- *Corresponding Author:
- Shaima Shereen
Department of Pharmacy Practice, Mesco College of Pharmacy, Hyderabad, Telangana 500006, India
E-mail: shaima.shereen404@gmail.com
| Date of Received | 14 October 2021 |
| Date of Revision | 29 November 2023 |
| Date of Acceptance | 21 March 2024 |
| Indian J Pharm Sci 2024;86(2):667-676 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of the study is to determine prevalence, severity, mechanism and potential risk factors of drug interactions among paediatrics of tertiary care hospital. A prospective and observational study was carried out for 3 mo. A total of 56 patients were included in the study. A data collection form was prepared to collect patient’s details. The prescriptions of each patient were scrutinized by entering into Micromedex 3.0 software to determine potential drug-drug interactions. Descriptive statistics was used to summarize patients’ characteristics. Chi-square test was done using statistical package for the social sciences version 22.0 to determine significant association between potential risk factors and drug-drug interactions. A p≤0.05 was considered to be statistically significant. A total of 56 drug-drug interactions were found ranging from 1-10 interactions in 19 paediatric patients. The prevalence of drug interactions was found to be 33.93 %. The mean and standard deviation of drug interactions was 2.95±2.82. Half of the drug interactions were found to be moderately severe (28 interactions (50 %)) with iron and pantoprazole being the most common moderately severe drug interaction. The most common mechanism of drug interaction was found to be pharmacokinetic type of mechanism (29 interactions (51.79 %)). Based on statistical analysis of potential risk factors of drug interactions, it was determined that parameters like age groups (p=0.002) and number of drugs prescribed per patient (p=0.003) were found to be statistically significant. Clinical pharmacists play a primary role in scrutinizing the pharmacotherapy given to paediatric patients in order to control drug-drug interactions.
Keywords
Drug interactions, pharmacotherapy, paediatrics, ciprofloxacin, pharmacokinetics
With speeding advancements in pharmacotherapy and discovery of new drugs, the pharmacotherapy process has become more complex. This has led to prescribing more drugs which in turn led to several drug-related problems. One such drug-related problem that has become prominent is drug-drug interactions[1]. Drug interaction refers to modification of response to one drug by another when they are administered simultaneously or in quick succession. The modification is mostly quantitative i.e., the response is either increased or decreased in intensity, but sometimes, it is qualitative i.e., an abnormal or a different type of response is produced. The possibility of drug interaction arises whenever a patient concurrently receives more than one drug, and the chances increase with the number of drugs taken[2].
There are several studies on drug interactions in adults[1-3]. But information about significant adverse drug reactions or drug interactions in special population such as paediatrics often remains incomplete or not available[4]. Paediatric population are at a higher risk of drug interactions due to their differences in rate and extent of organ function development and the distribution, metabolism, and elimination of drugs when compared with adults. These differences are present not only between adults and paediatrics but also among paediatric age groups. Paediatrics are defined as those younger than 19 y. Newborn infants born before 37 w of gestational age are termed premature; those from birth to 28 d of age are neonates; 1 mo-1 y are infants; those above (1-12) y are children and (13-18) y are adolescents. These differences among various paediatric age groups have led to complex and error prone medication use process[1-5]. Several previous studies have shown that the prevalence and risk of drug interactions is more prominent in hospitalized paediatric patients[6-8].
In order to control drug interactions among paediatric population it is important to know the risk factors that cause drug interactions. Studies have shown that age groups and polypharmacy are well known risk factors of drug interactions among paediatrics[6-10]. Studies have also shown that lack of proper communication and consensus between healthcare professionals is also adding to the existing problem[1,8,10]. This is where the role of clinical pharmacists becomes significant as clinical pharmacists gets an opportunity to work in a team and one of their functions is to report drugrelated problems which includes reporting drug interactions[6,11].
The aim and objective of the present study is to determine prevalence, severity, mechanism and potential risk factors of drug interactions among paediatrics of tertiary care hospital.
Materials and Methods
Study design and subjects:
A prospective and observational study was carried out at Nilofer Hospital, Hyderabad, India for 3 mo. The source population included all the hospitalized paediatric patients; however, the study population was based on the inclusion and exclusion criteria. The inclusion criteria included patients below 19 y, willing to participate, patients admitted in the Inpatient (IP) ward, with or without chronic illness, with or without comorbidities and with discharge summary. A total of 56 patients were included in the study. Exclusion criteria includes, patients of Outpatient (OP) and dermatology wards, patients admitted in Intensive Care Unit (ICU)/Neonatal Intensive Care Unit (NICU) and emergency wards and those not discharged or discharged before collecting or cross-checking the data.
Data collection:
A data collection form was prepared to collect patient data such as sociodemographic details (age, gender), clinical details (diagnosis along with or without comorbid conditions and with or without chronic illnesses) and drug therapy details (all the drugs prescribed with dose, dosage regimen, route of administration). The prescriptions of each patient were scrutinized by entering into Micromedex 3.0 software to determine potential drug-drug interactions. The levels of severity, degree of documentation, onset of action, type and description of mechanism and clinical management of drug-drug interactions were noted.
The severity of drug-drug interactions is classified into the following levels:
Contraindication: The drugs are contraindicated for concurrent use.
Major interaction: The interaction may be lifethreatening and/or require medical intervention to minimize or prevent serious adverse effects.
Moderate interaction: The interaction may result in an exacerbation of the patient’s condition and/or require an alteration in therapy.
Minor interaction: The interaction would have limited clinical effects. Manifestations may include an increase in the frequency or severity of side effects but generally would not require a major alteration in therapy.
Unknown interaction: In this case the severity of the interaction is unknown.
The documentation of drug-drug interactions is classified into the following degrees[12]:
Excellent: Controlled studies have clearly established the existence of the interaction.
Good: Documentation strongly suggests the interactions exists, but well-controlled studies are lacking.
Fair: Available documentation is poor, but pharmacologic considerations lead clinicians to suspect the interaction exists; or, documentation is good for a pharmacologically similar drug.
Unknown: In this case the drug-drug interactions were unknown.
The time of interaction and onset of related adverse events is classified into following types [3]:
Rapid: The effect of interaction occurs within 24 h of administration.
Delayed: The effect occurs if the interacting combination is administered for >24 h, i.e., days to weeks.
Not specified: The occurrence of the effect of interaction is not specified.
The mechanism of drug-drug interactions was classified into following types[13]:
Pharmacokinetic interactions: These interactions occur when one drug changes the systemic concentration of another drug, altering how much and for how long it is present at the site of action.
Pharmacodynamic interactions: These interactions occur when interacting drugs have either additive effects, in which case the overall effect is increased, or opposing effects, in which case the overall effect is decreased or even cancelled out.
Pharmaceutical interactions: These interactions occur when the formulation of one drug is altered by another before it is administered.
Unknown interactions: The mechanism of the interaction is unknown.
Statistical analysis:
Descriptive statistics such as range, mean, median and standard deviation were used to summarize patients demographic and clinical characteristics. Frequency tables along with their percentages were calculated using MS excel. A Chi-square test was used wherever appropriate to find a significant association between potential risk factors and drugdrug interactions. Odds Ratio (OR) and Confidence Interval (CI) of 95 % were used to see the strength of association. A p≤0.05 was considered to be statistically significant. The collected data was checked and assessed every day for completeness and accuracy before processing. Data was entered and statistical analysis was done using Statistical Package for the Social Sciences (SPSS) version 22.0 (copyright International Business Machines (IBM) Corporation and other(s) 1989, 2013).
Results and Discussion
The sample size was 56 paediatric patients which were divided into various variables to give a description of the paediatric patients (Table 1). Maximum number of paediatric patients were found in the age group of children (1-12) y which is about 31 paediatric patients (55.36 %). The range of age groups was from birth to 18 y. The mean, standard deviation and median of age groups is 9.3, 5.8 and 9 respectively. A majority of 41 paediatric patients (73.21 %) were found to be males. Most of the paediatric patients had no chronic illness (29 (51.79 %)) and had no comorbidity (31 (55.36 %)). The assessment of number of diseases/disorders diagnosed per patient showed that a majority of 31 paediatric patients (55.36 %) were diagnosed with only one disease/ disorder and a majority were diagnosed with blood disorders (22 (39.29 %)). A total of 338 drugs were prescribed among 56 paediatric patients with a mean of 6.04, standard deviation of 2.56 and median of 6. The range of drugs prescribed per patient was between 1-14 drugs. Majority of the paediatric patients were prescribed with >4 drugs (39 (69.64 %)) with six drugs being the maximum number of drugs prescribed in 15 paediatric patients (26.79 %).
| Variable | Frequency (%) in 56 paediatric patients |
|---|---|
| Age | |
| Neonates (from birth to 28 d) | 2 (3.57 %) |
| Infants (1 mo-1 y) | 3 (5.36 %) |
| Children (1-12) y | 31 (55.36 %) |
| Adolescent (13-18) y | 20 (35.71 %) |
| Gender | |
| Male | 41 (73.21 %) |
| Female | 15 (26.79 %) |
| Chronic illness | |
| Yes | 27 (48.21 %) |
| No | 29 (51.79 %) |
| Comorbidity | |
| Yes | 25 (44.64 %) |
| No | 31 (55.36 %) |
| Number of diseases diagnosed per patient | |
| 1 | 31 (55.36 %) |
| 2 | 21 (37.50 %) |
| 3 | 3 (5.36 %) |
| 4 | 1 (1.79 %) |
| Number of drugs prescribed per patient | |
| 1 to 4 drugs | 17 (30.36 %) |
| >4 drugs | 39 (69.64 %) |
| Diagnosis | |
| Blood disorders (DVT, malaria, thrombocytopenia, pancytopenia, anaemia, septicaemia, haemophilia, sepsis and septic shock) | 22 (39.29 %) |
| Liver disorders (hepatitis and jaundice) | 9 (16.07 %) |
| Fever diseases (viral haemorrhagic fever, viral pyrexia and dengue fever) | 8 (14.29 %) |
| Infectious diseases (HIV, meningitis and uterine tract infections) | 7 (12.5 %) |
| CNS disorders (seizures) | 6 (10.71 %) |
| Diabetes (diabetes mellitus type 1 and diabetic ketoacidosis) | 4 (7.14 %) |
| Respiratory disorders (pneumonia and pleural effusion) | 3 (5.36 %) |
| Nephrotic disease | 2 (3.57 %) |
| Others (menorrhagia, appendicitis, rickets, OP poisoning, grade 3 tonsillitis, GDD, anxiety and retardation with nocturnal enuresis) | 11 (19.64 %) |
Note: GDD: Global Developmental Delay
Table 1: Description of Patients
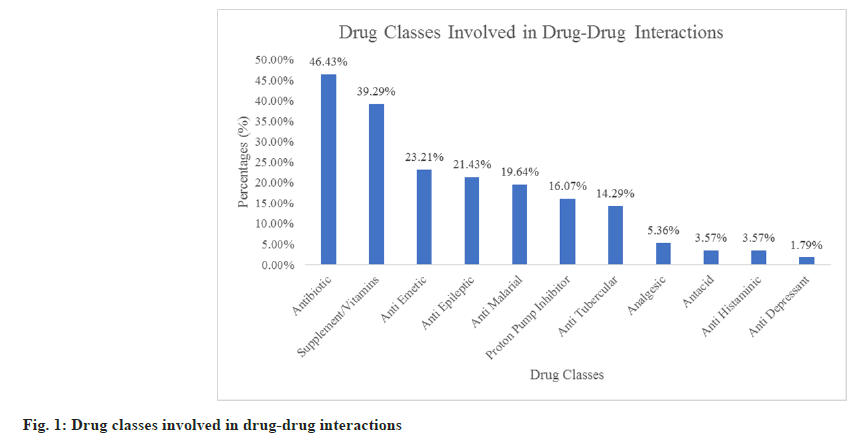
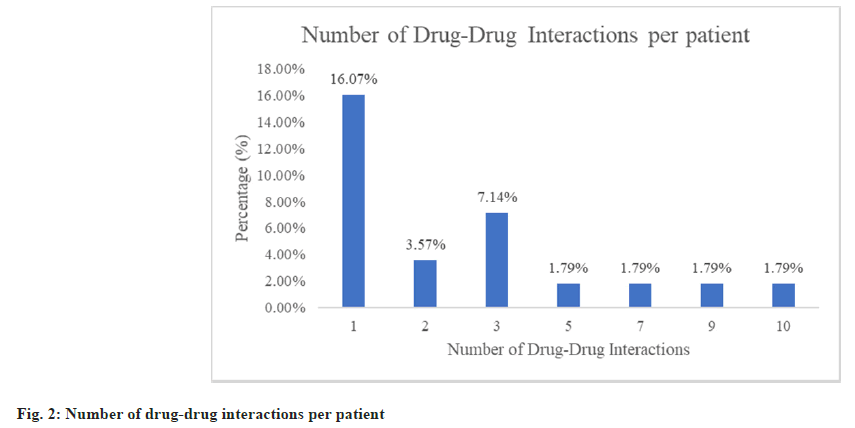
Fig. 1 shows the various drug classes involved in drug-drug interactions. Antibiotics were the most common drug class involved in drug-drug interactions which is 26 drug-drug interactions (46.43 %) followed by supplements/vitamins (39.29 %) which include lactated ringers solution, iron tablets, calcium tablets, zinc tablets and vitamin C tablets. A total of 56 drug-drug interactions were found ranging from 1-10 interactions in 19 paediatric patients. Thus, the prevalence of drug-drug interactions was found to be 33.93 % (number of patients with drug-drug interactions/ total number of patients×100=19/56×100=33.93 %). The mean, standard deviation and median of drugdrug interaction per patient was found to be 2.95, 2.82 and 2 respectively. A majority of 9 paediatric patients (16.07 %) were found with only one drugdrug interaction (fig. 2).
Table 2 shows the various levels of potential Drug- Drug Interactions (pDDIs) as per Micromedex 3.0. Based on severity of drug-drug interactions, half of the interactions were found with moderate severity (28 interactions (50 %)). There was only one contraindicated and minor interaction and no unknown interactions. A majority of 38 interactions (67.86 %) were fairly documented followed by good documentation (16 (28.57 %)) and there was no unknown documentation reported. The onset of half of the interactions were not specified (28 interactions (50 %)). Table 3 shows the description of most frequent drug-drug interactions and Table 4 shows their clinical management.
| Level | Frequency in 56 pDDIs | Frequency in 56 patients |
|---|---|---|
| Severity | ||
| Contraindicated | 1 (1.79 %) | 1 (1.79 %) |
| Major | 26 (46.43 %) | 11 (19.64 %) |
| Moderate | 28 (50 %) | 6 (10.71 %) |
| Minor | 1 (1.79 %) | 1 (1.79 %) |
| Documentation | ||
| Excellent | 2 (3.57 %) | 2 (3.57 %) |
| Good | 16 (28.57 %) | 4 (7.14 %) |
| Fair | 38 (67.86 %) | 13 (23.21 %) |
| Onset | ||
| Rapid | 14 (25 %) | 4 (7.14 %) |
| Delayed | 14 (25 %) | 2 (3.57 %) |
| Not Specified | 28 (50 %) | 13 (23.21 %) |
Table 2: Levels of Potential Drug-Drug Interactions
| Interaction | Frequency (%) in 56 pDDIs | Onset and documentation | Type and probable mechanism |
|---|---|---|---|
| Contraindicated | |||
| Lactated ringers solution+ceftriaxone | 1 (1.79 %) | Not specified and good | Pharmaceutical interactions and physical incompatibility |
| Major | |||
| Ciprofloxacin+metronidazole | 3 (5.36 %) | Not specified and fair | Pharmacodynamic and additive QT-interval prolongation |
| Moderate | |||
| Iron+pantoprazole | 6 (10.71 %) | Rapid and fair | Pharmacokinetic and reduced gastric pH, resulting in decreased absorption of iron |
| Minor | |||
| Calcium+iron Sucrose | 1 (1.79 %) | Delayed and fair | Pharmacokinetic and decreased iron absorption |
Table 3: Description of Most Frequent Drug-Drug Interactions
| Interaction | Clinical management |
|---|---|
| Contraindicated | |
| Lactated ringers solution+ceftriaxone | There is a risk of forming ceftriaxone-calcium precipitates. Do not mix or administer ceftriaxone concurrently with calcium-containing Intravenous (IV) solutions in the same IV administration line, including continuous calcium-containing infusions such as parenteral nutrition via a Y-site. However, in patients other than neonates, ceftriaxone and calcium-containing solutions may be administered sequentially if the infusion lines are thoroughly flushed between infusions with a compatible fluid |
| Major | |
| Ciprofloxacin+metronidazole | Metronidazole can cause QT-interval prolongation and has caused torsades de pointes with concomitant administration of another QT-interval prolonging drug. Susceptible patients may require Electrocardiogram (ECG) monitoring and avoidance of medications known to cause QT prolongation |
| Moderate | |
| Iron+pantoprazole | Absorption of iron may be affected due to the profound and long-lasting inhibition of gastric acid secretion by pantoprazole. Consider monitoring the patient for iron efficacy if pantoprazole is being used concurrently |
| Minor | |
| Calcium+iron sucrose | Concurrent administration of iron salts and aluminium, calcium or magnesium containing products is not recommended. If concurrent use cannot be avoided, iron salts should be taken at least 1 h before or 2 h after aluminium, calcium or magnesium containing products |
Table 4: Clinical Management of Most Frequent Drug-Drug Interactions
Ciprofloxacin and metronidazole were the most common drug-drug interaction found with major severity and has pharmacodynamic type of mechanism (3 (5.36 %)). Iron and pantoprazole were the most common drug-drug interaction found with moderate severity and has pharmacokinetic type of mechanism (6 (10.71 %)).
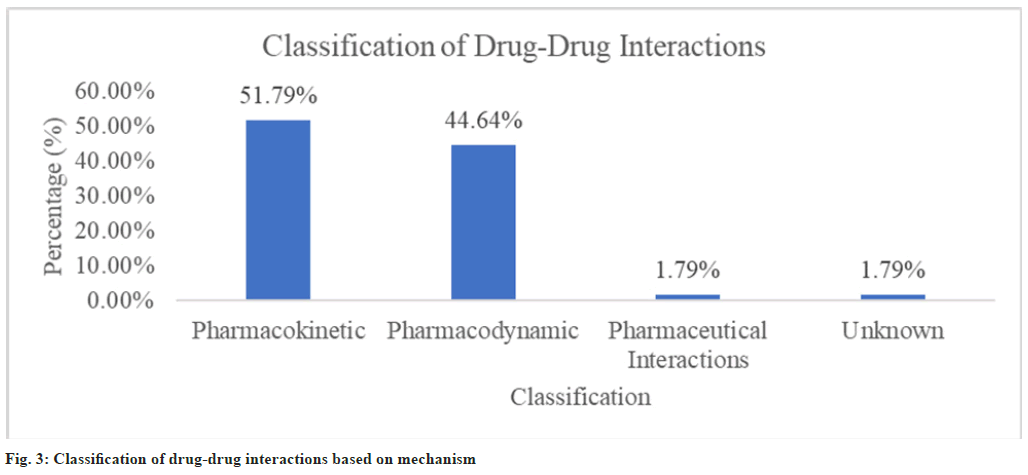
Classifying the drug-drug interactions based on mechanism (fig. 3) showed that 29 interactions (51.79 %) were in majority with pharmacokinetic type of mechanism followed by 25 interactions (44.64 %) with pharmacodynamic type of mechanism. There was only one pharmaceutical interaction (1 (1.79 %)) and only one unknown interaction (1 (1.79 %)). The statistical analysis of potential risk factors of drugdrug interactions showed that age groups (p=0.002) and number of drugs prescribed per patient (p=0.003) were found to be statistically significant (Table 5).
| Factor | Drug-drug interactions | OR (CI) | p value | |
|---|---|---|---|---|
| Present | Absent | |||
| Age | ||||
| Upto 12 y | 7 | 29 | 0.161 (0.047-0.543) | 0.002b |
| 13-18 y | 12 | 8 | ||
| Gender | ||||
| Male | 13 | 28 | 0.696 (0.204-2.369) | 0.561 |
| Female | 6 | 9 | ||
| Number of diseases diagnosed | ||||
| 1 disease | 9 | 22 | 0.613 (0.201-1.87) | 0.388 |
| >1 disease | 10 | 15 | ||
| Chronic illness | ||||
| Present | 9 | 18 | 0.95 (0.313-2.875) | 0.927 |
| Absent | 10 | 19 | ||
| Number of drugs prescribed per patient | ||||
| 1-4 drugs | 1 | 16 | 0.072 (0.008-0.605) | 0.003b |
| >4 drugs | 18 | 21 | ||
| Comorbidity | ||||
| Present | 10 | 15 | 1.629 (0.534-4.966) | 0.388 |
| Absent | 9 | 22 | ||
Note: bp≤0.05, was considered statistically significant
Table 5: Potential Risk Factors of Drug-Drug Interactions
In the present study, the paediatric patients were divided into a range of age groups from birth to 18 y of neonates (from birth to 28 d), infants (1 mo-1 y), children (1-12) y and adolescent (13-18) y where maximum number of paediatric patients were found in the age group of children (1-12) y. This trend was different from the results found in several previous studies[1,6,8,10,14,15] but in a study conducted by Ahmed et al.[16] the results were found similar. The mean of age in our study is 9.3 which was differing from earlier studies conducted by Mistry et al.[1] and Medina et al.[6]. However, the standard deviation of age which is 5.8 was slightly similar to the study conducted by Medina et al.[6]. Median of age in our study is 6 y which was contrasting to the results established by Getachew et al.[8]. and Ismail et al.[14].
Male patients were found in majority in our study, which was similar to the results of several previous studies[1,6,10,14,17,18]. Most of the paediatric patients were found without chronic illness. The trend of maximum number of patients found without any comorbidity was differing with the trend of a previous study conducted by Getachew et al.[8]
Majority of the patients were diagnosed with only one disease which was similar to the study conducted by Mistry et al.[1] where all the patients had one morbidity per prescription. In the study, blood disorders were concluded to be the most common diagnosis which was contradicting from the conclusions of earlier studies conducted by Mistry et al.[1] and Patel et al.[18]
A total of 338 drugs were prescribed among 56 paediatric patients out of which majority of the patients were prescribed with six drugs. This trend contradicted the results of earlier study conducted by Patel et al.[18]. Majority of the paediatric patients were prescribed with more than four drugs. This outcome was similar to various prior studies[8,14,15]. The range of drugs prescribed per patient was between 1-14 drugs which was differing in several previous studies[6,14,18]. In the present study, the mean and standard deviation of drugs prescribed per patient was found to be 6.04 and 2.56 respectively, which was varying in various earlier studies[1,6,19]. The median of the drugs prescribed per patient was found to be six drugs which was varying from the outcome of the study conducted by Ismail et al.[14].
A total of 56 drug-drug interactions ranging from 1-10 interactions per patient were found in 19 paediatric patients. The trend of range of drugdrug interactions per patient was contradicting from prior study conducted by Getachew et al.[8]. In our study, the mean and standard deviation of drugdrug interactions per patient were 2.95 and 2.82 respectively which was differing from the previous study conducted by Costa et al.[19]. Median of drugdrug interactions per patient was two interactions which differed from the outcome of study established by Ismail et al.[14]. Majority of the paediatric patients were found with only one drug-drug interaction per patient which was comparable to various previous studies[6-8,14,15].
The prevalence of drug-drug interaction was found to be 33.93 % which was greater in several prior studies and lower in several other prior studies[8,10,14-19]. The most common drug class involved in drug-drug interactions was found to be antibiotics followed by supplements/vitamins which was comparable to the outcome established by Nawaz et al.[15]. As per Micromedex, the level of severity, degree of documentation, onset of action, type and description of mechanism and clinical management of drugdrug interactions were noted. Half of the drug-drug interactions were found to be moderately severe which was similar in numerous past studies[1,7,8,10,14-17].
Majority of the drug-drug interactions were fairly documented and also the onset of action of half of the drug-drug interactions was established to be not specified as per Micromedex. This trend was varying with the study conducted by Ismail et al.[14]. Most common majorly severe and moderately severe interaction was ciprofloxacin and metronidazole, and iron and pantoprazole respectively, which was differing in numerous prior studies[1,8,10,14-19]. The type of mechanism was pharmacokinetic for the majority of drug-drug interactions. This result was comparable to the results discovered in few prior studies[10,15,16].
Various factors were statistically analysed to determine significant potential risk factors of drugdrug interactions which led to the conclusion that age groups (up to 12 y and 13-18 y) and number of drugs prescribed per patient were the potential risk factors that were statistically found to be significant. Several prior studies also reported number of drugs prescribed per patient to be statistically significant potential risk factor of drug-drug interactions[1,6-8,14-16,19]. There were several other prior studies that reported various age groups to be statistically significant potential risk factor of drug-drug interactions[6,8,19].
In order to avert the problem of drug interactions in a special population like paediatrics that have complicated pharmacotherapy, it is advisable to healthcare professionals, to use electronic interaction software such as Micromedex to effectively determine drug-drug interactions. This view is similar to that of previous study conducted by Mistry et.al.[1]; to cautiously use most involved drug or drug groups in drug-drug interactions by reducing or changing with alternative drugs. This view was similar in a few previous studies[1,18]; to create awareness by continued medical education to the physicians and clinical pharmacists so that they remain vigilant about various drug-drug interactions and suggest adequate therapy adjustments when appropriate. This view was similar in a few previous studies[6,16]; to take necessary precautions while prescribing medications by using computerized prescriptions, careful monitoring of drug therapy and timely identification of possible interactions by physicians and clinical pharmacists. This view was similar in a few previous studies[6,10,14,16].
To incorporate clinical pharmacists in the multidisciplinary team to avert the problem of drugdrug interactions. This view was similar in a few prior studies[6,16] and to create a separate system for reporting drug interactions. As clinical pharmacists get an opportunity to work in a team it becomes easier for them to report drug interactions and also to avoid any hurdles like lack of proper communication and consensus between healthcare professionals. This view was similar in a few previous studies[1,6,8,10,11,16].
Therefore, our study determined a significant amount of prevalence of drug interactions in paediatric patients and majority of the drug interactions were with moderate severity. A detailed description of drug interactions which included onset, mechanism and clinical management was also noted. Age groups and polypharmacy were statistically ascertained as potential risk factors of drug interactions. Our study recommends the use of electronic interaction software like Micromedex, continued medical education to healthcare professionals, use of computerized prescriptions and cautious monitoring of drugdrug interactions. Paediatrics being a sensitive population requires a separate system of reporting drug interactions managed by clinical pharmacists. Therefore, it becomes the primary role of clinical pharmacists to scrutinize the pharmacotherapy given to the paediatric patients in order to control the increase in the number of drug interactions. Hence, it is further recommended to incorporate clinical pharmacist in multidisciplinary team of healthcare professionals.
Acknowledgments:
I would like to give my special thanks to my study participants for their assistance and participation in the study. I would like to thank the health care professionals and staff of the hospital for assisting in patient data collection.
Ethical approval:
The study was approved by the Institutional Ethics Committee (IEC) of Mesco College of Pharmacy, Hyderabad, Telangana with the IEC approval number MCP/IEC/PD/PR/37.
Conflict of interests:
The authors declare no competing interests.
References
- Mistry M, Gor A, Ganguly B. Potential drug-drug interactions among prescribed drugs in paediatric outpatients department of a tertiary care teaching hospital. J Young Pharm 2017;9(3):371.
- Tripathi KD. Essentials of medical pharmacology. JP Medical Ltd; 2013.
- Diksis N, Melaku T, Assefa D, Tesfaye A. Potential drug-drug interactions and associated factors among hospitalized cardiac patients at Jimma university medical center, Southwest Ethiopia. SAGE Open Med 2019;7:2050312119857353.
[Crossref] [Google Scholar] [PubMed]
- World Health Organization. Safety of medicines: A guide to detecting and reporting adverse drug reactions: Why health professionals need to take action. World Health Organization; 2002.
- Chapter in a book: Milap CN, Carol T. Paediatrics. In: text book of pharmacotherapy: A pathophysiologic approach. McGraw-Hill; 2014. p. 95-104.
- Medina BF, Vázquez-Méndez E, Pérez-Guerrero EE, Sánchez-López VA, Hernández-Cañaveral II, Huerta-Olvera SG. Pilot study: Evaluation of potential drug-drug interactions in hospitalized pediatric patients. Pediatr Neonatol 2020;61(3):279-89.
[Crossref] [Google Scholar] [PubMed]
- Morales-Ríos O, Jasso-Gutierrez L, Reyes-Lopez A, Garduno-Espinosa J, Muñoz-Hernández O. Potential drug-drug interactions and their risk factors in pediatric patients admitted to the emergency department of a tertiary care hospital in Mexico. PloS One 2018;13(1):e0190882.
[Crossref] [Google Scholar] [PubMed]
- Getachew H, Assen M, Dula F, Bhagavathula AS. Potential drug-drug interactions in pediatric wards of Gondar university hospital, Ethiopia: A cross sectional study. Asian Pac J Trop Biomed 2016;6(6):534-8.
- World Health Organization. Medication safety in polypharmacy: Technical report. World Health Organization; 2019.
- Rao C, Shenoy V, Udaykumar P. Potential drug-drug interactions in the pediatric intensive care unit of a tertiary care hospital. J Pharmacol Pharmacother 2019;10(2):63-8.
- Ahmad A, Khan MU, Haque I, Ivan R, Dasari R, Revanker M, et al. Evaluation of potential drug-drug interactions in general medicine ward of teaching hospital in southern India. J Clin Diagn Res 2015;9(2):10.
[Crossref] [Google Scholar] [PubMed]
- IBM. IBM Watson Health. IBM Micromedex user guide; 2021;92-3.
- Snyder BD, Polasek TM, Doogue MP. Drug interactions: Principles and practice. Aust Prescr 2012;35(3).
- Ismail M, Iqbal Z, Khan MI, Javaid A, Arsalan H, Farhadullah H, et al. Frequency, levels and predictors of potential drug-drug interactions in a pediatrics ward of a teaching hospital in Pakistan. Trop J Pharm Res 2013;12(3):401-6.
- Nawaz HA, Khan TM, Adil Q, Goh KW, Ming LC, Blebil AQ, et al. A prospective study of medication surveillance of a pediatric tertiary care hospital in Lahore, Pakistan. Pediatr Rep 2022;14(2):312-9.
[Crossref] [Google Scholar] [PubMed]
- Ahmed S, Yesmine S, Rahman M, Shahriar M. Assessment of interactions of drugs prescribed for pediatric patients in Bangladesh. Bangladesh Pharm J 2021;24(2):91-8.
- Sripada R, Kumar SS, Devanna N, Reddy KR. A Study on the prevalence and severity of possible drug-drug interactions in pediatrics department at an Indian tertiary care teaching hospital. Int J Pharm Pharm Sci 2018;10(7):52.
- Patel S, Chaudhari M, Patel N. Evaluation of potential drug-drug interaction in indoor patients of pediatric department of tertiary care hospital. Natl J Physiol Pharm Pharmacol 2019;9(10):1012-8.
- Costa HT, Leopoldino RW, da Costa TX, Oliveira AG, Martins RR. Drug-drug interactions in neonatal intensive care: A prospective cohort study. Pediatr Neonatol 2021;62(2):151-7.
[Crossref] [Google Scholar] [PubMed]