- *Corresponding Author:
- Wei Wang
Department of Basic Medicine,
Jiangxi Medical College,
Shangrao, Jiangxi Province 334000,
China
E-mail: ww19801981@163.com
| This article was originally published in a special issue, “Trending Topics in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(1) Spl Issue “100-108” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the expression of lymphocyte cytoplasmic protein 1 in plasma and tumor tissues of patients with gastric cancer and to analyze the correlation between lymphocyte cytoplasmic protein 1 and clinicopathological indexes and its effect on tumor immune infiltration. The expression of lymphocyte cytoplasmic protein 1 in plasma samples of 61 patients with gastric cancer who underwent surgery from December 2019 to December 2020 was detected by enzyme-linked immunosorbent assay. The expression of lymphocyte cytoplasmic protein 1 was detected by immunohistochemical staining and western blot. To study the correlation between pathology and lymphocyte cytoplasmic protein 1 expression in gastric cancer and the effect of lymphocyte cytoplasmic protein 1 expression on tumor immune infiltration. The expression of lymphocyte cytoplasmic protein 1 was closely related to the tumor size, differentiation, lymph node metastasis, tumor, nodes and metastases stage and depth of invasion of gastric cancer (p<0.05). After the expression of lymphocyte cytoplasmic protein 1, the migration and infiltration ability of gastric cancer MKN45 cells and BGC823 cells were improved (p<0.05). The expression of lymphocyte cytoplasmic protein 1 is closely related to the pathology of gastric cancer and the high expression of lymphocyte cytoplasmic protein 1 can significantly enhance the immune infiltration ability of gastric cancer and then has the potential of drug therapeutic targets.
Keywords
Lymphocyte cytoplasmic protein 1, gastric cancer, tumor infiltration, tumor metastasis, immunohistochemical staining, western blot
Gastric cancer is one of the most common malignant tumor diseases. According to the data of the international agency for research on cancer, by 2021, the new cases of gastric cancer worldwide accounted for 6.8 % of all cancers, ranking the fifth in malignant tumors[1]. At the same time, the mortality rate of gastric cancer ranks second among the causes of cancer death[2], because the early symptoms of gastric cancer are not obvious and it is often considered that the diagnosis of chronic gastritis is delayed, and the treatment results are not satisfactory due to the late diagnosis[3]. Once the patients with obvious clinical signs especially those with abdominal masses and swollen supraclavicular lymph nodes, they have entered the middle and late stage[4]. At present, the cognition of the pathogenesis of gastric cancer is not clear, that is, the occurrence of gastric cancer is the comprehensive result of multi-step and multi factor[5]. Among them, the recurrence rate and survival rate of gastric cancer are closely related to tumor pathological type, tumor immune infiltration and tumor cell migration. In view of this, reasonable selection of comprehensive treatment scheme and timely and effective prediction of prognosis are the research hotspots of gastric cancer treatment[6]. As a kind of actin binding protein, the mechanism of Lymphocyte Cytoplasmic Protein 1 (LCP1) is reflected in the regulation of cell movement through interaction with actin and LCP1 plays an important role in the process of cell migration, and its high and low expression indexes can also help to evaluate the prognosis of tumor patients[7,8]. In recent years, research and development have shown that LCP1 is highly expressed in gastric cancer cells, but there are few reports on whether LCP1 expression is related to tumor size, degree of differentiation, lymph node metastasis, Tumor, Nodes and Metastases (TNM) stage, depth of invasion and prognosis of gastric cancer[9,10]. In view of this, this study focuses on analyzing the correlation between LCP1 expression and gastric cancer pathology, and the effect of LCP1 expression level on tumor immune infiltration, so as to provide corresponding theoretical basis for clinical diagnosis and treatment of gastric cancer.
Materials and Methods
Data collection:
Participants include 61 patients with gastric cancer admitted to our hospital from June 2020 to June 2021. The tissues of patients with gastric cancer (61 cases) and adjacent normal gastric tissues (61 cases) were collected for comparative experiments, and 20 healthy volunteers were selected as the control group. Inclusion criteria include patients with gastric cancer confirmed by clinicopathology; gastric cancer patients without any tumor treatment; gastric cancer patients with other malignant tumors; gastric cancer patients with complete clinicopathological data. Histological grading and tumor staging were performed according to the American Joint Committee on Cancer (AJCC) standard[11].
Test reagent:
SP-0023 immunohistochemical kit, mouse anti-human LCP1 monoclonal antibody and 3,3’-Diaminobenzidine (DAB) chromogenic reagent kit were purchased from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd. Follow the instructions of the kit for dyeing steps. The pathology department of our hospital provides 70 %, 80 % and 95 % ethanol; absolute ethanol; hematoxylin; deionized water and xylene.
Research methods:
Determination by Enzyme-Linked Immunosorbent Assay (ELISA): For ELISA determination, it is necessary to set the standard hole, add the sample diluent and incubate in the greenhouse for 120 min. Then, the primary antibody (anti-human LCP1 monoclonal antibody) working solution and enzyme labeled antibody working solution were added for 60 min incubation, and the dark reaction was carried out for 5~8 min. Finally, add the termination solution and test the absorbance (Optical Density (OD)) value, and carry out two measurements (standard, control and sample OD values) respectively. Plot with CurveExpert 1.3 software to obtain the LCP1 concentration twice and finally get its mean value[12].
Tissue immunohistochemical staining: For the detection of tissue immunohistochemical staining, it is necessary to repair the tissue antigen, add the primary antibody and Hydrogen Peroxide (H2O2) and incubate in the greenhouse; then, combined with DAB solution and MaxVisionTM reagent, carry out specific observation under the microscope. After that, water washing, hematoxylin re dyeing, xylene transparency, sealing and other operations are carried out; known positive samples were selected as positive control, and Phosphate Buffered Saline (PBS) buffer was selected as negative control instead of primary antibody[13]. Finally, two pathologists performed double-blind film reading.
Index evaluation includes positive cells which were scored by percentage. 0 points were positive cells ≤5 %, 1 point was positive cells between 5 %~25 % and 2 points were positive cells between 25 %~50 %; 3 points were positive cells ranged from 50 % to 75 %; 4 points were positive cells ≥75 %. Score of coloring intensity is given in detail, 0 points for no coloring; 1 point for light yellow; 2 points for yellow and 3 points for tan. The sum of positive cell score and staining intensity score ≤3 points is negative; the weak positive score was between 4 and 6; the score of strong positive was more than 6 points.
Tissue western blot detection: For tissue western blot detection, it is necessary to calculate the tissue protein concentration, complete the preparation of laminated gel and separation gel, add sample protein, add primary antibody beta (β)-actin was used as internal reference and target protein and anti-human LCP1 polyclonal antibody was added. Then, the primary antibody was developed overnight and the secondary antibody was added for color development, which was compared with the internal reference to determine the expression of LCP1 in the tissue. Finally, the expression of LCP1 in adjacent normal tissues and tumor tissues was semi quantitatively analyzed[14].
Statistical analysis:
The data analysis was completed by Statistical Package for the Social Sciences (SPSS) 21.0 statistical software. The statistical methods involved include two sample independent t-test, nonparametric test, chi square test and descriptive analysis α=0.05. The mean and Standard Deviation (SD) was used for descriptive analysis. Two sample independent t-test was used to analyze the quantitative data in accordance with the positive Pacific distribution; nonparametric test was used for grade data analysis; chi square test was used to compare and analyze the data of the two groups.
Results and Discussion
Relationship between LCP1 expression and clinicopathological features of gastric cancer is explained here. In this study, the correlation between plasma LCP1 concentration and gastric cancer pathology was analyzed from the indexes of gender, age, TNM stage, depth of invasion and lymph node metastasis. Table 1 shows the relationship between plasma LCP1 concentration and clinicopathological features of gastric cancer.
| Pathological features of gastric cancer | Number of cases | Plasma LCP1 concentration (Mean±SD (μg/l)) | p | |
|---|---|---|---|---|
| Gender | Male | 43 | 34.239±33.786 | 0.498 |
| Female | 18 | 38.078±27.530 | ||
| Age (years) | ≤60 | 33 | 39.990±31.825 | 0.185 |
| >60 | 28 | 32.513±29.610 | ||
| TNM staging | I+II | 32 | 31.089±26.153 | 0.004 |
| III+IV | 29 | 49.142±36.920 | ||
| Infiltration depth | T1+T2 | 22 | 39.382±32.648 | 0.314 |
| T3+T4 | 39 | 30.563±30.585 | ||
| Lymph node metastasis | Negative | 28 | 27.956±26.226 | 0.004 |
| Positive | 33 | 49.195±35.206 | ||
| Vascular tumor thrombus | Negative | 32 | 31.089±26.153 | 0.004 |
| Positive | 29 | 49.142±36.920 | ||
| Degree of differentiation | High | 16 | 28.445±23.322 | 0.001 |
| Low | 45 | 48.929±37.184 | ||
Table 1: Relationship between Plasma LCP1 Concentration and Clinicopathological Features of Gastric Cancer
As shown in Table 1, the increase of LCP1 concentration in plasma is closely related to tumor differentiation, lymph node metastasis, TNM stage and other indicators (p<0.05), but has no significant correlation with the depth of invasion, gender and age of patients with gastric cancer (p>0.05). Table 2 shows the comparison of serum LCP1 concentration between gastric cancer group and normal group.
| Group | Number of cases (n) | Plasma LCP1 concentration (Mean±SD (μg/l)) | p |
|---|---|---|---|
| Gastric cancer group | 61 | 49.195±35.206 | <0.001 |
| Normal control group | 20 | 3.956±26.226 |
Table 2: Comparison of Serum LCP1 Concentration between Gastric Cancer Group and Normal Group
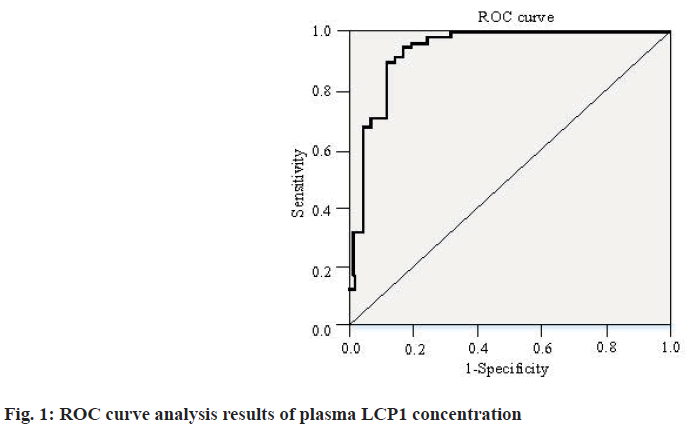
As shown in Table 2, the concentration of serum LCP1 in gastric cancer group was 49.195±35.206 μg/l, the serum LCP1 concentration in the normal group was 27.956±26.226. The serum LCP1 concentration in the gastric cancer group was higher than that in the control group (p<0.001). In addition, the Receiver Operating Characteristic (ROC) curve analysis results of plasma LCP1 concentration are shown in fig. 1.
It can be seen from fig. 1 that on the ROC curve of plasma LCP1 concentration, the specificity is 87.5 %, the sensitivity is 90.0 % and the Likelihood Ratio (LR=7.2) and Youden index reach 0.775. The results showed that the SD was 0.029 and Area Under the Curve (AUC)=0.930, with significant statistical difference (p<0.01). In addition, Table 3 shows the relationship between LCP1 expression and clinicopathological features of gastric cancer.
| Pathological features of gastric cancer | Number of cases | LCP1 low expression | LCP1 high expression | χ2 | p | |
|---|---|---|---|---|---|---|
| Gender | Male | 43 | 21 | 22 | 0.229 | 0.632 |
| Female | 18 | 10 | 8 | |||
| Age (years) | ≤60 | 33 | 19 | 14 | 1.313 | 0.252 |
| >60 | 28 | 12 | 16 | |||
| TNM staging | I+II | 32 | 23 | 9 | 11.939 | <0.001 |
| III+IV | 29 | 8 | 21 | |||
| Infiltration depth | T1+T2 | 22 | 17 | 5 | 8.050 | 0.005 |
| T3+T4 | 39 | 14 | 25 | |||
| Lymph node metastasis | Negative | 28 | 21 | 7 | 12.108 | <0.001 |
| Positive | 33 | 10 | 23 | |||
| Vascular tumor thrombus | Negative | 32 | 19 | 13 | 4.921 | 0.027 |
| Positive | 29 | 9 | 20 | |||
| Degree of differentiation | High | 16 | 8 | 8 | 0.006 | 0.939 |
| Low | 45 | 23 | 22 | |||
Table 3: Relationship between LCP1 Expression and Clinicopathological Features of Gastric Cancer
As shown in Table 3, the depth of invasion, lymph node metastasis and TNM stage of gastric cancer patients were closely correlated with their LCP1 expression (p<0.05). At the same time, there was no significant correlation between the expression of LCP1 and the degree of tumor differentiation, age and gender in patients with gastric cancer (p>0.05).
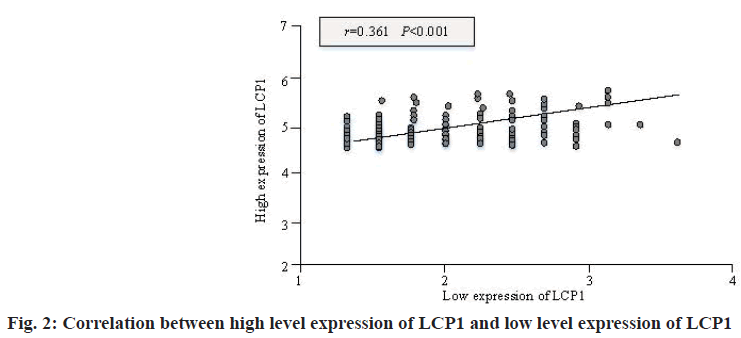
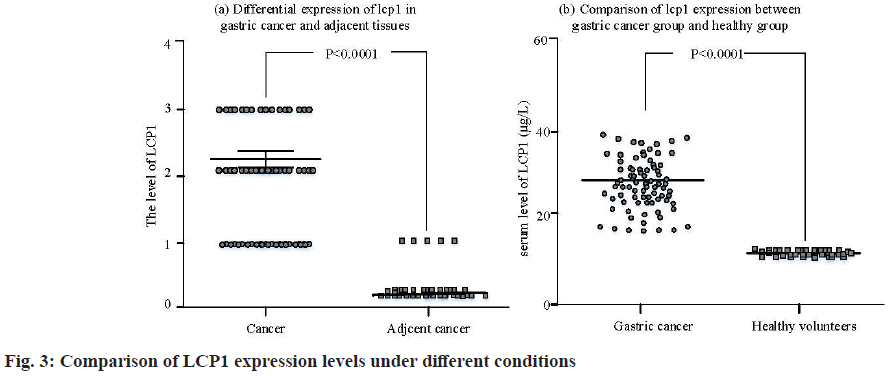
It can be seen from fig. 2 that there is a significant positive correlation between the high-level expression of LCP1 and the low-level expression of LCP1, with a statistical difference (r=0.361, p<0.001), which shows that the high-level expression of LCP1 is consistent with the low-level expression of LCP1. In addition, fig. 3 shows the comparison of LCP1 expression levels in different cases.
The expression level of LCP1 in the serum of patients with gastric cancer was significantly higher than that in the healthy group (p<0.001). According to the pathological score, there was significant difference in the expression of LCP1 in gastric cancer and adjacent tissues (p<0.01).
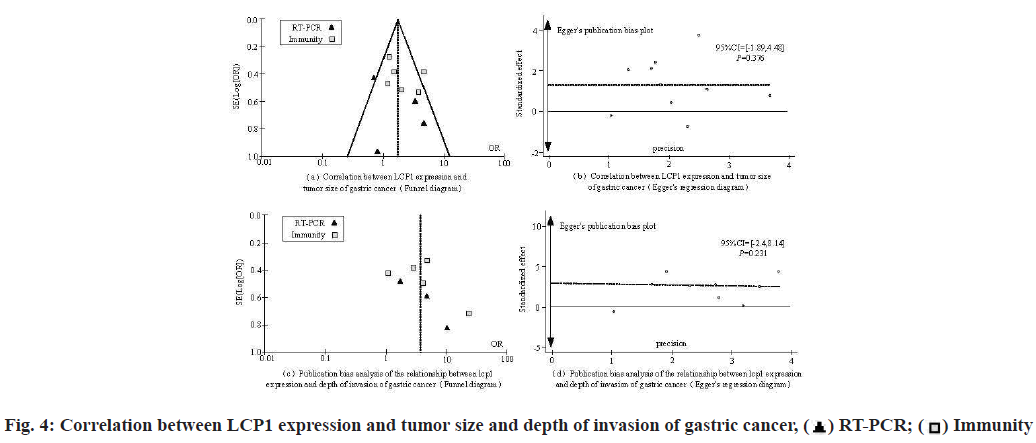
The potential publication bias of each index was preliminarily estimated by drawing a funnel chart and the bias of each index was evaluated by Egger’s test, no bias judgment (lower limit of 95 % Confidence Interval (CI)>1; p>0.1), otherwise it was regarded as biased. Fig. 4 shows the correlation between tumor size and depth of invasion of gastric cancer and LCP1 expression in patients with gastric cancer.
As shown in fig. 4, at the level of correlation analysis between tumor size and LCP1 expression in patients with gastric cancer, the lower limit of 95 % CI value is >1; p>0.1, showing no bias. In the correlation analysis between the depth of invasion and the expression of LCP1 in patients with gastric cancer, the lower limit of 95 % CI was >1; p>0.1, showing no bias.
As shown in fig. 5, at the level of correlation analysis between the degree of gastric cancer differentiation and LCP1 expression in patients with gastric cancer, the lower limit of 95 % CI value is >1; p>0.1, showing no bias; In the correlation analysis between lymph node metastasis and LCP1 expression in patients with gastric cancer, the lower limit of 95 % CI was >1; p>0.1, showing no bias.
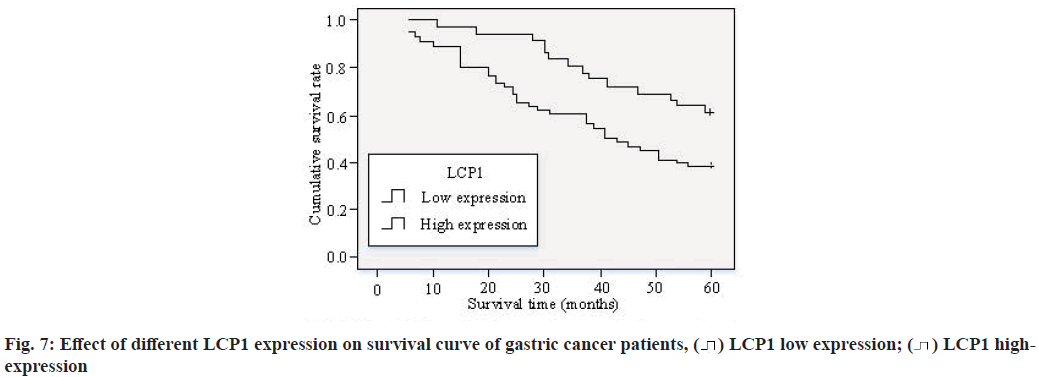
As can be seen from fig. 6a and fig. 6b, after the expression of LCP1 in Human Gastric Cancer cell line MKN45 cells and BGC823 cells, compared with the negative control group, the migration and infiltration ability of gastric cancer MKN45 cells and BGC823 cells were improved, with statistical difference (p<0.05). It can be seen from fig. 6c and fig. 6d that after LCP1 expression, there was no significant change in the proliferation ability of gastric cancer MKN45 cells and BGC823 cells compared with the negative control group. Fig. 7 shows the effect of different LCP1 expression on the survival curve of gastric cancer patients.
According to fig. 7, the 3 y and 5 y survival rates of gastric cancer patients with high expression of LCP1 were 82.9 % and 60 % respectively; the 3 y and 5 y survival rates of gastric cancer patients with low expression of LCP1 were 60 % and 37.8 % respectively. The expression of LCP1 in gastric cancer was significantly positively correlated with the prognosis of patients. The cumulative survival rate of gastric cancer patients with high expression of LCP1 was significantly higher than that with low expression of LCP1 (p<0.05).
Correlation analysis between LCP1 expression and clinicopathological features of gastric cancer is discussed below. Gastric cancer as one of the malignant tumor diseases that seriously threaten human quality of life and health by 2021, the new cases of gastric cancer accounted for 6.8 % of all cancers worldwide and the mortality of gastric cancer ranked second among malignant tumor diseases. It can be seen that gastric cancer is a threat to human life and health. Specifically, there are significant differences in the treatment schemes of gastric cancer patients due to the multi-step and multi factor pathogenesis of gastric cancer. With the high recurrence rate and high mortality of gastric cancer patients, how to effectively treat gastric cancer patients is important and key[15]. Combined with the current clinical practice of treating gastric cancer, there is still no clinical plan that can effectively identify early diagnosis, prevention and treatment of gastric cancer. However, the early diagnosis of gastric cancer is of great importance to improve the therapeutic effect, reduce mortality and incidence rate, and become a difficult problem to overcome in the global treatment of gastric cancer[16].
Combined with the clinical practice of treating gastric cancer, the important reason for the high mortality and high recurrence rate of gastric cancer patients is the invasion and metastasis of gastric cancer. Moreover, the invasion and metastasis of gastric cancer are the result of multi-step and multi factor interaction, different gastric cancer patients have different pathological characteristics and prognosis[17]. Then, there are significant differences in the clinical treatment of gastric cancer. However, with the deepening understanding of tumor occurrence, invasion and metastasis in current clinical medicine, tumor targeted treatment strategies for gastric cancer have been put forward one after another and have become a new way and important link for the treatment of patients with gastric cancer[18]. Its advantage is not only to help the prognosis of patients with gastric cancer, so as to develop targeted treatment strategies, but also to determine the targeted treatment of gastric cancer in time, so as to actively improve the quality of life of patients with gastric cancer.
The results showed that the expression of LCP1 in patients with gastric cancer was significantly correlated with the pathological characteristics of gastric cancer, such as TNM stage, depth of invasion and lymph node metastasis (p<0.05). At the same time, the expression of LCP1 was not significantly correlated with the degree of tumor differentiation, gender and age of patients with gastric cancer (p>0.05). At the same time, the expression level of LCP1 in the serum of patients with gastric cancer was significantly higher than that in the healthy group (p<0.001). According to the pathological score, there was significant difference in the expression of LCP1 in gastric cancer and adjacent tissues (p<0.01). The above results suggest that the expression of LCP1 may be involved in the occurrence and development of diffuse gastric cancer, and may provide a molecular target for the treatment of gastric cancer.
In summary, as actin binding protein, the action mechanism of LCP1 is reflected in the regulation of cell movement through interaction with actin and LCP1 plays an important role in the process of cell migration and its high and low expression indexes can also help to evaluate the prognosis of tumor patients[19]. Relevant studies have shown that[20], LCP1 is highly expressed in gastric cancer cells and LCP1 expression is correlated with the pathology of gastric cancer patients. Moreover, LCP1 expression can also help to evaluate the prognosis of tumor patients.
Effect and significance of LCP1 expression on invasion and migration of gastric cancer cells is explained in detail. For the clinical treatment of tumor, modern medicine shows that the determination of tumor markers plays an important role in modern tumor treatment. It can contribute to the early diagnosis and treatment of tumor patients, the benign or malignant judgment of tumor patients, and the formulation of prognosis plan for tumor patients[21]. Therefore, the demand for tumor markers with high sensitivity and specificity has become an important practical direction of modern clinical tumor treatment, and an important direction of experimental research and clinical exploration of modern oncology[22].
Relevant studies have shown that[23], the main reason for tumor cell invasion and metastasis is that the intracellular actin skeleton is closely related to cell migration adhesion. The actin skeleton structure can be regulated by a variety of actin binding proteins. Therefore, actin binding protein has become a research hotspot in preventing tumor invasion and metastasis in recent years[23]. Relevant studies have shown that[24], for a variety of tumor cells, the expression of LCP1 in their tissues shows a corresponding increasing trend[25]. Although the clinical value of LCP1 expression has not been confirmed in tumor treatment, the close relationship between tumor cell invasion and metastasis and LCP1 expression has been confirmed.
The results showed that the depth of invasion, lymph node metastasis and TNM stage were closely related to the expression of LCP1 in patients with gastric cancer (p<0.05). At the same time, after the expression of LCP1 in gastric cancer MKN45 cells and BGC823 cells, the migration and infiltration ability of gastric cancer MKN45 cells and BGC823 cells were improved compared with the negative control group (p<0.05). It can be said that the intracellular mechanism of LCP1 involved in the enhancement of tumor cell infiltration and migration has been gradually revealed. Although a variety of actin binding proteins regulate the dynamic cytoskeleton of actin, recent studies on the regulation of actin dynamic LCP1 show that activated LCP1 can induce cell adhesion and increase the binding between actin[26], and the invasion and metastasis of tumor cells are closely related to cell adhesion and migration. Therefore, it can be said that regulating actin binding protein is an important target for inhibiting tumor cell metastasis.
In summary, the expression of LCP1, an important actin binding protein, can significantly enhance the immune infiltration ability of gastric cancer and then has the potential of drug therapeutic targets[27]. LCP1 has strong tissue specificity and ectopic high expression in most tumor cells and is involved in tumor biological functions such as tumor invasion and metastasis. Clinically, it can also be used as an indicator to help to evaluate the prognosis of tumor patients[28]. At present, it is known that LCP1 plays different functions in different types of cells[29] and its specific role and mechanism in different types of tumors still need to be further clarified. However, the study on the regulatory mechanism and functional role of ectopic high expression of LCP1 in tumor cells has important clinical significance for the development of new targets of tumor therapeutic drugs with LCP1 as the core.
Conflict of interests:
The authors declared no conflict of interest.
References
- Jin Y, Tao L, Jin S, Cai W. Patterns of immune infiltration in gastric cancer and their clinical significance. Jpn J Clin Oncol 2021;51(7):1067-79.
[Crossref] [Google Scholar] [PubMed]
- Lu J, Huang XY, Wang YH, Xie JW, Wang JB, Lin JX, et al. POC1A acts as a promising prognostic biomarker associated with high tumor immune cell infiltration in gastric cancer. Aging (Albany NY) 2020;12(19):18982-9011.
[Crossref] [Google Scholar] [PubMed]
- Jiang J, Ding Y, Wu M, Lyu X, Wang H, Chen Y, et al. Identification of TYROBP and C1QB as two novel key genes with prognostic value in gastric cancer by network analysis. Front Oncol 2020;10:1765.
[Crossref] [Google Scholar] [PubMed]
- Jiang Y, Wang H, Wu J, Chen C, Yuan Q, Huang W, et al. Noninvasive imaging evaluation of tumor immune microenvironment to predict outcomes in gastric cancer. Ann Oncol 2020;31(6):760-8.
[Crossref] [Google Scholar] [PubMed]
- Zhong R, Chen D, Cao S, Li J, Han B, Zhong H. Immune cell infiltration features and related marker genes in lung cancer based on single-cell RNA-seq. Clin Transl Oncol 2021;23(2):405-17.
[Crossref] [Google Scholar] [PubMed]
- Huang SF, Lin JC, Shiau AC, Chen YC, Li MH, Tsai JT, et al. Optimal tumor coverage with different beam energies by IMRT, VMAT and TOMO: Effects on patients with proximal gastric cancer. Medicine 2020;99(47):e23328.
[Crossref] [Google Scholar] [PubMed]
- Guo Y, Liu X, Xu D, Huang C, Wang Z, Xia X, et al. Role of LATS1/2 in prognosis of advanced gastric cancer and its relationship with the tumor immune microenvironment. Front Oncol 2020;10:1406.
[Crossref] [Google Scholar] [PubMed]
- Danilova NV, Mikhailov IA, Oleynikova NA, Malkov PG, Chayka AV, Khomyakov VM, et al. Persistence of Epstein-Barr virus antigens in gastric cancer: Characteristic of inflammatory cellular reactions in tumor. Arkh Patol 2021;83(1):18-24.
[Crossref] [Google Scholar] [PubMed]
- Huang X, He C, Hua X, Kan A, Sun S, Wang J, et al. Bioinformatic analysis of correlation between immune infiltration and COVID-19 in cancer patients. Int J Biol Sci 2020;16(13):2464-76.
[Crossref] [Google Scholar] [PubMed]
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020;395(10224):565-74.
[Crossref] [Google Scholar] [PubMed]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181(2):271-80.
[Crossref] [Google Scholar] [PubMed]
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8(4):420-2.
[Crossref] [Google Scholar] [PubMed]
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382(18):1708-20.
[Crossref] [Google Scholar] [PubMed]
- Critchley-Thorne RJ, Simons DL, Yan N, Miyahira AK, Dirbas FM, Johnson DL, et al. Impaired interferon signaling is a common immune defect in human cancer. Proc Natl Acad Sci USA 2009;106(22):9010-5.
[Crossref] [Google Scholar] [PubMed]
- Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol 2020;21(3):335-7.
[Crossref] [Google Scholar] [PubMed]
- Zingone F, Savarino EV. Viral screening before initiation of biologics in patients with inflammatory bowel disease during the COVID-19 outbreak. Lancet Gastroenterol Hepatol 2020;5(6):525.
[Crossref] [Google Scholar] [PubMed]
- Chew V, Tow C, Teo M, Wong HL, Chan J, Gehring A, et al. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J Hepatol 2010;52(3):370-9.
[Crossref] [Google Scholar] [PubMed]
- Yang J, Li H, Hu S, Zhou Y. ACE2 correlated with immune infiltration serves as a prognostic biomarker in endometrial carcinoma and renal papillary cell carcinoma: Implication for COVID-19. Aging (Albany NY) 2020;12(8):6518-35.
[Crossref] [Google Scholar] [PubMed]
- Ihle CL, Owens P. Integrating the immune microenvironment of prostate cancer induced bone disease. Mol Carcinog 2020;59(7):822-9.
[Crossref] [Google Scholar] [PubMed]
- Goh M, Lim ZM, Koh V, Lum J, Zhang X, McGovern N, et al. Single-cell analysis of immune-microenvironment and immune-tumor interaction in human gastric cancers. J Clin Oncol 2019;37(4):29.
- Hua L, Xia H, Zheng W. The Association between immune infiltration and clinical phenotypes and prognosis of prostate cancer. Iran J Biotechnol 2020;18(4):e2538.
[Crossref] [Google Scholar] [PubMed]
- Javani AF, Fazilati M, Azadeh M, Ghaedi K. rs12977 of RAC1 is associated with the progress of gastric cancer in Iranian population. Gene Rep 2021;23:101069.
- Li JP, Zeng SH, Zhang YH, Liu YJ. Bioinformatics-based analysis of the association between the A1-chimaerin (CHN1) gene and gastric cancer. Bioengineered 2021;12(1):2874-89.
[Crossref] [Google Scholar] [PubMed]
- Xue C, Chen C, Gu X, Li L. Progress and assessment of lncRNA DGCR5 in malignant phenotype and immune infiltration of human cancers. Am J Cancer Res 2021;11(1):1-13.
[Google Scholar] [PubMed]
- P?un I, Costin AI, Constantin VD, Lomaca I, Iano?i NG, Socea B, et al. Gastric cancer–histopathological correlations between tumor and non-tumor gastric mucosa changes. Rom J Morphol Embryol 2020;61(4):1129-41.
[Crossref] [Google Scholar] [PubMed]
- Ho KH, Huang TW, Shih CM, Lee YT, Liu AJ, Chen PH, et al. Glycolysis-associated lncRNAs identify a subgroup of cancer patients with poor prognoses and a high-infiltration immune microenvironment. BMC Med 2021;19(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Wang J, Qian Z, Han Y. CCR5 is associated with immune cell infiltration and prognosis of lung cancer. J Thorac Oncol 2019;14(5):e102-3.
[Crossref] [Google Scholar] [PubMed]
- Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer 2012;12(4):298-306.
[Crossref] [Google Scholar] [PubMed]
- Edlund K, Madjar K, Mattsson JS, Djureinovic D, Lindskog C, Brunnström H, et al. Prognostic impact of tumor cell programmed death ligand 1 expression and immune cell infiltration in NSCLC. J Thorac Oncol 2019;14(4):628-40.
[Crossref] [Google Scholar] [PubMed]
 Immunity
Immunity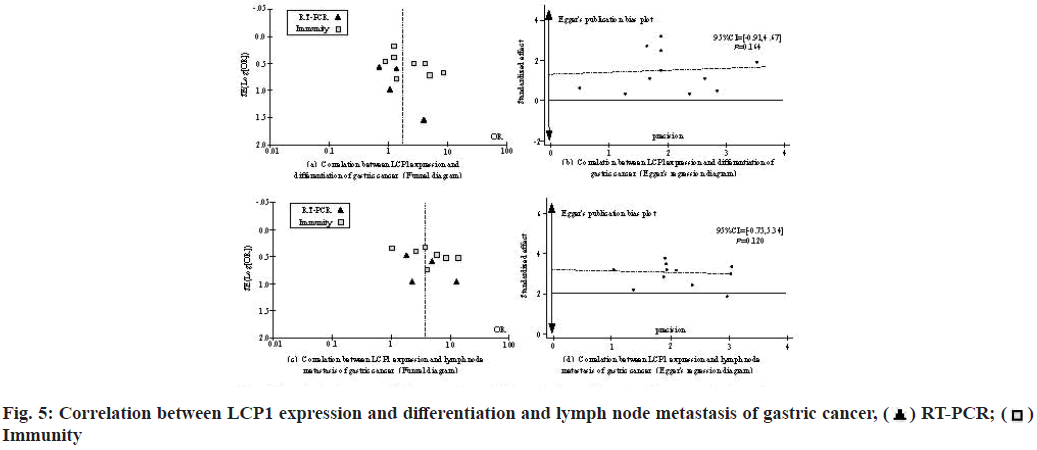
 Immunity
Immunity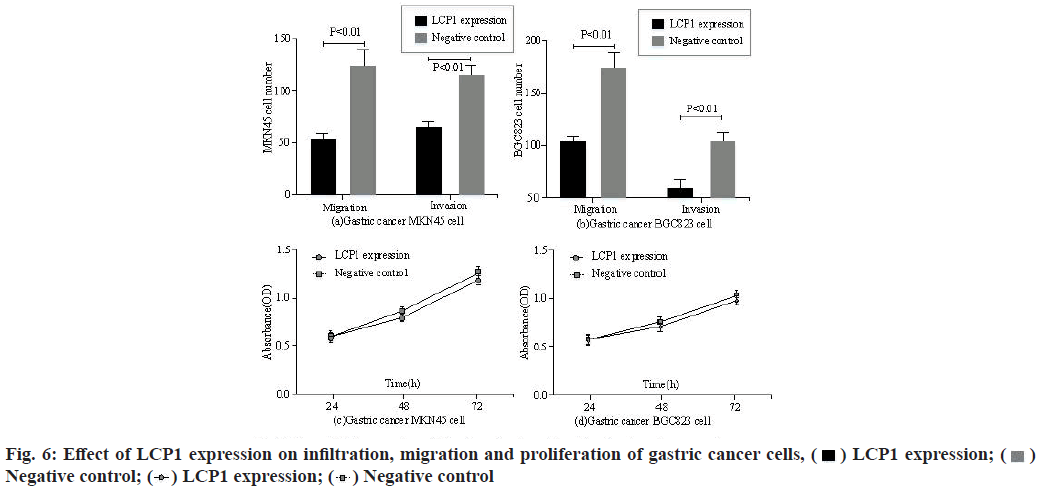

 LCP1 highexpression
LCP1 highexpression



