Hardness (diametric crushing strength) is a force required to break a tablet across the diameter. The hardness of a tablet is an indication of its strength. The tablet should be stable to mechanical stress during handling and transportation. The degree of hardness varies with the different manufactures and with the different types of tablets. The hardness was tested using Pfizer hardness tester. Ten tablets from each batch were tested and the average of ten values was found. The force was measured in kilograms per centimetre square [12].
Disintegration time
The disintegration time of the tablet was measured by using disintegration apparatus. Water is used as buffer and the temperature maintained is 37° [13].
In vitro dissolution studies
In vitro drug release of the samples was carried out using USP ? type II dissolution apparatus (paddle type). The dissolution medium, 900 ml of phosphate buffer (pH 7.4) solution, was placed into the dissolution flask maintaining the temperature of 37±0.5° at 50 rpm. One stavudine tablet was placed in each flask of dissolution apparatus. The apparatus was allowed to run for 120 min. Sample measuring 5 ml were withdrawn after every 5, 10, 20, 40, 60, 90 and 120 min. The fresh dissolution medium was replaced every time with the same quantity of the sample [14]. The collected samples were analysed at 266 nm using dissolution medium as blank. The cumulative percentage drug release was calculated.
Wetting time and water absorption ratio
Wetting time of dosage form is related with the contact angle. Wetting time of the mouth dissolving tablets is another important parameter, which needs to be assessed to give an insight into the disintegration properties of the tablets; a lower wetting time implies a quicker disintegration of the tablet. The wetting time of the tablet can be measured using a simple procedure.
Two circular tissue papers of 10 cm diameter are placed in a petridish having the same inner diameter. 10 ml of phosphate buffer solution, 6.8 pH containing water is added to petridish. A tablet is carefully placed on the surface of the tissue paper so that complete tablet was not immersed in the solution. Then, the time required for buffer to reach an upper surface of the tablet is noted as wetting time [15]. Wetting time test was performed on three tablets of each batch, the average was taken as wetting time.
Water absorption ratio (R) is calculated using the formula, R=((Wa-Wb)/Wb)×100, where, Wa is the weight of tablet after absorption and Wb is the weight of tablet before absorption [16].
In vivo drug release kinetics
Two groups of rabbit were taken for the study. Each group consists of three animals. To the first group 2.1 mg of conventional stavudine tablets and to the second group stavudine oral disintegration tablet were given orally. Blood was collected from the marginal ear vein of the rabbit after 30, 60, 90 and 120 min. Before collecting blood 50 μl of 15% sodium citrate was added to the eppendorf tubes as an anticoagulant. Then it is centrifuged at 2000 rpm for 10 min to separate the plasma [17]. The drug concentration in the plasma was determined by high performance liquid chromatography (HPLC) at 266 nm. The relative bioavailability was determined using the formula: F=(AUCtest×Dstd)/(AUCstd×Dtest), where, AUC is the area under the curve of plasma profile and D is the dose given.
Chromatographic technique
Plasma concentrations of stavudine were determined following the HPLC procedure as described previously [18]. The mobile phase consisted of methanol:distilled water:acetic acid in 23:77:0.2 (v/v) ratio and was delivered to the system at a flow rate of 1 ml/min. The column used for the study was a reverse phase column (Waters, Sunfire C18, 5μ, 4.6×250 mm). The detector was a 2489 UV/Vis detector and the detection wavelength was 266 nm. The calibration curve showed excellent linearity over the concentration range of 5-30 μg/ml. The correlation coefficient (r) and the determination coefficient (r2) of the curve were 0.999 and 0.997, respectively. All intra- and inter-day coefficients of variation (CV) were less than 10%.
Results and Discussion
The frictional force in powder blends can be measured by angle of repose. The angle repose for the powder blend was found to be in the range 22-34°. Formulations S1, S7 and S11 which showed angle of repose values ≤25° were found to have excellent flow. Formulations S3, S4, S5, S6, S8, S9, S10 and S12 which showed angle of repose values 25-30° were found to have good flow. Formulations S2, S13 and S14 which showed angle of repose values 30-40° were found to have passable flow (Table 2).
| Formulation | Angle of repose | Bulk density (g/ml) | Tapped density (g/ml) | Compressibility index | Hausner ratio |
|---|---|---|---|---|---|
| S1 | 22°37”±67” | 0.54±0.004 | 0.63±0.009 | 14.28±0.533 | 1.232±0.007 |
| S2 | 31°24”±46” | 0.58±0.003 | 0.71±0.006 | 18.1±0.399 | 1.140±0.005 |
| S3 | 30°28”±87” | 0.54±0.003 | 0.66±0.004 | 14.7±0.245 | 1.102±0.003 |
| S4 | 28°42”±58” | 0.58±0.002 | 0.67±0.003 | 16.1±0.116 | 1.095±0.002 |
| S5 | 28°18”±76” | 0.55±0.003 | 0.69±0.002 | 17.1±0.173 | 1.150±0.002 |
| S6 | 27°19”±43” | 0.53±0.005 | 0.65±0.007 | 17.1±0.324 | 1.099±0.005 |
| S7 | 24°16”±65” | 0.59±0.003 | 0.73±0.004 | 16.9±0.236 | 1.193±0.003 |
| S8 | 26°14”±86” | 0.57±0.004 | 0.67±0.003 | 16.1±0.369 | 1.173±0.006 |
| S9 | 26°00”±35” | 0.58±0.004 | 0.68±0.004 | 14.2±0.202 | 1.246±0.003 |
| S10 | 29°21”±55” | 0.52±0.006 | 0.64±0.006 | 15.4±0.465 | 1.184±0.009 |
| S11 | 24°24”±28” | 0.57±0.003 | 0.66±0.009 | 15.8±0.256 | 1.165±0.007 |
| S12 | 26°21”±93” | 0.53±0.002 | 0.65±0.003 | 16.9±0.585 | 1.178±0.002 |
| S13 | 34°20”±33” | 0.59±0.005 | 0.68±0.005 | 17.2±0.287 | 1.221±0.008 |
| S14 | 31°21”±76” | 0.52±0.007 | 0.61±0.006 | 19.1±0.135 | 1.197±0.007 |
Average value of three determinations with SD
Table 2: Physical Properties of Different Powder Blends
Interparticulate interactions influence the bulking properties of powder. A comparison of the bulk density and tapped density can give a measure of the relative importance of this interaction in a given powder; such a comparison is often used as an index of the ability of the powder to flow. The bulk density of the powder formulation was in the range of 0.52±0.009 to 0.59±0.005 g/ml; the tapped density was in the range of 0.613±0.011 to 0.73±0.06 g/ml, which indicates that the powder was not bulky. The Carr?s index was found to be in the range of 14.2±0.94 to 19.1±1.71, Hausner ratio was found to be in the range of 1.099±0.02 to 1.246±0.011, indicating good compressibility of the powder blend (Table 2). These values indicate that the prepared powder will show good uniformity of weight of tablet when punched.
A total of 14 formulations of oral disintegrating tablets of stavudine were prepared by direct compression method using superdisintegrants such as sodium starch glycolate and crospovidone in different ratios. During preparation, the lubricating agent and sweetening agent were kept constant to avoid any possible influence by these ingredients. Saccharin sodium which is 450 times sweeter than sucrose was included in all the formulations within the permissible limit (0.5 mg/kg) to mask the bitter taste which may be helpful for increasing the patient compliance.
Weight variation test was performed as per Indian Pharmacopoea 2007 (IP); the test ensured that the fill in the die cavity was uniform for all the batches. Any variation in the weight of tablet (for any reason) leads to either under medication or over medication. So, every tablet in each batch should have a uniform weight. Deviation within the IP permissible limit of 7.5% is allowed. Corrections were made during the compression of tablets to get uniform weight. The percent deviation calculated was less than 7.5% of the average weight of the tablet. Hence, all batches comply with the test for weight variation as per IP (Table 3). The average thickness of tablets which were measured using Vernier callipers was recorded as 0.3±0.05. The friability of all the batches except S6, S9 and S12 formulations was found to be less than 1% ranging from 0.442 to 0.937%, thereby all the batches were found to pass the test for friability of tablets as per IP (Table 3). The hardness of all batches was found to be in range of 3.1-4.3 kg/ cm2, and it was kept constant in this range during compression (Table 3).
| Formulation | Weight variation test | Friability test | Hardness test | Disintegration test | Wetting time | Water absorption ratio |
|---|---|---|---|---|---|---|
| (mg) | (%) | (kg/cm2) | (s) | (min) | (%) | |
| S1 | 147.2±3.6 | 0.442±0.05 | 2.8±0.6 | 56±2 | 0.52±0.7 | 14.98±0.86 |
| S2 | 93.1±2.2 | 0.879±0.17 | 3.1±0.5 | 98±2 | 1.3±0.3 | 15.34±0.34 |
| S3 | 119.6±5.7 | 0.782±0.9 | 2.6±0.4 | 119±4 | 2.2±0.6 | 14.32±0.74 |
| S4 | 121.3±3.4 | 0.829±0.10 | 3.1±0.7 | 84±3 | 1.4±0.4 | 14.73±0.54 |
| S5 | 127.5±6.2 | 0.556±0.01 | 3.2±0.9 | 121±3 | 1.6±0.1 | 15.43±0.33 |
| S6 | 132.1±8.6 | 1.447±0.13 | 3.4±0.1 | 105±4 | 2.1±0.7 | 13.87±0.63 |
| S7 | 128.9±2.3 | 0.623±0.2 | 3.4±0.1 | 92±2 | 1.08±0.6 | 16.02±0.46 |
| S8 | 129.0±4.6 | 0.523±0.7 | 3.5±0.8 | 87±4 | 1.4±0.6 | 15.21±0.57 |
| S9 | 157.2±3.4 | 2.059±0.12 | 3.1±0.7 | 78±2 | 2.6±0.54 | 15.92±0.43 |
| S10 | 161.3±1.8 | 0.482±0.7 | 2.8±0.9 | 112±4 | 1.03±0.7 | 14.27±0.57 |
| S11 | 148.7±4.6 | 0.937±0.4 | 3.2±0.1 | 103±3 | 2.0±0.6 | 14.82±0.74 |
| S12 | 152.1±3.5 | 1.212±0.19 | 3.5±0.8 | 98±4 | 2.4±0.6 | 13.93±0.23 |
| S13 | 177.6±4.8 | 0.542±0.13 | 3.4±0.4 | 89±3 | 1.8±0.5 | 15.42±0.67 |
| S14 | 179.2±2.4 | 0.482±0.15 | 3.9±0.5 | 115±3 | 2.2±0.4 | 14.76±0.34 |
Average value of three determinations with SD
Table 3: Quality Control Tests for Different Stavudine Formulations
The disintegration test was performed as per IP. The disintegration time range for all batches was found to be 56±2 to 121±3 s. Among the various batches, formulation made with the combination of crospovidone and sodium starch glycollate superdisintegrants (S1) has the least disintegration time of 56±2 s. Whereas, formulations S3 and S4 which lacks in sodium starch glycollate and crospovidone, respectively, showed increased disintegration times, 119±4 and 84±3 s, respectively. Formulations S9, S5 and S6 showed an increased disintegration time due to the presence of starch in the formulations and also due to the absence of sodium starch glycollate and crospovidone in formulations S5 and S6, respectively. Even though formulation S8 lacked in sodium starch glycollate the disintegration time is found to be lower than the disintegration time for formulation S3 because of the presence of PVP which enhanced the disintegration time. Eudragit RS 100 and Eudragit RL 100 increased the disintegration time of the formulations S11, S12, S13 and S14 and this is because Eudragit is less permeable to water and hence an increased disintegration time. Formulation S5 has the highest disintegration time of 121±3 s because of the lack of sodium starch glycollate and presence of starch. The disintegration times of various formulations have been mentioned in Table 3.
Wetting time test was performed to find out the time taken for the water to wet the whole tablet and the wetting time range for all batches was found to be in the range of 52±3 to 126±5 s. Formulations S1, S9, S10, S13 and S14 has similar concentrations of the drug, superdisintegrants, flavouring agent and talc but vary in the presence or absence of starch, PVP, Eudragit RS 100 and Eudragit LS 100. These formulations shows a wetting time of 0.52±0.7, 2.6±0.54, 1.03±0.7, 1.8±0.5 and 2.2±0.4 s and these values indicate the increased wetting time due to the presence of starch (S9), PVP (S10), Eudragit RS 100 (S13) and Eudragit LS 100 (S14), respectively. When compared to formulations S1 and S3, absence of superdisintegrant, sodium starch glycollate in formulation S3 lead to an increase in wetting time from 0.52±0.7 to 2.2±0.6 s. Water absorption ratio was determined and its range for all batches was found to be 13.87±1.29 to 16.02±1.31% (Table 3).
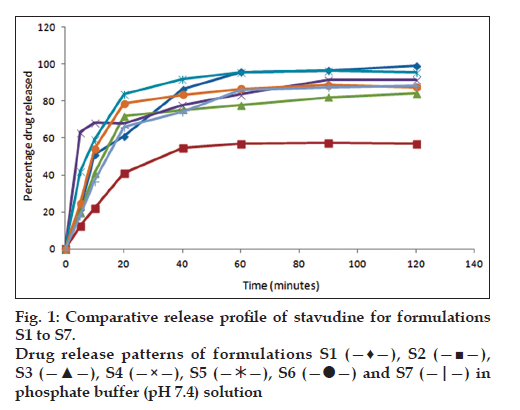
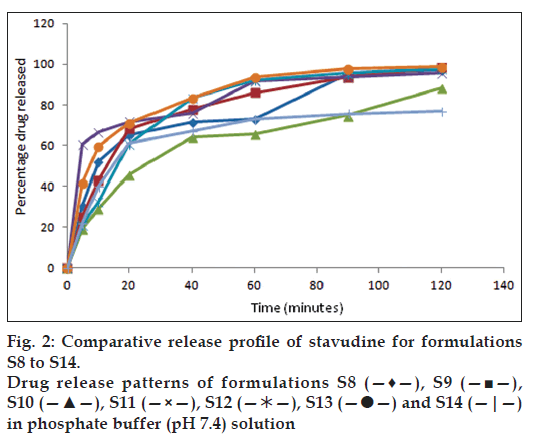
All the formulations were subjected to in vitro dissolution studies and the percentage of drug release was calculated and figs. 1 and 2 represent the in vitro release profiles for formulation S1 to S7 and S8 to S14, respectively. Among the 14 formulations high percentage of drug release in S1 formulation was found to be 99.2% in 100 min and disintegration time was found to be low (56 s). The percentage of drug release was found to be less for S2 formulation (57.6%) in 100 min and the disintegration time was found to be high (98 s) when compared with S1 formulation as the quantities of the superdisintegrants used were reduced by half. The formulation S3 containing single superdisintegrant crospovidone showed high disintegration time (119 s) in 100 min and low percentage of drug release (84.3%) when compared to the formulation S4 containing sodium starch glycollate alone as a superdisintegrant. In the presence of dry binder starch or PVP the formulations containing crospovidone (S5 and S8) showed comparatively high percentage of drug release than the formulations containing sodium starch glycollate (S6 and S7). In the presence of both the superdisintegrants crospovidone and sodium starch glycollate the formulation containing starch as binder (S9) showed comparatively high percentage of drug release (98.4%) in 100 min and low disintegration time (78 s) than the formulation containing PVP as binder. The polymers Eudragit RS 100 and Eudragit RL 100 were used in the formulation S11 to S14 for sustaining the drug release. In the formulations containing Eudragit RS 100, with one single superdisintegrant S11 showed low percentage of drug release (95.7%) and high disintegration time (103 s) when compared with the formulation containing two superdisintegrants S13. When Eudragit RL 100 was used, the formulation with single superdisintegrant S12 showed comparatively high percentage of drug release (97.8%) and low disintegration time (89 s) than the formulation containing two superdisintegrants S14. The results from the formulation S11 to S14 are not encouraging when compared to the formulation S1. Regression value of the formulation S1 for first order kinetics (0.972) is higher than zero order kinetics (0.696). This confirms that drug release kinetics follow first order kinetics. High percentage of drug release and lowest time of disintegration was achieved in S1 formulation due to the presence of higher quantities of superdisintegrants. Therefore, this best formulation was selected for animal studies.
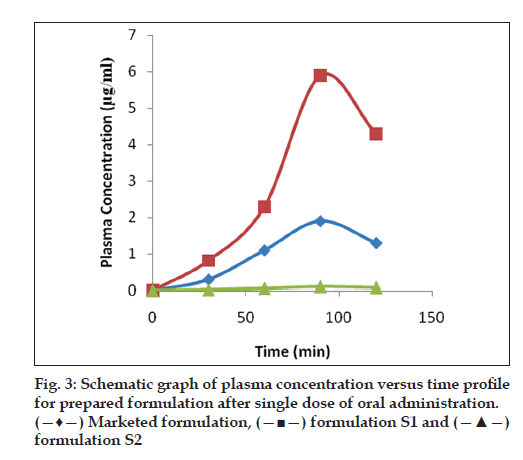
The Cmax for marketed formulation and formulation S1 was found to be 1.9±0.011 and 5.9±0.081 s, respectively. The Tmax for both marketed and formulation S1 was found to be 90 min. The AUC for the marketed formulation and formulation S1 was found to be 114 and 322.8, respectively. Hence, the relative bioavailability in rabbits for formulation S1 (stavudine+crospovidone+sodium starch glycollate) was found to be 2.83 times greater than that of conventional tablets (fig. 3). This confirms that the hepatic metabolism was reduced when the drug is given in the form of oral disintegration tablets.
The ODT stavudine formulations were prepared by direct compression technique using crospovidone and sodium starch glycollate as superdisintegrants in different ratios. The wetting time and disintegration time was found to be less in the formulation S1. Profound in vitro release in 120 min (99.3±0.46%) was found in S1 formulation (stavudine+crospovidone+sodium starch glycollate) due to the inclusion of combination of superdisintegrants in the concentration of 20%. The result outcome confirms inclusion of starch at 7% concentration increases the disintegration time as seen in formulation S9 because of the binding nature of starch with the drug. Formulation S14 which has Eudragit RL 100 at 20% concentration did not show encouraging in vitro results inspite of the presence of both the superdisintegrants, sodium starch glycollate and crosspovidone. Hence, addition of Eudragit RL 100 is not advisable for the formulation of oral disintegration tablets. The relative bioavailability for the optimized formulation S1 was found to be 2.83 times greater than the marketed capsule formulation, which confirms the decreased hepatic metabolism the of the drug. However, the formulation S2 (stavudine+crospovidone+sodium starch glycollate) which has 10% of superdisintegrants did not give encouraging bioavailability results. Saccharin sodium included in the formulation will help to overcome the bitter taste of stavudine; however, the prepared tablets should be evaluated for taste by panel testing in healthy human volunteers. From this study, we perceive the possibility of commercializing ODT formulation of stavudine as it has much better release profile and enhanced bioavailabilty when compared to the marketed capsule formulations. Moreover, this approach may increase drug bioavailability and patients compliance which is an important prerequisite for HIV management.
References
- Mallet L. Caring for the elderly patient. J Am Pharm Assoc (Wash) 1996;NS36:628.
- Krause J, Breitkreutz J. Improving drug delivery in paediatric medicine. Pharm Med 2008;22:41-50.
- Fu Y, Yang S, Jeong SH, Kimura S, Park K. Orally fast disintegrating tablets: Developments, technologies, taste-masking and clinical studies. Crit Rev Ther Drug Carrier Syst 2004;21:433-76.
- Shoukri RA, Ahmed IS, Shamma RN. In vitro and in vivo evaluation of nimesulide lyophilized orally disintegrating tablets. Eur J Pharm Biopharm 2009;73:162-71.
- Clarke A, Brewer F, Johnson ES, Mallard N, Hartig F, Taylor S, et al. A new formulation of selegiline: Improved bioavailability andselectivity for MAO-B inhibition. J Neural Transm 2003;110:1241-55.
- Schiermeier S, Schmidt PC. Fast dispersible ibuprofen tablets. Eur J Pharm Sci 2002;15:295-305.
- Fini A, Bergamante V, Ceschel GC, Ronchi C, de Moraes CA. Fast dispersible/slow releasing ibuprofen tablets. Eur J Pharm Biopharm 2008;69:335-41.
- Kawtikwar PS, Zade PS, Sakarkar DM. Formulation, evaluation and optimization of fast dissolving tablet containing tizanidine hydrochloride. Int J Pharm Tech Res 2009;1:34-42.
- Jain CP, Naruka PS. Formulation and evaluation of fast dissolving tablets of valsartan. Int J Pharm Pharm Sci 2009;1:219-26.
- Mahapatra AK, Murthy PN, Sahoo J. Formulation design and optimization of mouth dissolving tablets of levocetirizine hydrochloride using sublimation technique. Indian J Pharm Educ Res 2009;43:39-45.
- Modasiya MK, Patel VM, Shah DA. Design and characterization of fast disintegrating tablets of piroxicam. Int J Pharm Tech Res 2009;1:353-67.
- Mulla JA, Dasankoppa FS, Vilas GJ, Sholapur HP. Fast dissolving tablets of promethazine: A novel oral formulation for the treatment of fractionated radiotherapy induced nausea and emesis. Indian Drugs 2008;45:314-27.
- Mundada AS, Meshram DR, Banbale HB, Bhalekar MR, Avari JG. Formulation and evaluation of dispersible taste masked tablet of roxithromycin. Asian J Pharm 2008;2:116-9.
- Patel B, Patel D, Patel C. Development and in vitro evaluation of fast dissolving tablets of glipizide. Int J Pharm Pharm Sci 2009;1:145-50.
- Radke RS, Jadhav JK, Chajeed MR. Formulation and evaluation of orodispersible tablets of baclofen. Int J Chem Tech Res 2009;1:517-21.
- Rangasamy M, Ayyasamy B, Raju S, Gummadevelly S. Design and evaluation of the fast dissolving tablet of terbutaline sulfate. Asian J Pharm 2009;3:215-7.
- Shirsand SB, Suresh S, Swamy PV, Kumar DN, Rampure MV. Design and evaluation of fast dissolving tablets of clonazepam. Indian J Pharm Sci 2008;70:791-5.
- Contreras J, González HM, Menéndez R, López M. Development and validation of a reversed-phase liquid chromatographic method for analysis of D4T (stavudine) in rat plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2004;801:199-203.