- *Corresponding Author:
- So-Young Park
Laboratory of Pharmacognosy, College of Pharmacy, Dankook University, Cheonan 31116, Korea
E-mail: soypark23@dankook.ac.kr
| Date of Received | 09 May 2022 |
| Date of Revision | 17 March 2023 |
| Date of Acceptance | 19 June 2023 |
| Indian J Pharm Sci 2023;85(3):732-739 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Osteoporosis is one of the most common bone diseases in the elderly. Osteoporosis related bone fracture could seriously lower the quality of the elderly’s life. Thus, in this study, natural products which can treat or prevent osteoporosis without severe side effects have been searched. The ethanol extract of Paeonia lactiflora Pall (Paeoniceae) significantly increased the differentiation of MC3T3-E1 preosteoblastic cells determined by alkaline phosphatase assay. The bioassay-guided isolation of ethyl acetate fraction allowed to isolated 6 paeoniflorin derivatives and 2 catechins such as 4-O-methyl-moudanpioside C (1), 4-O-methylbenzoyloxypaeoniflorin (2), oxypaeoniflorin (3), (+)-catechin (4), catechuic acid (5), paeoniflorin (6), benzoylpaeoniflorin (7), albiflorin (8). Among them, 4-O-methylmoudanpioside C (1), 4-O-methylbenzoyloxypaeoniflorin (2), oxypaeoniflorin (3), benzoylpaeoniflorin (7) and albiflorin (8) significantly increased the ALP activity. Particularly, 4-O-methylmoudanpioside C (1), 4-O-methylbenzoyloxypaeoniflorin (2) were the most active. Furthermore, 4-O-methylbenzoyloxypaeoniflorin (2) significantly inhibited the osteoclastic differentiation of Raw264.7 cells. Taken together, the extract of Paeonia lactiflora and its active constituents, particularly, 4-O-methylbenzoyloxypaeoniflorin (2) could have a benefical effect on bone formation and promote bone health.
Keywords
Paeonia lactiflora, paeoniflorin derivatives, osteoblastic differentiation, osteoclastic diffferentiation, 4-O-methylbenzoyloxypaeoniflorin
Osteoporosis is one of the most common bone diseases in the elderly[1]. In early stage, patients with osteoporosis have few symptoms, but as the disease progresses, patients start to have symptoms such as back pain, loss of weight over time and bone fracture. Bone fracture could seriously lower the quality of the elderly’s life[2].
The common medications for osteoporosis include vitamin D, bisphosphonates and selective estrogen receptor modulators. However, these drugs are known to cause serious side effects. For example, bisphosphonates can cause gastrointestinal trouble, microfracture and jaw necrosis. On the other hand, selective estrogen receptor modulators are reported to cause venous thromboembolism, myocardial infarction and stroke[3]. Therefore, natural substances which can prevent or treat osteoporosis with minimum side effects could be beneficial.
Alkaline Phosphatase (ALP), an enzyme anchored in the membrane of osteoblast, plays an important role in the mineralization of bone and the expression of ALP is maximized during the osteoblast differentiation[4]. Thus, the analysis of ALP activity is frequently used as a specific marker of differentiation of osteoblasts in vitro.
Paeonia lactiflora Pall (Paeoniaceae) (P. lactiflora) has been used as a traditional medicine to promote the blood circulation and regulate menstruation in addition to anti-inflammatory agents and analgesics. In addition, P. lactiflora has been reported to contain various chemical constituents including monoterpenes and monoterpene glycosides. For example, paeoniflorin, albiflorin and paeoniflorin derivatives including paeonin A, mudanpioside A, benzoylpaeoniflorin, galloylpaeoniflorin, galloyloxypaeoniflorin and 6-O-benzoylalbiflorin were reported to be isolated from P. lactiflora[5,6]. A previous study reported that 3 chemical constituents including paeoniflorin, albiflorin, and 6'-O-ß- D-glucopyranosylalbiflorin from P. lactiflora stimulated the differentiation of osteoblast[7]. In that report, 6'-O-β-D-glucopyranosylalbiflorin was the most active, but paeoniflorin and albiflorin were less effective. This suggest that P. lactiflora might include other bioactive paeoniflorin derivatives that stimulate the differentiation of osteoblast.
In this study, the ethanol extract of P. lactiflora roots and its ethyl acetate fraction were applied to ALP assay in order to determine the effects on osteoblast differentiation using pre-osteoblastic mouse preosteoblast MC3T3-E1 cells. Then, the active constituents were isolated based on the bioassay-guided isolation method using diverse column chromatographies. The structures of isolated compounds were elucidated by the comparison of Nuclear Magnetic Resonance Spectroscopy (NMR) and Mass Spectrometry (MS) data with references. Finally, the effects of these compounds on osteoblast and oteoclast differentiation were evaluated.
Materials and Methods
General experimental procedures:
Minimum Essential Medium Alpha Modification (α-MEM) and trypsin Ethylenediaminetetraacetic acid were purchased from Welgene (Gyeongsan, Korea). Fetal Bovine Serum was purchased from Gibco (Carlsbad, CA, USA). ALP assay kit was purchased from Takara (Tokyo, Japan). ALP yellow liquid substrate reagents (p-nitrophenylphosphate (pNPP)) was purchased from Sigma Aldrich (Missouri, CA, USA). 3-(4,5-Dimethylthiazol- 2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) and Dimethyl Sulfoxide (DMSO) were purchased from Biosesang (Seongnam, Korea). Phosphate Buffered Saline (PBS) was purchased from Hyclone (Pittsburgh, PA, USA). Thin Layer Chromatography (TLC) plate being used were TLC silica gel 60 F254 (Merck, Frankfurt, Germany), and TLC silica gel 60 F RP-18 F 254s (Merck, Frankfurt, Germany). Stationary phases used for open column chromatography was Silica gel Si 60 (70- 230 mesh, Watchers, Toyko, Japan) and Sephadex LH–20(GE Healthcare, Danderyd, Sweden). Highperformance liquid chromatography (HPLC) analysis was performed by Capcellpak UG 80 columns (Shiseido, Tokyo, Japan). Concentration of solvent extract was performed using a rotary evaporator from N-1200A (EYELA, Tokyo, Japan) and miVac Duc-22060-B00 (Genevac, Ipswich, UK). Compound anlysis was performed using Alliance HPLC system Waters 2695 separation module, Waters 996 PDA (Waters, Milford, MA, USA). The microplate reader was E-max Precision microplate reader (Molecular Devices, San Jose, CA, USA).
Plant material, Extraction and isolation:
The roots of P. lactiflora were purchased from a commercial market (Samhong herb market, Seoul, Korea) in 2017. A voucher specimen has been deposited in Pharmacognosy Laboratory of College of Pharmacy, Dankook University, Korea (PL-2017-10). The pulverized roots of P. lactiflora root (3 kg) were extracted with 80 % ethanol (3×30 l, 24 h each time). After filteration, the ethanol extract was concentrated under vacuum to yield 814 g. Then, the ethanolic extract was suspended in minimum amount of distilled water and partitioned based on solvent-polarity using n-hexane, dichloromethane (CH2Cl2), ethyl acetate and water. All fractions were concentrated under vacuum and 4 fractions including n-hexane (2.5 g), dichloromethane (13.2 g), ethyl acetate (90.2 g), water (622 g) fractions were obtained. The ethyl acetate fraction was fractionated using silica gel column chromatography (C.C) with two solvent mixtures (chloroform:MeOH=10:1.5) and 13 fractions (PA 1~PA 13) were obtained. Then, the active fractions were applied to repeted C.C using Sephadex LH-20 and silica gel as stationary phases. As the results, 8 compounds were isolated and their stuructures were elucidated based on NMR and MS data by comparison with previous reports. The isolated compounds were as follows. 4-O-methyl-moudanpioside C (1, 8.1 mg), 4-O-methylbenzoyloxypaeoniflorin (2, 10.2 mg), oxypaeoniflorin (3, 12.1 mg), (+)-catechin (4, 10.2 mg), catechuic acid (5, 12.1 mg), paeoniflorin (6, 10.3 mg), benzoylpaeoniflorin (7, 12.1 mg), albiflorin (8, 10.2 mg).
Cell culture and cell cytotoxicity test:
MC3T3-E1 cells were grown in α-MEM with 10 % FBS (NM). The cells were incubated in the humidified atmosphere with 5 % CO2 at 37°. The cell cytotoxicity was determined using MTT assay. Briefly, MC3T3-E1 cells were plates in a 96 well plate with the density of 5.0×103 cells/well. After 24 h, the medium (NM) was changed to Osteoblast Differentiation Medium (ODM) consisting of α-MEM with 10 % FBS, 10 μM β-glycerophosphate and 50 μg/ml ascorbic acid, and test samples were added to each well. After 2 d, the media in the well (ODM) were changed to fresh ODM. After additional 2 d, the cells were incubated with 10 μl of 5 mg/ml MTT solution in PBS for 3 h at 37°. After removing the medium, 100 μl DMSO was added to each well to dissolve the MTT formazan crystals. After 1 h, the absorbance was measured at 540 nm using E-max Precision microplate reader (Molecular Devices).
ALP activity:
The cells were seeded in a 12 well plate with the density of 5.0×104 cells/well. After 24 h, the cells were treated with test samples in ODM. 2 d later, media in the well were changed to fresh ODM. After 2 d, the cells were lysed with 10 % NP-40 and centrifuged at 13 200 rpm for 10 min. Then, the supernatant was transferred into a 96 well plate and treated with pNPP reagent. After 1 h, the absorbance was measured at 405 nm using E-max Precision microplate reader.
Osteoclastic differentiation of RAW264-7 cells:
Cells were cultured in DMEM supplemented with antibiotics and 10 % FBS, and maintained at 37° in 5 % CO2 humidified air. Cells were seeded into 24- well plates with 2×102 cells per well. Media was changed with α-MEM with antibiotics and 10 % FBS containing 100 ng/ml Receptor Activator of Nuclear factor-κB Ligand (RANKL) (ODM) and 20 μg/ml test compounds which already showed the beneficial effect on osteoblastic differentiation. The media (ODM) was changed every other day for 12 d, and osteoclastic differentiation was evaluated by determining Tartrate-Resistant Acid Phosphatase (TRAP) activity. The cultured cells were lysed with 2 % NP-40 and supernatants including acid phosphatase were reacted with pNPP in the acidic condition. Then, the absorbance was measured at 405 nm by BioT ek.
Statistical analyses:
Data are presented as mean±standard deviation. Two or more group comparisons were evaluated by one-way analysis of variance followed by Tukey post hoc text (SPSS version 17.0, Armonk, NY, USA). Differences between values were considered statistically significant when the p value was below 0.05 (*p<0.05).
Results and Discussion
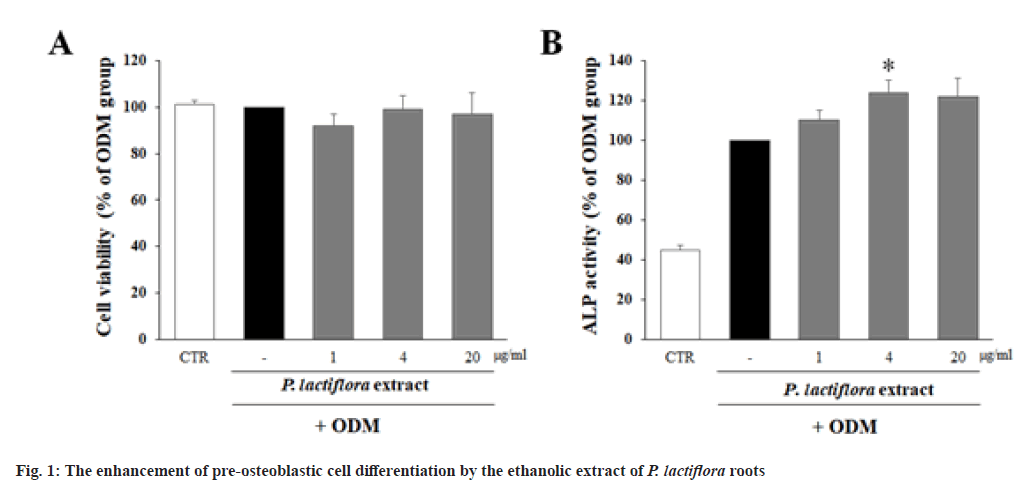
In order to determine the non-cytotoxic concentration of the ethanolic extract of P. lactiflora roots, the viability of MC3T3-E1 cells treated with the extract of P. lactiflora roots for 24 h was determined by MTT assay. The viability of all the groups treated with the ethanolic extract of P. lactiflora roots up to 20 μg/ml were over 80 %. These results indicate that the cytotoxicity was not observed in any treatment groups (fig. 1A).
Then, the effect of ethanol extract of P. lactiflora roots (1, 4 and 20 μg/ml) on the differentiation of pre-osteoblast was determined by ALP assay using the pre-osteoblastic MC3T3-E1 cells. As shown in fig. 1B, the cells incubated in ODM showed the higher ALP activity compared to control group incubated in NM, indicating ODM induced the deffrentiation of MC3T3-E1 cells to osteoblasts. Furthermore, the treatment of cells with the extract of P. lactiflora roots in ODM have a tendency to increase ALP activity compared to DMSO-treated ODM group. Particularly, the treatment with 4 μg/ml of P. lactiflora root extract significantly increased ALP activity.
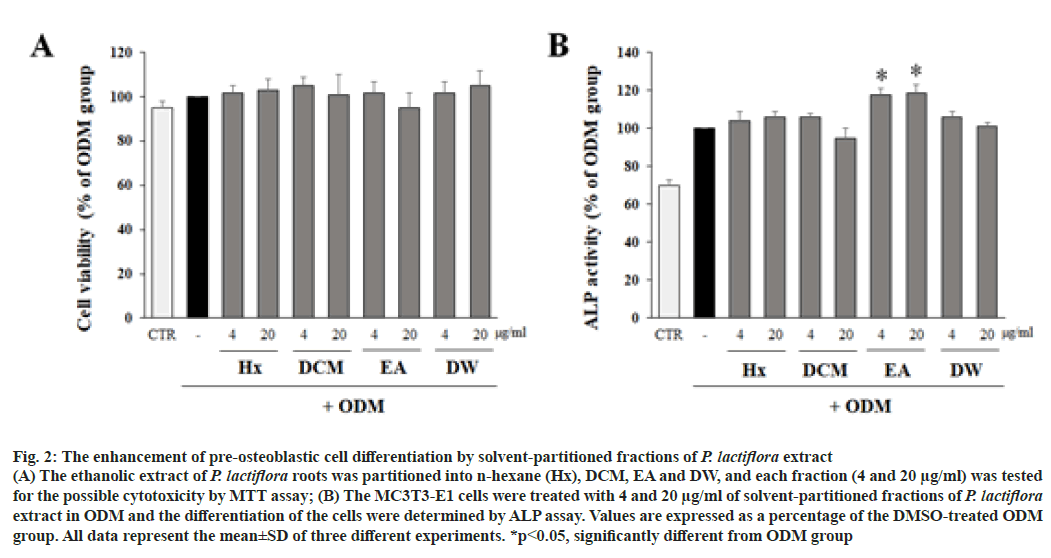
In order to isolate the active constituents responsible for the osteoblastic differentiation in P. lactiflora, the ethanol extract of P. lactiflora roots were partitioned based on solvent polarity. Four fractions including n-Hexane (Hx), Dichloromethane (DCM), Ethyl Acetate (EA) and Distilled Water (DW) fractions (4 and 20 μg/ ml) were tested for their possible cytotoxicity. As shwon in fig. 2A, the viabilities of cells treated with 4 fractions were all over 80 %. As the results, cytotoxicity was not observed in any treatment groups.
Then, the effect of solvent-partitioned fractions of P. lactiflora extract on the differentiation of pre-osteoblastic cells was determined by ALP assay. The concentrations of each treatment group were 4 and 20 μg/ml. As shown in fig. 2B, the Ethyl Acetate (EA) fraction significantly increased ALP activity compared to DMSOtreated ODM group, but other fractions did not.
Fig. 2: The enhancement of pre-osteoblastic cell differentiation by solvent-partitioned fractions of P. lactiflora extract
(A) The ethanolic extract of P. lactiflora roots was partitioned into n-hexane (Hx), DCM, EA and DW, and each fraction (4 and 20 μg/ml) was tested for the possible cytotoxicity by MTT assay; (B) The MC3T3-E1 cells were treated with 4 and 20 μg/ml of solvent-partitioned fractions of P. lactiflora extract in ODM and the differentiation of the cells were determined by ALP assay. Values are expressed as a percentage of the DMSO-treated ODM group. All data represent the mean±SD of three different experiments. *p<0.05, significantly different from ODM group
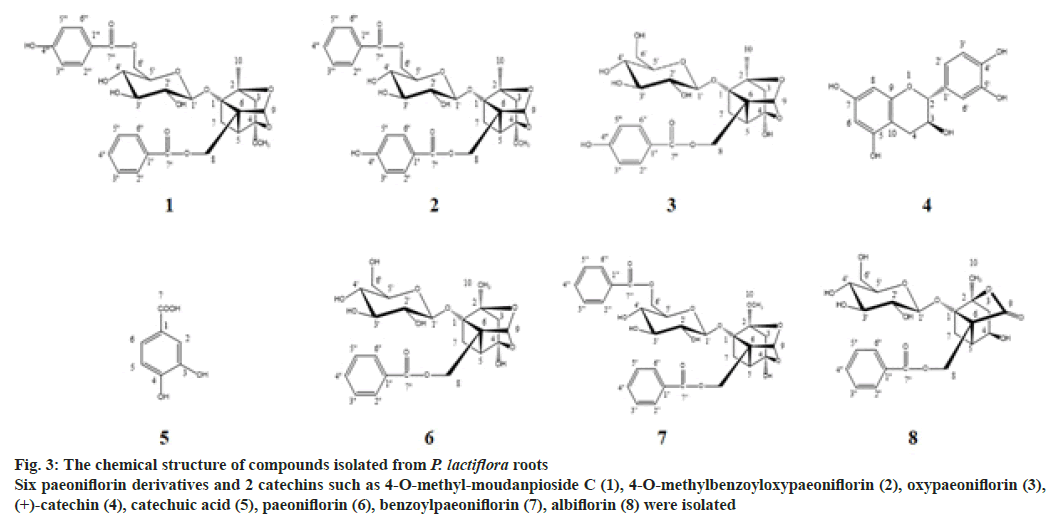
EA fraction was applied to diverse column chromatography using silica gel, C18, and sephadex LH-20 as stationary phages with the combination of organic solvent as elution phases to isolate active constituents. As the results, 8 compounds were isolated and the strutures of those compouds (fig. 3) were elucidated as 4-O-methyl-moudanpioside C (1), 4-O-methylbenzoyloxypaeoniflorin (2), oxypaeoniflorin (3), (+)-catechin (4), catechuic acid (5), paeoniflorin (6), benzoylpaeoniflorin (7), albiflorin (8), based on 1D and 2D NMR data compared with the references[8-14].
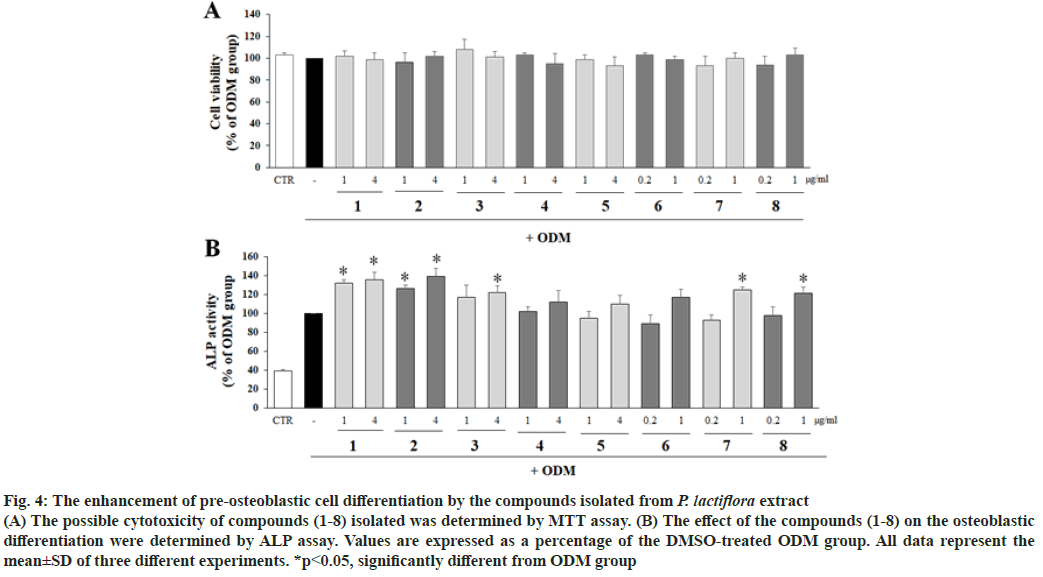
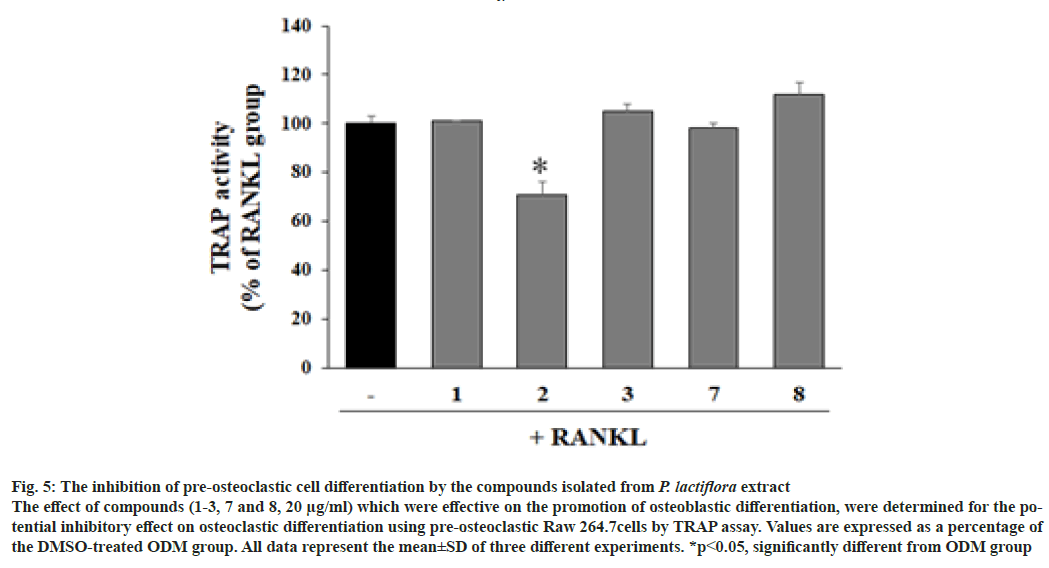
To evaluate the effects of isolated compounds on the differentiation of pre-osteoblastic cells, the MC3T3-E1 cells were treated with the compounds and ALP activity was measured with the non-cytotoxic concentrations. ALP activity were significantly increased with the treatment of 4-O-methyl-moudanpioside C (1), 4-O-methylbenzoyloxypaeoniflorin (2), and albiflorin (8) significantly increased ALP activity compared to ODM group at 1 and 4 μg/ ml, whereas oxypaeoniflorin (3) at 4 μg/ml and benzoylpaeoniflorin (7) at 1 μg/ml significantly upregulated ALP activity compared to DMSOtreated ODM group. However, (+)-catechin (4), catechuic acid (5), and paeoniflorin (6) exhibited no enhancement on ALP activity (fig. 3B) compared to ODM group. The active compounds enhancing osteoblastic differentiation were tested for their effect on osteoclastic differentiation with Raw264.7 cells by TRAP assay (fig. 4). As shown in fig. 5, the TRAP activity was not significantly altered by the treatment with 4-O-methyl-moudanpioside C (1), oxypaeoniflorin (3), benzoylpaeoniflorin (7) and albiflorin (8) (20 μg/ml). However, 4-O-methylbenzoyloxypaeoniflorin (2) significantly decreased TRACP activity indicating the inhibitory effect on osteoclastic differentiation.
Fig. 3: The chemical structure of compounds isolated from P. lactiflora roots
Six paeoniflorin derivatives and 2 catechins such as 4-O-methyl-moudanpioside C (1), 4-O-methylbenzoyloxypaeoniflorin (2), oxypaeoniflorin (3), (+)-catechin (4), catechuic acid (5), paeoniflorin (6), benzoylpaeoniflorin (7), albiflorin (8) were isolated
Fig. 4: The enhancement of pre-osteoblastic cell differentiation by the compounds isolated from P. lactiflora extract
(A) The possible cytotoxicity of compounds (1-8) isolated was determined by MTT assay. (B) The effect of the compounds (1-8) on the osteoblastic differentiation were determined by ALP assay. Values are expressed as a percentage of the DMSO-treated ODM group. All data represent the mean±SD of three different experiments. *p<0.05, significantly different from ODM group
Fig. 5: The inhibition of pre-osteoclastic cell differentiation by the compounds isolated from P. lactiflora extract
The effect of compounds (1-3, 7 and 8, 20 μg/ml) which were effective on the promotion of osteoblastic differentiation, were determined for the potential inhibitory effect on osteoclastic differentiation using pre-osteoclastic Raw 264.7cells by TRAP assay. Values are expressed as a percentage of the DMSO-treated ODM group. All data represent the mean±SD of three different experiments. *p<0.05, significantly different from ODM group
Bone regeneration is a complex process regulated by interactions between osteoblasts and osteoclasts. Briefly, osteoblasts are activated and secret cytokines, which stimulate the differentiation of osteoblasts, and osteoblasts resorb the bone. After macrophages clear off the resorption areas, differentiated osteoblasts form the new bone. In this study, the extract of P. lactiflora and its active constituents significantly increased ALP activity in pre-osteoblastic MC3T3-E1 cells, which is expressed largely by active osteoblasts and considered as a unique marker of osteoblast differentiation.
The roots of P. lactiflora have been reported to have diverse pharmacological activities such as anti-inflammatory and immunomodulatory effects[15]. In addition, chemical constituents included in P. lactiflora roots were also reported to have various biological activities, including anti-inflammatory[16], anti-oxidant[17] and anticonvulsant[ 18] activities. Paeoniflorin is a major constituent included in P. lactiflora roots, and the isolation of diverse paeoniflorin derivatives were also reported. In this study, the bioassayguided isolation of ethyl acetate fraction using diverse open column chromatography allowed to isolate 6 paeoniflorin derivatives and 2 catechins including 4-O-methylmoudanpioside C (1), 4-O-methylbenzoyloxypaeoniflorin (2), oxypaeoniflorin (3), (+)-catechin (4), catechuic acid (5), paeoniflorin (6), and benzoylpaeoniflorin (7) and albiflorin (8). Among them, 4-O-methylmoudanpioside C (1), 4-O-methylbenzoyloxypaeoniflorin (2), oxypaeoniflorin (3), benzoylpaeoniflorin (7) and albiflorin (8) significantly increased ALP activity. These active compounds enhancing osteoblastic differnetiation have a common chemical structures such as benzoyl moiety, monoterpene moiety and glycoside in a molecule[13]. previously reported the isolation of paeoniflorin derivatives including 6'-O-ß- D-glucopyranosylalbiflorin, albiflorin and paeoniflorin, as active constituents enhancing osteoblastic differentiation in P. lactiflora. These active compounds include benzoyl moiety, monoterpene moiety and glycoside in a molecule in common, whereas other compounds which don’t have a benzoyl moiety didn't exhibit any significant effects on ALP activity. In accordance with the previous report, in this study, all of the active compounds include benzoyl moiety, monoterpene moiety and glycoside. In addition, the active compounds with additional benzoyl group such as 4-O-methylmoudanpioside C (1), 4-O-methylbenzoyloxypaeoniflorin (2) and benzoylpaeoniflorin (7) have a tendency to increase better osteoblastic differentiation than molecules with one benzoyl moeity. Furthermore, 4-O-methylbenzoyloxypaeoniflorin (2) having additional benzoyl moeity significantly inhibited the osteoclastic differentiation.
Paeoniflorin (6), oxypaeoniflorin (3), benzoylpaeoniflorin (7), and albiflorin (8) have been isolated from P. lactiflora and their anti-inflammatory effects have been reported previously[16,19,20]. Paeoniflorin has been demonstrated to have strong antioxidant effect in addition to antiasthma and antithrombosis activities[21-23]. (+)-Catechin (4) has been reported to have antioxidant, antiinfluenza, and antimicrobial activities[24-26]. Lastly, albiflorin (4) has reported antithrombotic, neuroprotective and antidepressant activities[27-29]. However, the beneficial effect of 4-O-methylbenzoyloxypaeoniflorin (1), 4-O-methylbenzoyloxypaeoniflorin (2) and benzoylpaeoniflorin (7) on the osteoblastic differentiation and the inhibitory effect of 4-O-methylbenzoyloxypaeoniflorin (2) on the osteoclastic differentiation were first reported in this study.
Taken together, these results suggest that the extract of P. lactiflora roots and its active constituents including 4-O-methylbenzoyloxypaeoniflorin (2) have potential to be developed as preventives or therapeutics of osteoporosis. However, further mechanism study and animal study should be performed in order to confirm the beneficial effects.
Author Contributions:
The authors are grateful to Center for Bio-Medical Engineering Core Facility for providing NMR experiments.
Author contributions:
Eun-Sang Cho and Min Sung Ko contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Kanis JA, Melton 3rd LJ, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res 1994;9:1137-41.
[Crossref] [Google Scholar] [PubMed]
- Siris ES, Adler R, Bilezikian J, Bolognese M, Dawson-Hughes B, Favus MJ, et al. The clinical diagnosis of osteoporosis: A position statement from the National Bone Health Alliance Working Group. Osteoporos Int 2014;25:1439-43.
[Crossref] [Google Scholar] [PubMed]
- Mo D, Fleseriu M, Qi R, Jia N, Child CJ, Bouillon R, et al. Fracture risk in adult patients treated with growth hormone replacement therapy for growth hormone deficiency: A prospective observational cohort study. Lancet Diabetes Endocrinol 2015;3(5):331-8.
[Crossref] [Google Scholar] [PubMed]
- Stein GS, Lian JB, Owen TA. Relationship of cell growth to the regulation of tissue?specific gene expression during osteoblast differentiation. FASEB J 1990;4(13):3111-23.
[Crossref] [Google Scholar] [PubMed]
- Zhao DD, Jiang LL, Li HY, Yan PF, Zhang YL. Chemical components and pharmacological activities of terpene natural products from the genus Paeonia. Molecules 2016;21(10):1362-76.
[Crossref] [Google Scholar] [PubMed]
- Wu S, Wu D, Chen Y. Chemical constituents and bioactivities of plants from the genus Paeonia. Chem Biodivers 2010;7(1):90-104.
[Crossref] [Google Scholar] [PubMed]
- Dolder S, Hofstetter W, Wetterwald A, Mühlbauer RC, Felix R. Effect of monoterpenes on the formation and activation of osteoclasts in vitro. J Bone Miner Res 2006;21(4):647-55.
[Crossref] [Google Scholar] [PubMed]
- Ding L, Jiang Z, Liu Y, Chen L, Zhao Q, Yao X, et al. Monoterpenoid inhibitors of NO production from Paeonia suffruticosa. Fitoterapia 2012;83(8):1598-603.
[Crossref] [Google Scholar] [PubMed]
- Lee SC, Kwon YS, Son KH, Kim HP, Heo MY. Antioxidative constituents from Paeonia lactiflora. Arch Pharm Res 2005;28:775-83.
[Crossref] [Google Scholar] [PubMed]
- Davis AL, Cai Y, Davies AP, Lewis JR. 1H and 13C NMR assignments of some green tea polyphenols. Magn Reson Chem 1996;34(11):887-90.
- Son BW, Park JH, Zee OP. Catechin glycoside from Ulmus davidiana. Arch Pharm Res 1989;12:219-22.
- Abdel-Raziq MS, Bar FM, Gohar AA. Alpha-amylase inhibitory compounds from Musa cavendishii. Br J Pharm Res 2016;13(4):1-10.
- Pham Hai Y, Phan Van K, Nguyen Xuan N, Nguyen Huu T, Tran Hong Q, Chau Van M, et al. A new monoterpene glycoside from the roots of Paeonia lactiflora increases the differentiation of osteoblastic MC3T3-E1 cells. Arch Pharm Res 2007;30:1179-85.
[Crossref] [Google Scholar] [PubMed]
- Xianxiang X, Yangzheng W. Studies on the separation of monoterpene glycosides from Paeonia veitchii Lynch. herbs. Pharm Chem J 2016;50:568-72.
- He DY, Dai SM. Anti-inflammatory and immunomodulatory effects of Paeonia lactiflora Pall., a traditional Chinese herbal medicine. Front Pharmacol 2011;2:10.
[Crossref] [Google Scholar] [PubMed]
- Wang QS, Gao T, Cui YL, Gao LN, Jiang HL. Comparative studies of paeoniflorin and albiflorin from Paeonia lactiflora on anti-inflammatory activities. Pharm Biol 2014;52(9):1189-95.
[Crossref] [Google Scholar] [PubMed]
- Ryu G, Park EK, Joo JH, Lee BH, Choi BW, Jung DS, et al. A new antioxidant monoterpene glycoside, α-benzoyloxypaeoniflorin from Paeonia suffruticosa. Arch Pharm Res 2001;24:105-8.
[Crossref] [Google Scholar] [PubMed]
- Abdel-Hafez AA, Meselhy MR, Nakamura N, Hattori M, Watanabe H, Murakami Y, et al. Anticonvulsant activity of paeonimetabolin-I adducts obtained by incubation of paeoniflorin and thiol compounds with Lactobacillus brevis. Biol Pharm Bull 1999;22(5):491-7.
[Crossref] [Google Scholar] [PubMed]
- Zhang MH, Feng L, Zhu MM, Gu JF, Wu C, Jia XB. Antioxidative and anti-inflammatory activities of paeoniflorin and oxypaeoniflora on AGEs-induced mesangial cell damage. Planta Med 2013;79(14):1319-23.
[Crossref] [Google Scholar] [PubMed]
- Oh GS, Pae HO, Choi BM, Jeong S, Oh H, Oh CS, et al. Inhibitory effects of the root cortex of Paeonia suffruticosa on interleukin-8 and macrophage chemoattractant protein-1 secretions in U937 cells. J Ethnopharmacol 2003;84(1):85-9.
[Crossref] [Google Scholar] [PubMed]
- Kim ID, Ha BJ. The effects of paeoniflorin on LPS-induced liver inflammatory reactions. Arch Pharm Res 2010;33:959-66.
[Crossref] [Google Scholar] [PubMed]
- Zhang T, Yang Z, Yang S, Du J, Wang S. Immunoregulatory effects of paeoniflorin exerts anti-asthmatic effects via modulation of the Th1/Th2 equilibrium. Inflammation 2015;38:2017-25.
[Crossref] [Google Scholar] [PubMed]
- Ye J, Duan H, Yang X, Yan W, Zheng X. Anti-thrombosis effect of paeoniflorin: Evaluated in a photochemical reaction thrombosis model in vivo. Planta Med 2001;67(08):766-7.
[Crossref] [Google Scholar] [PubMed]
- Rossetto M, Vanzani P, Mattivi F, Lunelli M, Scarpa M, Rigo A. Synergistic antioxidant effect of catechin and malvidin 3-glucoside on free radical-initiated peroxidation of linoleic acid in micelles. Arch Biochem Biophys 2002;408(2):239-45.
[Crossref] [Google Scholar] [PubMed]
- Mantani N, Imanishi N, Kawamata H, Terasawa K, Ochiai H. Inhibitory effect of (+)-catechin on the growth of influenza A/PR/8 virus in MDCK cells. Planta Med 2001;67(03):240-3.
[Crossref] [Google Scholar] [PubMed]
- Friedman M, Henika PR, Levin CE, Mandrell RE, Kozukue N. Antimicrobial activities of tea catechins and theaflavins and tea extracts against Bacillus cereus. J Food Prot 2006;69(2):354-61.
[Crossref] [Google Scholar] [PubMed]
- Xie P, Cui L, Shan Y, Kang WY. Antithrombotic effect and mechanism of radix paeoniae rubra. BioMed Res Int 2017;2017:9475074.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Fei X, Tao W, Li J, Shen H, Wang X, et al. Neuroprotective effect of modified xijiao dihuang decoction against oxygen-glucose deprivation and reoxygenation-induced injury in PC12 cells: involvement of TLR4-MyD88/NF-κB signaling pathway. Evid Based Complement Alternat Med 2017;2017: 3848595.
- Wang YL, Wang JX, Hu XX, Chen L, Qiu ZK, Zhao N, et al. Antidepressant-like effects of albiflorin extracted from Radix paeoniae Alba. J Ethnopharmacol 2016;179:9-15.
[Crossref] [Google Scholar] [PubMed]