- *Corresponding Author:
- Swati Dhande
Department of Pharmacology, Bharati Vidyapeeth College of Pharmacy, Navi Mumbai, Maharashtra 400614, India
E-mail: swati.dhande@bvcop.in
| Date of Received | 23 December 2021 |
| Date of Revision | 06 September 2022 |
| Date of Acceptance | 01 August 2023 |
| Indian J Pharm Sci 2023;85(4):1163-1168 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The goal of this research is to examine the pharmacokinetics of Zandopa® in rats after oral administration of Zandopa® herbal formulation. Acclimatization of rats was done for 7 d before the study. The study consisted of 24 rats which had been separated equally into two groups i.e. vehicle control and Zandopa® control group with 12 animals in each group. The drug was administered via oral route in male Sprague Dawley rats. The vehicle control group rats were dosed with 1 % carboxymethyl cellulose and Zandopa® control group with 775 mg/kg of Zandopa® in 1 % carboxymethyl cellulose. Food was with-held for a further 1 h. The blood sample was taken between 0, 0.5, 1, 2, 4, 6, 8, 12 and 24 h time intervals after treatment with the respective drug. Plasma samples were prepared by centrifugation and concentration was measured by high performance liquid chromatography. Urine was collected at a time interval of 0-1, 1-4, 4-8, 8-24, 24-48 h after treatment with the respective drug, and kinetic parameters were measured. Then animals were sacrificed using isoflurane overdose and the liver was isolated and stored for metabolism study. The Cytochrome P-450 concentration and Cytochrome P-450 content was found to be 0.0012 mM and 1.2 nmol/mg/protein compared with the vehicle control group. The cumulative amount excreted was found to be 64.66 mg. The study of the pharmacokinetics of Zandopa® in rats was shown to be significant as compared to the vehicle control group.
Keywords
Zandopa®, high performance liquid chromatography, absorption, excretion, metabolism
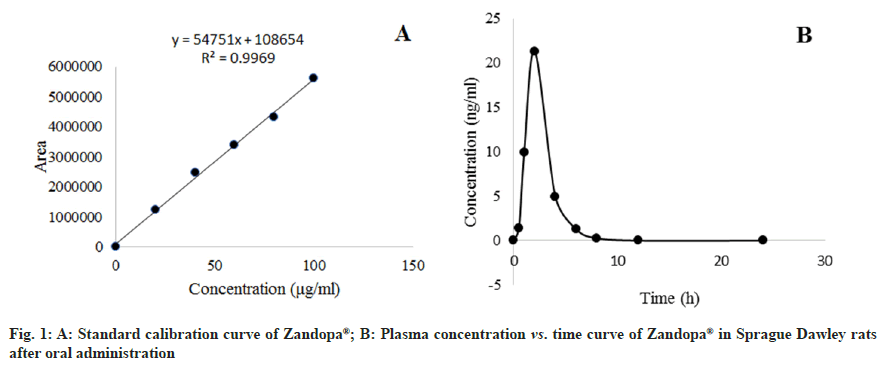
Except for Allopathy, herbal drugs account for a significant portion of all recognized Indian health systems, including Ayurveda, Yoga, Unani, Siddha, Homeopathy and Naturopathy[1]. Herbal formulation is more effective than conventional medicines. It is cheaper and with fewer side effects. Many of them are also known to have healing properties that were used for centuries before modern pharmaceuticals were developed. Herbal medicines are often prescribed for common ailments like cold, cough, pain and anxiety[2]. A natural remedy, herbal formulation, or herbal medicine is any mixture of botanical products that are used for medicinal purposes. There are many herbs that can be used in natural remedies and formulations[3,4]. Levodopa is said to be one of the components of Mucuna pruriens[5]. In Ayurveda, the beans are used to heal neurological ailments and arthritis as well as being a potent aphrodisiac. Mucuna pruriens seed powder is sold under the brand name Zandopa®. It contains natural levodopa, which is more effective. There are many studies, Mucuna pruriens seed extract has proven to be a successful treatment for Parkinson's disease[6]. The activity of the body on the medication, which includes absorption, distribution, metabolism, and excretion, is known as pharmacokinetics. Absorption is the movement of drug molecules from the site of administration to blood circulation. Once absorbed, a drug may be distributed to the various organs of the body. Distribution describes the reversible diffusion or transfer of drug molecules from intravascular space to body tissues. Metabolism describes the conversion of drugs into metabolites by enzymes. The major site of metabolism is the liver. Elimination is the irreversible loss of a drug from the body by metabolism or excretion. Studying the pharmacokinetics of any drug requires the measurement of drug concentration in a suitable biological fluid such as plasma, serum, blood or urine at appropriate time intervals using a valid analytical method[7]. The activity of the body on the medication, which includes absorption, distribution, metabolism, and excretion, is known as pharmacokinetics[7]. Herbal medications are mostly utilised in drug development, determining drug concentration in the body, and determining drug efficacy. Today, they have become more popular for various medicinal purposes[8]. Though herbal formulations are said to be safe, there is a need for detailed pharmacokinetic profiling of herbal formulations to enhance its effect and its active function in the body. A pharmacokinetics approach to herbal formulations reveals the dynamic processes of active components including their absorption, distribution, metabolism, and excretion which offer guidance for rational uses. This approach may help for better therapeutic outcomes with the herbal formulation and help understand the risk to benefit aspect when to be administered concomitantly with other medications. One such attempt is made in this study wherein the pharmacokinetic profiling of Zandopa® was carried out. This study will enlighten and form the basis for understanding and predicting the therapeutic effect of this formulation alone or the probable interaction when administered concomitantly with the available Allopathic drug regimen. Zandopa® herbal formulation manufactured by Emami Limited, Silavasea, marketed by Zandu, batch Number-EN0010 (7.5 g powder of Mucuna pruriens containing 250 mg levodopa) was purchased from the medical store. In this study analytical-grade chemicals and reagents are used. Male Sprague Dawley rats weighing 120-150 g were taken from the Bombay Veterinary College Parel, Mumbai. In the animal house of Bharati Vidyapeeth College of Pharmacy, Navi Mumbai, the rats were housed in a 12:12 h light-dark cycle, at a temperature of 23±2° and a humidity of 50-65 %. Throughout the study, the rats were fed with standard rat pellets and water ad libitum. The Institutional Animal Ethics Committee approved the study (Protocol Number: BVCP/IAEC/02/2020), and all animal studies being performed in accordance with Committee for the Purpose of Control and Supervision of Experiments on Animals regulations (CPCSEA). The study consisted of 24 rats which were divided equally into two groups i.e. vehicle control and Zandopa® control group with 12 animals in each group. The drug was administered via oral route in male Sprague Dawley rats. The vehicle control group rats were dosed with 1 % carboxymethylcellulose (CMC) and Zandopa® control group with 775 mg/kg of Zandopa® in 1 % CMC. In the oral study, approximately 500 μl of the blood samples were collected from retro-orbital plexus of rats at 0, 0.5, 1, 2, 4, 6, 8, 12 and 24 h after treatment with the respective drug. Blood samples were collected in pre-labeled Ethylenediaminetetraacetic Acid (EDTA) tubes at respective sampling time points. The blood samples were centrifuged for 5 min at 14 000 revolutions per minute (rpm) at 4° to collect the plasma. A Shimadzu High Performance Liquid Chromatography (HPLC) system with a 600E HPLC pump, RP-18, 5 μm, 250×4.6 mm (Supelco) column, an autosampler, and Ultraviolet (UV) detector was used for chromatography. The analyte was detected at 280 nm, and the column temperature was maintained at 40°. The separation was carried out at a flow rate of 0.5 ml/min using a mobile phase comprising methanol, acetonitrile, and water (pH 4.0) in a 20:30:50 ratio[9]. The calibration curve was constructed using Zandopa® standard solution. The Zandopa® retention time was found to be 7.6 min. In each marked tube, a methanolic solution of Zandopa® (25 μl) at a concentration of 100 μg/ml was added to 250 μl of plasma (in quadruplicates) except to the control tubes. For each configuration of the solvent system, 25 μl of methanol was supplied to the control tube. On a vortex mixer, tubes were vortexed for 1 min. The tubes were divided into 3 groups for extraction using 4 different solvents viz., methanol, acetonitrile, tetrahydrofuran and diethyl ether. Each tube was filled with 1 ml of each solvent and vortexed thoroughly over 2 min at high speed. The tubes were centrifuged for 10 min at -5° at 5500 rpm. The organic layers were decanted into a separate set of tubes that had already been labelled. The tubes were dried at 35° in a solvent evaporator. The remaining residues in the tubes were dissolved in 0.5 ml mobile phase and vortexed for 1 min. The samples were filtered into HPLC vials using a 0.45 μm syringe filter, and the HPLC procedure was used to analyse them. Because acetonitrile provided the best recovery of Zandopa® from the plasma matrix, it was chosen as the recovery solvent for further testing. Plasma samples (250 μl) were extracted with 500 μl acetonitrile. Using the HPLC method, the concentration of Zandopa® in plasma was calculated. The samples were tested using HPLC to determine the concentration of Zandopa® in the plasma at various time points after drug administration. The different concentrations of Zandopa® were made by dissolving 1 mg of Zandopa® in 1 ml of methanol to make a 1 mg/ml solution. The stock was serially diluted with methanol to produce concentrations ranging from 0.1 μg/ml to 100 μg/ml. The study of various concentrations produced a calibration curve. The sample concentration was calculated from the peak area using the linear regression equation established from the calibration curve. Plasma concentration of Zandopa® was calculated using calibration curve (peak area vs. concentration) using Microsoft excel 2019 version 2110 (Build 14527.20276). The plasma concentration-time profile was used to determine Cmax and Tmax. From 0 to the last blood sample time (t), Area under the ROC Curve (AUC) values were determined using a linear trapezoidal method. The terminal plasma elimination half-life (t1/2) was computed as 0.693/λz, where λz was calculated using linear regression of the plasma concentration-time curve's terminal log-linear phase. After scarification of animals, the liver was isolated immediately. The liver was given thorough washing with ice-cold saline and refrigerated until further analysis. The excised livers were carefully chopped with scissors, and homogenized in a homogenizer with equal volumes i.e. for 4 g of liver 4 ml of icecold 10 mM Tris-HCL buffer with 0.25 M sucrose, pH 7.4. The homogenate was centrifuged at 13 000 rpm for 10 min at 4° in a cooling centrifuge, and the precipitate was discarded. To obtain a final concentration of 10 mM, 1.47 g calcium chloride was added to the supernatant (10 ml) with steady stirring. After stirring for 15-20 min, the solution was centrifuged at 25 000 rpm for 10 min at 4°. To get the microsomes, the supernatant was removed and the residue was homogenised in 100 mM Tris- HCL buffer containing 20 % w/v glycerol and 10 mM EDTA at pH 7.4. The microsomes were stored in a deep freezer until use. The whole microsomal isolation procedures were carried out in an ice bath at a temperature of 0-4°[10]. Dilute microsomes in 0.2 M phosphate buffer, pH 7.4, with 0.5 % Triton X-100 and 1 mM EDTA to 1 mg/ml. The mixture was thoroughly mixed before being divided into two 3 ml glass cuvettes. By heating formic acid with concentrated sulfuric acid, carbon monoxide gas was produced. 30 to 40 carbon monoxide bubbles were sprayed into the sample and reference cuvettes containing the microsomal preparations. To achieve a reduced carbon monoxide vs oxidised carbon monoxide difference spectrum, sodium dithionite (4 mg) was applied just to the sample cuvette. The extinction coefficient (450-490 nm) for cytochrome P450 (CYP) is determined using the formula CYP concentration (mM)=A/ε, where, A=A 450-A 490, the absorbance difference between 450 and 490 nm and ε=the extinction coefficient (450-490 nm) having the value 91 mM-1cm-1. To get the CYP content in the original undiluted sample, multiply the CYP concentration (mM) in the diluted sample by the dilution factor[11]. Total urine samples were taken from respective groups at 0-1, 1-4, 4-8, 8-24, and 24- 48 h following an oral dosage of 775 mg/kg and analysed for drug concentration using a UV spectrophotometer at 280 nm. To eliminate the matrix, a urine sample was diluted 10 fold with deionized water. The addition of acetonitrile to urine samples precipitated proteins; 1 ml of urine was combined with 9 ml of acetonitrile to precipitate proteins from the specimen, which was vortexed for 3 min. The dilution procedure at the experimental stage was taken into account in the calculations. Thus, the data collected was the volume of urine collected and the drug concentration in urine during each interval[12,13]. The rate of excretion and the total amount excreted were estimated after analysing urine data (Table 1). Statistical evaluation among groups was carried out using Paired T-test and p<0.05 was considered significant. The linear regression equation was Y=54751 x+108654 with (r2 = 0.9969). The linear range of Zandopa® was found to be sufficient for this approach to be employed in current pharmacokinetic research (fig. 1A). With diverse solvents, such as methanol, acetonitrile, tetrahydrofuran, and diethyl ether, the mean percentage recovery of Zandopa® was 64.07 %, 72.5 %, 34.43 %, and 21.54 %, respectively. Because acetonitrile yields the highest recovery of Zandopa®, it was chosen as the solvent for extracting Zandopa® from the plasma matrix produced after oral dosing. After oral administration of Zandopa® to rats, the concentration in plasma was measured at various time intervals (fig. 1B). At 2 h after oral treatment, the highest plasma concentration of Zandopa® was reported to be 21.34±1.20 ng/ml. The AUC(0-t) of Zandopa® in Sprague Dawley rats following oral treatment was 53.64±1.45 ng.h/ml. The pharmacokinetic parameters obtained were t1/2 as 1.06±0.01 h, Tmax at 2 h and Cmax as 21.34±1.20 ng/ml upon oral administration of Zandopa® (Table 2). In the vehicle control group, CYP concentration and CYP content was found to be 0.0025 mM and 2.3 nmol/mg/protein respectively. In the Zandopa® control group, CYP concentration and CYP content was found to be 0.0012 mM and 1.221 nmol/mg/ protein respectively (Table 3).
| Parameters | Groups | |
|---|---|---|
| Zandopa® control group | Vehicle control group | |
| T1/2 (h) | 1.06±0.01* | 0 |
| Tmax (h) | 2.00±0* | 0 |
| Cmax (ng/ml) | 21.34±1.20* | 0 |
| AUCo-t (ng.h/ml) | 53.64±1.45* | 0 |
Note: T½: half-life; Tmax: time of peak concentration; Cmax: peak plasma concentration; AUC: area under curve; Value is expressed as mean SEM; n=3, where n is the number measurement in a triplicate set of animals; *p<0.05 as compared with Zandopa® control group by Paired T-test
Table 1: The Absorption Studies of Zandopa® in Sprague Dawley Rat Plasma after Oral Administration
| Groups | CYP concentration | CYP content |
|---|---|---|
| (mM) | (nmol/mg/protein) | |
| Vehicle | 0.0025±0.00023 | 2.302±0.1608 |
| Zandopa® | 0.0012±0.00013* | 1.221±0.0876* |
Note: Value is expressed as mean SEM; n=12, where n is the number of animals per group; *p<0.05 as compared with Zandopa® control group by Paired T-test
Table 2: Cyp Concentration and Cyp Content in Vehicle Control and Zandopa® Control Group
| Time interval (h) | Urine Concentration | Amount Excreted | Cumulative Amount Excreted | Rate of Excretion |
|---|---|---|---|---|
| (mg/ml) | ΔU (mg) | U (mg) | ΔU/Δt (mg/h) | |
| Vehicle | ||||
| 0-1 | 0.001±0.001 | 0.003±0.001 | 0.002±0.001 | 0.000±0.001 |
| 1-4 | 0.003±0.001 | 0.007±0.001 | 0.009±0.001 | 0.002±0.001 |
| 4-8 | 0.011±0.009 | 0.023±0.019 | 0.033±0.018 | 0.002±0.001 |
| 8-24 | 0.158±0.014 | 0.316±0.029 | 0.348±0.039 | 0.014±0.001 |
| 0-1 | 0.002±0.001 | 0.003±0.001 | 0.002±0.001 | 0.000±0.001 |
| Zandopa® | ||||
| 0-1 | 07.34±0.44 | 14.70±0.88 | 14.66±0.88* | 4.89±0.29* |
| 1-4 | 13.17±0.44 | 26.30±0.88 | 41.00±1.73* | 6.58±0.22* |
| 4-8 | 10.34±0.72 | 20.70±1.45 | 58.33±4.48* | 1.29±0.09* |
| 8-24 | 02.34±0.44 | 04.70±0.88 | 63.00±5.03* | 0.19±0.04* |
| 24-48 | 0.84±0.17 | 01.70±0.34 | 64.66±5.17* | 0.03±0.01* |
Note: Value is expressed as mean SEM; n=3, where n is the number measurement in a triplicate set of animals; *p<0.05 as compared with Zandopa control group by Paired T-test
Table 3: Data Analysis of Vehicle Control and Zandopa® Control Group in Urine
The total amount excreted was found to be 64.66 mg and 0.43 mg in the Zandopa® control group and vehicle control group respectively. There was a significant difference found between the vehicle and Zandopa® control group for total amount excreted and rate of excretion. The administration of a drug aids in the achievement of a certain therapeutic aim[14]. The current study looked at the pharmacokinetics of Zandopa® following oral treatment in Sprague Dawley rats. The current Zandopa® pharmacokinetic study was split into three sections that is absorption, metabolism, and excretion. Acetonitrile was chosen as the recovery solvent for oral pharmacokinetic studies because it showed the highest percentage recovery of Zandopa®. The drug concentration in the samples was below the detection limit after 10 h of oral treatment. The rate at which a drug is eliminated from the body is affected by its metabolic biotransformation, which can have a considerable impact on its efficacy and safety[15]. The CYP enzyme substrates account for around 60 % of all marketed drugs[16]. In this study, 53 % of the reduction was found in the levels of the CYP enzyme as compared to the vehicle control group indicating the metabolism of the drug took preferentially by CYP enzymes. In the Zandopa® control group, CYP content was found which is nearly half of the value of the vehicle control group. In this study, a significant (p<0.05) drug in the urine sample of the Sprague Dawley rats was found. The rate of excretion for the Zandopa® control group was more prominent than the vehicle control group after the oral administration. The cumulative amount excreted in the Zandopa® control group shows a significant difference as compared to the vehicle control group. By correlating enzyme activity of a specific CYP, rat liver microsomes are beneficial in identifying crucial CYP engaged in the biotransformation of the medication. The liver is the primary site of drug metabolism, the majority of drugs must pass through it. A set of CYP enzymes is the primary mechanism by which the liver metabolises drugs[11]. Levodopa present in Zandopa® formulation is decarboxylated extensively by the high affinity amino acid decarboxylase enzyme also called dopa decarboxylase in the first pass through the liver. The CYP metabolism is a major metabolic pathway for the clearance of orally administered levodopa[10]. Hence, we can correlate why the CYP concentration and CYP content was found decreased as compared to normal in this study. In conclusion, the role of pharmacokinetics in drug development is increasingly important. In herbal drugs, the availability of accurate pharmacokinetic and metabolic data is a must. Thus, in the present study, we were able to outline the maximum concentration, half-life, absorption pattern in terms of area under the curve, metabolism by CYP enzyme and preferential excretion by urine. This data will serve as basics when the interaction of Zandopa® is to be predicted with other classes of drugs or food on concomitant use and hence design the therapeutic regimen for Parkinson’s patients.
Conflict of interests:
The authors declared no conflict of interests.
References
- Vaidya AD, Devasagayam TP. Current status of herbal drugs in India: An overview. J Clin Biochem Nutr 2007;41(1):1-1.
[CrossRef] [Google Scholar] [PubMed]
- Ekor M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 2014;4:177.
[CrossRef] [Google Scholar] [PubMed]
- Pandey MM, Rastogi S, Rawat AK. Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid Based Complement Alternat Med 2013;2013.
[CrossRef] [Google Scholar] [PubMed]
- Sofowora A, Ogunbodede E, Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med 2013;10(5):210-29.
[CrossRef] [Google Scholar] [PubMed]
- Maldonado RG. Mucuna and Parkinson’s disease: Treatment with natural levodopa. In: Parkinson's Disease-Understanding Pathophysiology and Developing Therapeutic Strategies 2018.
- Lampariello LR, Cortelazzo A, Guerranti R, Sticozzi C, Valacchi G. The magic velvet bean of Mucuna pruriens. J Tradit Complement Med 2012;2(4):331-9.
[CrossRef] [Google Scholar] [PubMed]
- Bhattaram VA, Graefe U, Kohlert C, Veit M, Derendorf H. Pharmacokinetics and bioavailability of herbal medicinal products. Phytomedicine 2002;9:1-33.
[CrossRef] [Google Scholar] [PubMed]
- Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules 2016;21(5):559.
[CrossRef] [Google Scholar] [PubMed]
- Ahmad MZ, Hamid KA, Effendi TJB. A validated high-performance liquid chromatographic method for the determination of levodopa in rat plasma its application in pharmacokinetic studies. Eng Ind Appl 2012;9:134–9.
- Walawalkar P, Serai P, Iyer K. Isolation and catalytic competence of different animal liver microsomal fractions prepared by calcium-aggregation method. Indian J Pharm Sci 2006;68(2).
- Hill JR. In vitro drug metabolism using liver microsomes. Curr Protoc Pharmacol 2003;23:1-11.
- Ertokus GP. The determination of Parkinson’s drugs in human urine by applying chemometric methods. Int J Anal Chem 2019;2019.
[CrossRef] [Google Scholar] [PubMed]
- David WA. Analysis of Urine Data. Analysis 2010;5:1–23.
- Jia L, Liu X. The conduct of drug metabolism studies considered good practice (II): In vitro experiments. Curr Drug Metab 2007;8(8):822-9.
[CrossRef] [Google Scholar] [PubMed]
- Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 2013;138(1):103-41.
[CrossRef] [Google Scholar] [PubMed]
- Ghiculescu RA. Therapeutic drug monitoring: Which drugs, why, when and how to do it. Aust Prescr 2008;31(2).