- *Corresponding Author:
- Y. C. Lee
Department of Internal Medicine, Research Center for Pulmonary Disorders, Chonbuk National University Medical School, 560-182, Republic of Korea
E-mail: leeyc@jbnu.ac.kr
| Date of Submission | 16 June 2016 |
| Date of Revision | 04 April 2017 |
| Date of Acceptance | 15 October 2017 |
| Indian J Pharm Sci 2017;79(6): 994-1000 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Type 2 helper T cell-predominant asthma is a refractory type of asthma that has motivated biomedical industries to develop novel drugs. The phosphoinositide 3-kinase pathway has been implicated in asthma and chronic obstructive pulmonary disease. IC87114 (2-[(6-aminopurin-9-yl) methyl]-5-methyl-3-[2-methylphenyl]quinazolin-4-one) was recently developed as a PI3Kδ inhibitor. In a neutrophil type 2 helper T cell dominant asthma model, pharmacokinetic analysis of IC87114 was performed. Following intratracheal administration of 1 mg/kg IC87114 to ovalbumin/lipopolysaccharide-sensitized/challenged mice, airway hyper-responsiveness and structural analysis was performed and the plasma concentration time course and bronchoalveolar lavage fluid concentration of IC87114 were described using a compartment model with a first-order elimination rate constant. Plasma had a three-fold higher Cmax compared to bronchoalveolar lavage fluid. The half-life of IC87114 in plasma and bronchoalveolar lavage fluid was 2.37 and 10.25 h, respectively. Enhanced airway hyper-responsiveness and increased peribroncholar and perivascular inflammatory cell infiltrates in the lungs were significantly reduced in the IC87114-administered group compared with the ovalbumin/lipopolysaccharide-exposed group. Modeling approaches sufficiently explained the relationship between systemic circulation and bronchoalveolar lavage fluid concentration of IC87114 with Kbronchoalveolar lavage/plasma (approximately 0.38 h-1) in ovalbumin/lipopolysaccharide-sensitized/challenged mice. Our results will be useful for understanding the relationship between the pharmacokinetics and pharmacodynamics of IC87114.
Keywords
IC87114; pharmacokinetic; bronchoalveolar lavage fluid; compartmental
Asthma is a chronic airway disease that originates in childhood and is related to allergies, dysregulated type 2 helper T cells (TH2) inflammatory response, and increased eosinophils. Classically, the best treatment for asthma is to target inflammation. However, there is accumulating evidence regarding the complexity of asthmatic populations with steroid-resistant asthma and difficult or refractory asthma that do not exhibit typical asthmatic features [1,2]. In fact, severe asthma might be mediated by neutrophils [3,4]. Thus, unmet medical needs related to refractory asthma have motivated biomedical industries to develop novel drugs.
The phosphoinositide 3-kinase (PI3K) pathway has been implicated in many diseases including cancer, rheumatoid arthritis, asthma, and chronic obstructive pulmonary disease. PI3Ks phosphorylate inositol lipids within cell membranes as an early step in the signalling cascades initiated by numerous ligand-receptor interactions [5]. Thus, PI3Ks have been the focus of intensive drug discovery and development efforts. The discovery of the quinazolinone purine series, which is exemplified by ICOS Corporation (Bothell, WA) compound IC87114 (2-[(6-aminopurin-9-yl) methyl]-5-methyl-3-[2-methylphenyl]quinazolin-4- one) [6], indicates the feasibility of targeting PI3Ks. In an ovalbumin (OVA) model of asthma, treatment with PI3Kδ inhibitor IC87114 reduces eosinophil recruitment [7,8]. More recently, it has become apparent that pharmacological inhibition of PI3Kδ with IC87114 also reduces IL-17 levels in an asthma mouse model [9]. In addition, IC87114 blocks TNF1α-stimulated elastase exocytosis from neutrophils [10].
Pharmacokinetic studies are required to develop practical guidelines for newly developed agents. Although pharmacokinetic studies of IC87114 have been performed [11-13], they focused on sensitive liquid chromatography with tandem mass spectrometry (LCMS/ MS) method development, and thus, in this study, pharmacokinetic analysis was performed with normal rats to obtain analytical information.
Intratracheal drug delivery is preferred for asthma patients, although systemic distribution is observed under organ-specific delivery conditions. In this study, we performed a pharmacokinetic study of IC87114 following intratracheal administration of 1 mg/kg in a neutrophil-dominant TH2-type asthma model. In addition, we developed a pharmacokinetic model to explain the relationship between plasma concentration and bronchoalveolar lavage (BAL) fluid concentration.
Materials and Methods
IC87114 was purchased from Calbiochem (San Diego, CA, USA). Acetaminophen, phosphate-buffered saline (PBS), OVA, lipopolysaccharides (LPS), and methacholine (MeCh) were purchased from Sigma (St. Louis, MO, USA). Isoflurane was purchased from Hana Pharm. Co. Ltd (Seoul, South Korea). Similarly, ketamine was purchased from Yuhan Pharmaceuticals (Seoul, South Korea) and xylazine was purchased from Bayer Korea (Seoul, South Korea) respectively. Water was purified using the Milli-Q system from Millipore (Bedford, MA, USA). LC-MS-grade acetonitrile, methanol, and formic acid were purchased from Fisher Scientific (Fair Lawn, NJ, USA).
Experimental animals
All experiments were conducted using adult (8-10 w old, 20-23 g weight) female C57B/L6 mice purchased from Damul Sciences (Dajeon, Korea). The mice were placed in cages (5 mice per cage) and were kept in a fully acclimatized room at constant temperature and humidity on a 12:12-h light/dark cycle, with ad libitum feeding. The animal ethics committee at Chonbuk National University of Korea approved all experiments (CBU 2013-0025) on the basis of the 3 R’s (replacement, refinement and reduction). Animals were deeply anesthetized with isoflurane before being killed by cervical dislocation. Efforts were made to minimize the number of animals used and their suffering.
Mouse asthma model protocol
All mice were sensitized intranasally with 75 μg OVA plus 10 μg LPS on days 0, 1, 2, 3 and 7 and challenged with OVA alone on days 14, 15, 21 and 22. On day 22 (3 h after the last airway challenge with OVA), 1 mg/kg IC87114 or vehicle (0.05 % dimethyl sulfoxide, DMSO) diluted with 0.9 % NaCl, was administered in a volume of 30 μl by a nonsurgical intratracheal method [14].
Nonsurgical intratracheal method
Mice were given a single intratracheal dose of IC87114 (1 mg/kg). We selected this dose based on previous reports confirming its efficacy in asthma models [8,10]. Mice were lightly anesthetized with gaseous isoflurane (Matrix, Orchard Park, NY) and placed in a supine position on an angled board with their necks extended for intratracheal administration. The tongue of the mice was gently pulled outwards using forceps. With the other hand, a 1-ml syringe containing the drug, fitted with a bent gavage needle, was inserted into the trachea and the plunger was pushed to deliver the drug. Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) intraperitoneally before collecting blood. Blood samples were collected retroorbitally using a capillary tube 0, 0.083, 1, 2, 4, 8, 12, 18, 24, 48 and 72 h after dosing and mice were euthanized using cervical dislocation. The chest cavity was exposed to allow expansion, after which the trachea was carefully incubated and catheter secured with ligatures. One millilitre of 0.9 % NaCl was slowly infused into the lungs and withdrawn. Six animals were sacrificed at each time point.
Measurement of airway hyper reactivity (AHR)
AHR was assessed by whole-body plethysmography (OCP 3000, Allmedicus, Seoul, South Korea), and airflow obstruction was induced with a MeCh aerosol spray. Each group of mice, DMSO or OVA/LPS with or without IC87114 was exposed for 3 min to aerosolized saline followed by exposure to increasing concentrations of aerosolized MeCh (0, 6.25, 12, 25 and 50 mg/ml) dissolved in isotonic saline. Following each nebulization, the enhanced pause (PE) was recorded for 3 min. PE values measured during each 3-min sequence were averaged and evaluated for each dose of MeCh. PE data were plotted as the change from baseline per dose of MeCh in PBS (0, 6.25, 12, 25, and 50 mg/ml) nebulized through the inlet of the main chamber. Data were recorded using FinePoint software version v2.4.6 (Buxco® Research Systems). Each dose was nebulized for 2 min and the ensuing airway response was recorded for 5 min. The percent increase in PE over baseline PE was calculated for each concentration of MeCh, and the values were used to compare results among experimental groups.
Analysis of BAL samples
Mice were euthanized via cervical dislocation. The abdominal cavity was opened and the chest cavity was dissected to expose the lung and trachea for expansion. The trachea was exposed by a 1 cm incision on the ventral neck skin for insertion of the cannula. A 1-ml volume of 0.9 % NaCl was slowly infused into the lungs and withdrawn. Six animals were sacrificed at each time point. BAL samples (1 ml) were centrifuged (600× g, 3 min) and the supernatants were stored at –20° for cytokine analysis. The cell pellets from both samples were pooled for total counts (model Z1, Beckman-Coulter, Miami, FL, USA) after lysis of erythrocytes (Zap-Oglobin II, Beckman-Coulter, Fullerton, CA, USA). Slides were loaded with 1×105 cells, centrifuged (Eppendorf, 5415R; 700× g, 3 min), and stained with Diff-Quick (Baxter, Detroit, MI, USA). Differentials (300 cells) were counted under light microscopy (Jena, Germany). The concentration of IC87114 in plasma and BAL fluid was measured using a previously reported LC-MS/MS method [12].
Non-compartmental pharmacokinetic analysis
The validated method was applied to a pharmacokinetic study in mice. Blood and BAL fluid samples were collected at 0, 0.083, 1, 2, 4, 8, 12, 18, 24, 48 and 72 h. Non-compartmental pharmacokinetic analyses were performed with WinNonlin Standard Edition software, version 5.3 (Pharsight Corp., Mountain View, CA, USA). The area under the plasma and BAL fluid concentration versus time curves from time zero to the last measurable concentration (AUCt) was calculated using the linear trapezoidal rule and extrapolated to infinity (AUC∞). The peak plasma and BAL fluid concentration (Cmax) and the time to reach Cmax (Tmax) of IC87114 were taken directly from the data. A terminal elimination rate constant (λz) was calculated using log-linear regression of the terminal phase, and the elimination half-life (t1/2) was calculated as 0.693/λz. Apparent total body clearance (Clt/F) and volume of distribution (Vz/F) were calculated as dose/ AUC∞ and dose/(Kel×AUC∞), respectively. All results are presented as the mean±standard deviation (SD).
Compartmental pharmacokinetic analysis
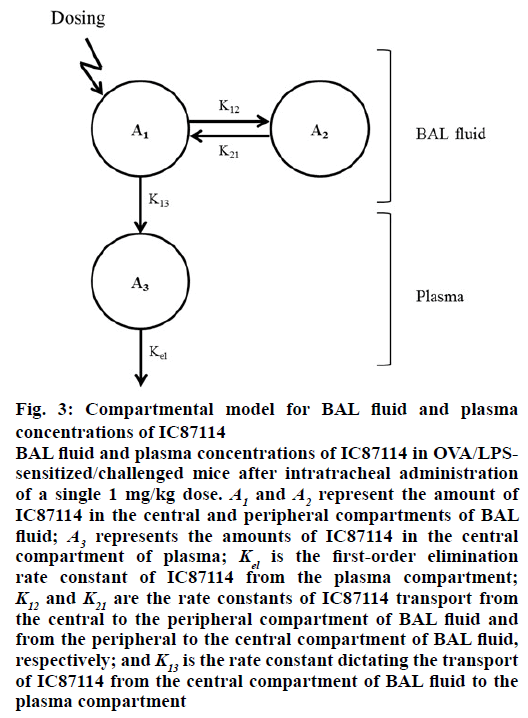
The relationship between plasma concentration and BAL fluid concentration of IC87114 after intratracheal administration in OVA/LPS-sensitized/challenged mice was explained using a BAL fluid-plasma compartment model with first-order rate elimination kinetics. The differential equations used to describe the changes in the amounts of IC87114 in the different compartments following intratracheal administration are given by Eqns. 1, 2, and 3: dA1/dt=K21×A2– (K12+K13)×A1, dA2/dt=K12×A1–K21×A2, dA3/dt=K13×A1– Kel×A3, where A1 and A2 represent the amount of IC87114 in the central and peripheral BAL fluid compartments; A3 represents the amount of IC87114 in the central plasma compartment; Kel is the first-order elimination rate constant of IC87114 from the plasma compartment; K12 and kcp are the rate constant of transport of IC87114 from the central to the peripheral BAL fluid compartment and from the peripheral to the central BAL fluid compartment, respectively; and K13 is the rate constant dictating the transport of the drug from the central BAL fluid compartment to the plasma compartment.
Equations were solved numerically and fitted to the data using ADAPT 5 (Biomedical Simulation Resource, Los Angeles, CA, USA) with maximum likelihood estimation under the assumption that the SD of the measurement error was a linear function of the measured quantity. The BAL fluid concentration (Cb) and plasma concentration (Cp) of IC87114 are as Eqns. 4 and 5: Cb=A1/V1; Cp=A3/V3, where V1 and V2 are the volume of distribution of IC87114 for BAL fluid and plasma, respectively. The final model accommodated the relationship between BAL fluid and plasma concentration of IC87114 in the asthma model.
The following information, obtained using ADAPT 5, was used to evaluate the goodness of fit and the quality of the parameter estimates: visual examination of the distribution of residuals, Akaike’s information criterion, coefficient of variation of parameter estimates, parameter correlation matrices, and sums of squares of residuals. We used a coefficient of variation of <0.5 and a correlation coefficient threshold of 0.9 as criteria for evaluating the numerical identifiability of parameter estimates [15].
Results and Discussion
The calibration curves were linear over the following concentration ranges: 0.01-1000 ng/ml for plasma/ BAL fluid. The calibration curves of IC87114 were satisfactory, with correlation coefficients of 0.9993 and 0.9998 for plasma and BAL fluid, respectively.
Intra- and inter-day precision and accuracy results are presented in Table 1. Precision is presented in terms of the percent relative SD and the accuracy as percent bias. The intra-day precision for the lowest concentration of IC87114 (1 ng/ml) was 9.75 % for plasma and 6.58 % for BAL fluid. Likewise, the inter-day precision was 13.44 % for plasma and 9.79 % for BAL fluid. The intra-day and inter-day variation and accuracy for various concentrations of IC87114 were all within acceptable range. The average extraction recoveries of IC87114 were 93.9-99.9 % for plasma and 93.9-102 % for BAL fluid (Table 2). The matrix effects of IC87114 were 96.4-98.8 % and 97.9-101.0 % for plasma and BAL fluid, respectively.
| Sample | Concentration | Intra-assay | Inter-assay | ||
|---|---|---|---|---|---|
| (ng/ml) | Precision (%RSD) | Accuracy (%bias) | Precision (%RSD) | Accuracy (%bias) | |
| Plasma | 0.1 | 9.75 | 5.01 | 13.44 | 11.01 |
| 10 | 1.26 | 3.32 | 6.73 | 6.78 | |
| 1000 | 1.11 | 2.13 | 3.92 | 3.74 | |
| BAL fluid | 0.1 | 6.58 | 8.60 | 9.79 | 9.12 |
| 10 | 2.12 | 5.67 | 3.18 | 6.30 | |
| 1000 | 1.18 | 3.10 | 2.75 | 5.45 | |
Table 1: Intra and Inter-Day Precision and Accuracy of IC87114 Quantitation
| Concentration | Recovery (%) | Matrix effect (%) | ||
|---|---|---|---|---|
| (ng/ml) | Plasma | BAL fluid | Plasma | BAL fluid |
| 0.05 | 97.8±5.6 | 100.1±5.1 | 96.4±3.2 | 98.2±7.6 |
| 0.5 | 99.9±6.4 | 102.0±3.1 | 98.8±2.5 | 97.9±3.7 |
| 2 | 93.9±2.2 | 93.9±2.6 | 98.1±4.3 | 101.0±7.2 |
Data are presented as mean±standard deviation (SD) for n=5 observations
Table 2: Recovery and Matrix Effect of IC87114 in Plasma and BAL Fluids Samples
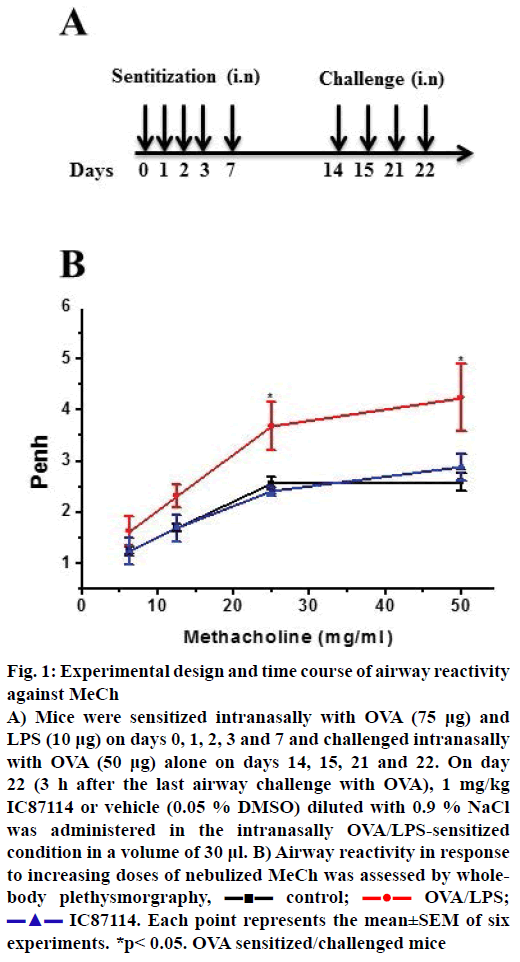
In an initial study of airway hyper-responsiveness, mice underwent the allergy protocol (n=6 per group) followed by aspiration after the last allergen challenge (Figure 1A). In order to determine the effects of IC87114 (a PI3Kδ inhibitor), mice were sampled at 0, 5 min, 1, 4, 8, 12, 18, 24, 48, and 72 h after IC87114 administration. Airway responses to MeCh (12.5 mg/ml) were measured. One hour before exposure to MeCh, the OVA/LPS and OVA/LPS with IC87114 groups showed asthma-like symptoms (data not shown). Expectedly, PE values were significantly increased in response to MeCh in OVA/LPS-sensitized/challenged mice. In contrast, IC87114 exposure significantly reduced PE values in OVA/LPS-exposed mice.
Figure 1: Experimental design and time course of airway reactivity against MeCh
A) Mice were sensitized intranasally with OVA (75 μg) and LPS (10 μg) on days 0, 1, 2, 3 and 7 and challenged intranasally with OVA (50 μg) alone on days 14, 15, 21 and 22. On day 22 (3 h after the last airway challenge with OVA), 1 mg/kg IC87114 or vehicle (0.05 % DMSO) diluted with 0.9 % NaCl was administered in the intranasally OVA/LPS-sensitized condition in a volume of 30 μl. B) Airway reactivity in response to increasing doses of nebulized MeCh was assessed by wholebody plethysmorgraphy,  control;
control;  OVA/LPS;
OVA/LPS;  IC87114. Each point represents the mean±SEM of six
experiments. *p< 0.05. OVA sensitized/challenged mice
IC87114. Each point represents the mean±SEM of six
experiments. *p< 0.05. OVA sensitized/challenged mice
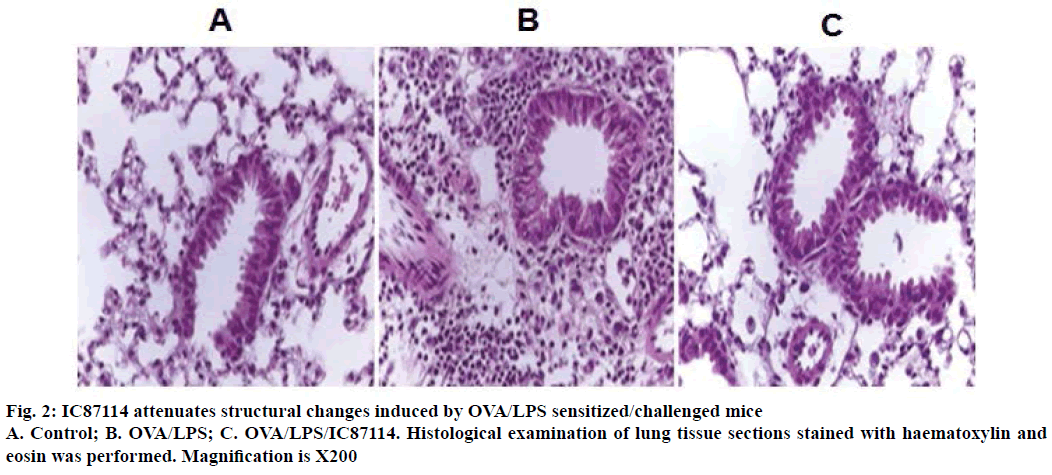
The reduction in airway hyper-responsiveness described above correlated with histological changes in the lung parenchyma. Lungs from OVA/LPSsensitized/ challenged mice exhibited widespread peribronchiolar and perivascular inflammatory cell infiltrates compared with controls (Figure 2). However, the administration of IC87114 resulted in a significant reduction in inflammatory cell infiltration and inhibition of morphological alterations. Together, these results indicate that treatment with IC87114 efficiently inhibited infiltration of inflammatory cells and attenuated allergic airway inflammation.
Cmax values were 816.89±331.96 and 340.24± 204.21 μg/ml for plasma and BAL fluid. Plasma pharmacokinetic data were slightly higher in the present study compared to data observed in a previous study by Marahatta et al. [12]. The plasma sample had a 2.4- fold higher drug concentration compared to BAL fluid. Tmax was delayed in BAL fluid by 0.15 h compared to plasma samples. Plasma IC87114 reached Cmax at 0.57 h post dose and then was eliminated with a terminal half-life of 2.37 h. Subsequently, the ∞BAL fluid concentration of IC87114 gradually decreased with a t1/2 of 10.25 h. AUC72h was 5-fold lower in BAL fluid. Clearance and volume of distribution were 489.25±188.77 ml/h/kg and 1628.58±586.41 ml/kg. Thappali et al. [13] reported a Cmax of 1374 ng/ml at a dose 5 mg/kg. In this study, AUC 0-∞ was 20143 h×ng/ ml, similar to a recent study in a healthy model [12]. The non-compartmental pharmacokinetic parameters of IC87114 are listed in Table 3. Similarly, final estimates of the compartmental parameters are listed in Table 4.
| Parameter (units) | Plasma | BAL fluid |
|---|---|---|
| Tmax (h) | 0.57±0.39 | 0.72±0.99 |
| Cmax (ng/ml) | 816.89±331.96 | 340.24±204.21 |
| AUC72h (h×ng/ml) | 2252.05±699.03 | 422.08±96.92 |
| AUCinf (h×ng/ml) | 2256.76±691.36 | 473.39±185.74 |
| λz (h-1) | 0.31±0.09 | 0.09±0.06 |
| t1/2 (h) | 2.37±0.64 | 10.25±6.42 |
| Clt/F (ml/h/kg) | 489.25±188.77 | N.A. |
| Vz/F (ml/kg) | 1628.58±586.41 | N.A. |
Table 3: Non-Compartmental Pharmacokinetic Parameters of IC87114
| Parameter (units) | Estimates | %CV |
|---|---|---|
| K12 (h-1) | 4.05 | 23.57 |
| K21 (h-1) | 0.77 | 29.37 |
| K13 (h-1) | 1.64 | 26.43 |
| Kel (h-1) | 1.39 | 1.71 |
| V1F(l) | 5.61 | 3.76 |
| V2/F (l) | 0.20 | 24.82 |
Table 4: Final Estimated Parameters from the BAL Fluid–Plasma Compartment Model in Ova-Sensitized/Challenged Mice
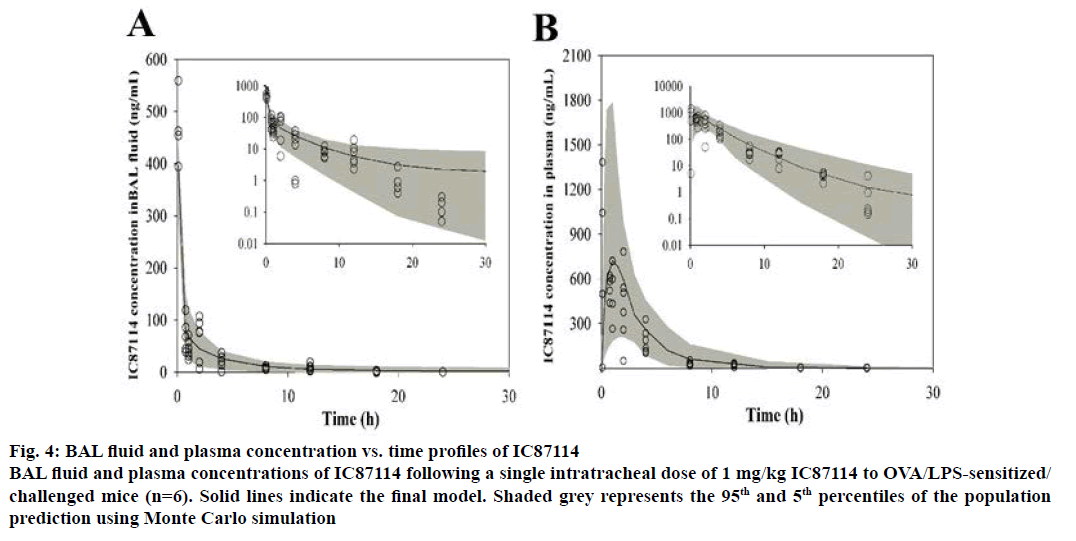
In order to analyse the pharmacokinetics and bronchopulmonary disposition of IC87114, we established a BAL fluid-plasma compartment model using first-order kinetics (Figure 3). The plasma and BAL fluid concentration-time profiles of IC87114 after a single intratracheal dose of 1 mg/kg in OVA/LPSsensitized/ challenged mice are shown in Figure 4. The solid lines represent the resulting fit of the average plasma and BAL fluid concentration-time profiles for IC87114, demonstrating that the measured data were consistent with the compartment model. In addition, we found that a BAL fluid-plasma compartment model with a first-order elimination constant best described the time course of drug concentrations in both BAL fluid and plasma.
Figure 3: Compartmental model for BAL fluid and plasma concentrations of IC87114
BAL fluid and plasma concentrations of IC87114 in OVA/LPSsensitized/challenged mice after intratracheal administration of a single 1 mg/kg dose. A1 and A2 represent the amount of IC87114 in the central and peripheral compartments of BAL fluid; A3 represents the amounts of IC87114 in the central compartment of plasma; Kel is the first-order elimination rate constant of IC87114 from the plasma compartment; K12 and K21 are the rate constants of IC87114 transport from the central to the peripheral compartment of BAL fluid and from the peripheral to the central compartment of BAL fluid, respectively; and K13 is the rate constant dictating the transport of IC87114 from the central compartment of BAL fluid to the plasma compartment
Figure 4: BAL fluid and plasma concentration vs. time profiles of IC87114
BAL fluid and plasma concentrations of IC87114 following a single intratracheal dose of 1 mg/kg IC87114 to OVA/LPS-sensitized/challenged mice (n=6). Solid lines indicate the final model. Shaded grey represents the 95th and 5th percentiles of the population prediction using Monte Carlo simulation
Compartmental pharmacokinetic parameters were calculated using ADAPT 5 software. The BAL fluidplasma compartment model provided a good fit for the data. Time versus BAL fluid concentration curves showed a clear distribution in the peripheral compartment. Thus, a high Kcp/Kpc ratio (estimated to be 5.28) was established to explain rapid distribution into the peripheral compartment [16]. In addition, the relationship between BAL fluid and plasma concentration of IC87114 was explained using the K13 parameter, which was estimated to be 1.64 (26.43 %).
To evaluate the quality of the final model, Monte Carlo simulation with 1000 subjects was performed. The mean pharmacokinetic parameter vector and diagonal elements of the variance components of the analysis were embedded in the PRIOR subroutine of ADAPT 5 software using programs by D’Argenio and Schumitzky [17]. Population simulations were performed using the process noise option. Figure 4 shows the modelpredicted IC87114 concentration averages in BAL fluid and plasma, which described the experimental data well between the 5th and 95th percentiles of the prediction interval.
The pharmacokinetics and bronchopulmonary disposition of IC87114 were investigated using noncompartmental and compartmental approaches in OVA/LPS-sensitized/challenged mice. A BAL fluidplasma compartment model with first-order elimination kinetics was used to describe the relationship between systemic circulation and bronchopulmonary disposition after intratracheal administration of 1 mg/kg IC87114 in OVA/LPS-sensitized/challenged mice. These findings regarding the pharmacokinetic characteristics of IC87114 in a disease animal model will be useful for investigating the relationship between exposure and response to IC87114.
Acknowledgments
This work was supported by the Ministry for Health and Welfare, Republic of Korea (Korea Healthcare Technology R&D Project (A121931).
Conflict of interest
The authors declare that they have no conflict of interest.
Financial support and sponsorship
Nil.
References
- Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society. Am J Respir Crit Care Med 2000;162:2341-51.
- Gamble J, Stevenson M, McClean E, Heaney LG. The prevalence of nonadherence in difficult asthma. Am J Respir Crit Care Med 2009; 180:817-22.
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodelling. Am J Respir Crit Care Med 2000;161:1720-45.
- Chesné J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med 2014;190:1094-101.
- Trevor JL, Deshane JS. Refractory asthma: mechanisms, targets, and therapy. Allergy 2014;69:817-27.
- Myou S, Leff AR, Myo S, Boetticher E, Tong J, Meliton AY, et al. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. J Exp Med 2003;198:1573-182.
- Foster JG, Blunt MD, Carter E, Ward SG. Inhibition of PI3K signalling spurs new therapeutic opportunities in inflammatory/autoimmune diseases and haematological malignancies. Pharmacol Rev 2012;64:1027-54.
- Lee KS, Lee HK, Hayflick JS, Lee YC, Puri KD. Inhibition of phosphoinositide 3-kinase delta attenuates allergic airway inflammation and hyperresponsiveness in murine asthma model. FASEB J 2006;20:455-65.
- Lee KS, Park SJ, Kim SR, Min KH, Jin SM, Puri KD, et al. Phosphoinositide 3-kinase-delta inhibitor reduces vascular permeability in a murine model of asthma. J Allergy Clin Immunol. 2006;118:403-9.
- Park SJ, Lee KS, Kim SR, Min KH, Moon H, Lee MH, et al. Phosphoinositide 3-kinase δ inhibitor suppresses interleukin-17 expression in a murine asthma model. Eur Respir J 2010;36:1448-59.
- Puri KD, Doggett TA, Douangpanya J, Hou Y, Tino WT, Wilson T, et al. Mechanisms and implications of phosphoinositide 3-kinase delta in promoting neutrophil trafficking into inflamed tissue. Blood 2004;103:3448-56.
- Marahatta A, Bhandary B, Lee YC, Kim SR, Chae HJ. Development and validation of a highly sensitive LC-MS/MS method for quantification of IC87114 in mice plasma, bronchoalveolar lavage and lung samples: Application to pharmacokinetic study. J Pharm Biomed Anal 2014;89:197-202.
- Thappali SR, Varanasi KV, Veeraraghavan S, Vakkalanka SK, Mukkanti K. Simultaneous quantitation of IC87114, roflumilast and its active metabolite roflumilast N-oxide in plasma by LC-MS/MS: application for a pharmacokinetic study. J Mass Spectrom 2014; 47:1612-19.
- Kwak YG, Song CH, Yi HK, Hwang PH, Kim JS, Lee KS, et al. Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma. J Clin Invest 2003;111:1083-92.
- https://bmsr.usc.edu/files/2013/02/ADAPT5-User-Guide.pdf.
- Ananthakrishnan R, Gona P. Pharmacological modeling and biostatistical analysis of a new drug. Open Access J Clin Trials 2010; 2:59-82.
- D'Argenio DZ, Schumitzky A. A program package for simulation and parameter estimation in pharmacokinetic systems. Comput Programs Biomed 1979;9(2):115-34.