- *Corresponding Author:
- S. Muthukrishnan
Department of Pharmacy, Annamalai University, Chidambaram-608 002, India
E-mail: smuthukrishnan.smk@gmail.com
| Date of Submission | 07 August 2016 |
| Date of Revision | 08 October 2017 |
| Date of Acceptance | 26 April 2018 |
| Indian J Pharm Sci 2018;80(3):525-532 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Schleichera oleosa (Lour) Oken, family Sapindaceae, is medium to large sized tree widely distributed in the tropics and subtropics of India. In the present study, physicochemical parameters, fluorescence, phytochemical constituents and thin layer chromatogram of the leaves of the plant were investigated. The total, acid-insoluble, water-soluble and sulphated ash values and the moisture content of the leaf powder of Schleichera oleosa were found to be 9.06, 7.16, 5.73, 13.5 and 5.21 %, respectively. The fluorescent characteristics of the leaf powder with various chemical reagents were examined under visible light and UV light. Percent yields of petroleum ether, chloroform, ethyl acetate, ethanol and aqueous leaf extracts were 3.26, 1.35, 1.70, 7.73 and 4.21 % respectively. A significant amount of phenolic and flavonoid content was detected. Preliminary phytochemical analysis revealed the presence of alkaloids, carbohydrates, flavonoids, glycosides, phenols, saponins and steroids in ethanol and aqueous extracts. The ethyl acetate, ethanol and aqueous extracts were subjected to TLC for identification of bioconstituents, which revealed various colored phytochemical compounds with different Rf values in different solvent systems. The results indicated that the leaves contain an appreciable number of bioactive compounds and presence of these phytochemicals especially the phenols and flavonoids could be the reason behind the use of S. oleosa in ethnomedicine for the management of various ailments. These results could help in standardization, identification and in carrying out further research on S. oleosa leaf-based herbal drugs.
Keywords
Physicochemical standards, fluorescence, extractive values, phytochemical studies, flavonoids, phenolic compounds, TLC, Rf values
Plant-based drugs have been used worldwide in traditional medicines for treatment of various diseases. World plant biodiversity is the largest source of herbal medicine and still about 60-80 % world population relies on plant-based medicines, which are being used since the ancient ages as traditional medicine. India is the largest producer of medicinal herbs and appropriately called the botanical garden of the world. It is now clear that the medicinal value of these plants lies in the bioactive phytochemical constituents that produce definite physiological effects on the human body. These natural compounds also formed the base of modern drugs as we are using today [1-3].
Schleichera oleosa (Lour.) Oken, commonly known as Lac tree or Kusum, is a forest species of tropical and subtropical region. The tree is utilized for multifarious purposes and production of natural, biodegradable and commercially important lac resins that serve as a livelihood support to millions of poor farmers [4]. It also has many medicinal uses and is used in traditional medicine for several indications. The powdered seeds are applied to wounds and ulcers of cattle to remove the maggots. The bark is used as an astringent and against skin inflammations, ulcers, itching, acne and other skin infections [5]. It is generally used as an analgesic, antibiotic and against dysentery [6]. Recently, it was reported that the bark along with water is used to treat menorrhea [7].
Hence the present investigation was taken up to study the physicochemical characteristics, phytochemical constituents, fluorescence and thinlayer chromatography (TLC) analysis of leaves of S. oleosa (Lour.) Oken extracted with petroleum ether, chloroform, ethyl acetate, ethanol and water and also to perform qualitative and quantitative analysis of some secondary metabolites and minerals present in this plant [8].
Materials and Methods
Fresh leaves of S. oleosa were collected during May 2015 from the forest regions of Kanyakumari District, Tamil Nadu. The plant material was identified and authenticated in the Botany Laboratory, Central Council for Research in Ayurveda and Siddha (Government of India), Tirunelveli, Tamil Nadu. Voucher specimens were preserved in the department herbarium library (PS-08/14/1/5/15).
Taxonomy and nomenclature
Synonym(s): Schleichera Trijuga Willd & Klein, family: Sapindaceae. Vernacular/common names: lac tree (English), kusum (India), pongro (French, Khmer of Cambodia), gum-lac tree (Filipino), kasambi (Indonesian), kusambi (Malay), takhro (Thai) [9]. Scientific synonyms are Cussambium oleosum O. Kuntze, Pistacia oleosa Lour. Schleichera trijuga Wild. [4]. The vernacular names are Kusum, Kusam (Hindi); Sagada (Kannada); Pava, puvan (Tamil), Pusuku (Telugu) [4].
Processing of the sample
Fresh leaves of plants were washed well using tap water followed by washing twice using distilled water and were dried in the shade for a period of 10-15 d, at an ambient temperature of 33°. After drying, leaf materials were crushed into small pieces. The dried samples were ground properly to obtain a coarse powdered, which was stored at room temperature till used in the investigation [10,11].
Macroscopic evaluation
The leaves were collected from the surrounding environment. The fresh leaves were deep green in colour. The leaves were separated from the stem and shoots, cleaned manually and kept over a dry plastic sheet and macroscopic characters of the fresh leaves were noted. A magnifying glass and scale were used to measure the parameters like morphology, length, width, shape, surfaces, venation, presence and absence of petiole, the apex, margin, base, lamina and texture [12-14].
Fluorescence analysis
The fluorescence analysis of the plant was done by placing dry, powdered leaves on a slide and observing the colour changes under visible and UV lights after treating with several drops of specific reagents such as ammonia, acetic acid, 1 N hydrochloric acid, 1 N nitric acid, 1 N sulphuric acid, 10 % ferric chloride, I2 solution and 1 N sodium hydroxide. Similarly, different solvent crude extracts were observed without adding any reagents under visible and UV light. The development of colour was noted within 1-2 min in order to avoid drying and resultant colour change [12,15,16].
Preparation of extracts
The dried, powdered leaf material (500 g) was extracted successively with petroleum ether, chloroform, ethyl acetate, ethanol and water in the increasing order of polarity by hot percolation method using a Soxhlet apparatus. After distillation, the resultant extracts were collected in a clean dry glass beaker. The extracts were evaporated to dryness in a Buchi rotary vacuum evaporator (Rotavapor R-210, Switzerland) at 40-50° and the final extracts were kept at low temperature for further investigations [17,18].
Physicochemical standardization
Standardization of the extracts of S. oleosa leaves was carried out as per guidelines of WHO and different procedures listed in the pharmacopeia. The standardization studies on different physicochemical parameters including extractive values in different solvents, total ash, water-soluble ash, acid-insoluble ash, moisture content and loss on drying (LOD) were carried out [19,20]. Cold maceration estimated water and alcohol-soluble extracts according to the method prescribed by World Health Organization (WHO). All estimations were performed six times and the result, which was presented as mean±standard error of the mean (SEM).
Total ash value
The total ash value gave an estimate of the total material remained after ignition. About 2 g of the dried crude drug was accurately weighed in a silica crucible and incinerated gently at first and gradually increased the temperature to 675±25°, until free from carbon. Then the ash was cooled in a desiccator and weighed. The percent of ash was calculated with reference to the air dried powder. The procedure was repeated to get the constant weight [21].
The total ash was boiled with 25 ml of water for 5 min and filtered through ash less filter paper (Whatman No. 41). The insoluble matter was collected in a filter paper followed by washing with hot water. The filter paper was ignited in silica crucible for 15 min at a temperature not exceeding 450°. The remaining ash was cooled in a desiccator and weighed. Subtracting the weight of the water-insoluble matter from the weight of the total ash yielded the weight of water-soluble ash. Percent watersoluble ash in the dried powder was calculated [21].
Sometimes, variable inorganic constituents like calcium oxalate, silica and carbonate content of the crude drug affected the total ash value. Such variables were removed by treating the total ash powder with hydrochloric acid. The acid-insoluble ash measures the amount of silica, especially as sand and siliceous earth present in the plant material. The acid-insoluble ash was determined from the total ash obtained. It was boiled for five minutes with 25 ml of 2 M hydrochloric acid and filtered through an ash less filter paper (Whatmann No. 41). The collected insoluble matter was washed with hot water, ignited in a silica crucible and cooled in desiccator. The residue was weighed and the acid-insoluble ash of the plant material reference to the air-dried powder was determined [21].
Sulphated ash value
A silica crucible was heated to redness for 10 min, allowed to cool in a desiccator and weighed. About 1 g of the dried crude powder was accurately weighed in a silica crucible and ignited gently at first until the substance is thoroughly charred. Then cooled and the residue was moistened with 1 ml sulphuric acid, heated gently until the white fumes no longer evolved and ignited at 800°±25° until all black particles have disappeared. Then the crucible was allowed to cool, few drops of sulphuric acid were added and heated. The process was repeated and weighed [21].
Determination of LOD
The test for LOD determines both water and volatile matter. About 5 g of the powdered plant material was accurately weighed in a flat tarred weighing bottle. The bottle was dried in an oven at 105º for 1 h. It was cooled in a desiccator and again weighed. The LOD was calculated with reference to the amount of airdried powder taken [21].
Determination of crude fibre content
Estimation of crude fibre content represented the measurement of the content of cellulose, lignin and cork cells in the plant tissue. Here the plant material was defatted and boiled with dilute acid and alkali to eliminate the soluble material. The excess of crude fibre might indicate adulteration with woody tissues like kernels. The crude fibre content was determined by the method recommended in USP XX. About 2 g of the coarse powder of the plant material was accurately weighed and extracted with ether. The marc was boiled with 200 ml of 1.25 % sulphuric acid for 30 min under reflex in a 500 ml flask. The mixture was then filtered through tarred filter and the residue was washed with boiled water until free from acid. The residue was again transferred into the flask and boiled with 200 ml 1.25 % sodium hydroxide solution under reflex for 30 min. Then the liquid was filtered quickly through a tarred filter. The residue was washed with boiling water until it became neutral. Then the residue was dried in a hot air oven at 110° and weighed. After weighing the residue, it was incinerated and weighed again. The difference in the weight of the residue before and after incineration provided the crude fibre content of the plant material [22].
Determination of swelling index
The swelling index is the swelling of 1 g of plant material under specified conditions. Plant materials may have specific therapeutic or pharmaceutical utility because of their swelling properties, especially the gums and those containing mucilage, pectin or hemicellulose. About 1 g of coarse powder of the plant material was transferred into a 25 ml glass stoppered measuring cylinder containing 25 ml of water. The mixture was shaken thoroughly every 10 min for 1 h. Then it was allowed to stand for 3 h at room temperature. After that, the volume occupied by the plant material, including any sticky was measured. This method was used to determine the amount of active constituent present in the plant material, which are soluble in particular solvent [23].
Extractive value
This method was used to determine the amount of active constituents present in the plant material, which are soluble in given solvent. Extraction of any crude drug with a particular solvent yielded a solution containing different phytoconstituents soluble in that solvent [24]. About 5 g of powder was accurately weighed and placed in a round bottom flask containing 100 ml of ether, which was subjected to continuous extraction in a Soxhlet apparatus for 20 h. Then the extract was transferred into a porcelain dish, allowed to evaporate spontaneously followed by drying it over phosphorous pentoxide for 18 h and weighed. Finally, the percent ether-soluble extractive was calculated [24].
Five grams of the powder was macerated with 100 ml of alcohol of the specified strength in a closed flask for 24 h shaking frequently during 6 h and allowed to stand for 18 h. It was filtered rapidly taking precautions against loss of alcohol; 25 ml of the filtrate was evaporated to dryness in a tarred flat bottomed shallow dish, dried at 105° and weighed. The percent of alcoholsoluble extractive was calculated with reference to the air-dried drug [24].
About 5 g of the powder was added to 50 ml of water at 80° in a stopper flask. It was shaken well and allowed to stand for 10 min, cooled to 15° and to it 2 g of kieselghur was added and filtered. About 5 ml of the filtrate was transferred to a tarred evaporating basin (7.5 cm in diameter), the solvent was evaporated on a water bath, drying was continued for half an hour, finally it was dried in a hot air oven for 2 h and weighed. The percent of water-soluble extractive was calculated with reference to the dried drug [24].
Phytocemical screening of the plant material
Phytochemical screening was performed to detect the presence of secondary metabolites that could be responsible for curing ailments. The phytochemical screening of the plant extract was carried out for all the extracts, as per reported standard methods [25-27].
Quantitative phytochemical test
Alkaloids were estimated by weighing 5 g of the sample into a 250 ml beaker and 200 ml of 10 % acetic acid in ethanol was added and covered and allowed to stand for 4 h. This was filtered and the extract was concentrated on a water bath to one fourth of the total volume. Concentrated ammonium hydroxide was added drop-wise to the extract until the precipitation was completed. The whole solution was allowed to settle and the precipitate was collected and washed with dilute ammonium hydroxide and then filtered. The residue was the alkaloid matter, which was dried and weighed [28].
Twenty grams of sample powder was put into a conical flask and 100 ml of 20 % aqueous ethanol was added. The samples heated over a water bath for 4 h with continuous stirring at about 55°. The mixture was filtered and the residue re-extracted with another 200 ml of 20 % ethanol. The extract was reduced to 40 ml over water bath at about 90°. The concentrate was transferred into a 250 ml separating funnel and 20 ml of diethyl ether was added and shaken vigorously. The aqueous layer was recovered. The purification process was repeated by extracting with 60 ml n-butanol. The combined n-butanol extracts and washed twice with 10 ml of 5 % aqueous sodium chloride. After evaporation, the samples were dried in the oven to a constant weight and the saponin content was calculated [29].
Total flavonoids were estimated by extracting 10 g of the plant sample repeatedly with 100 ml of 80 % aqueous methanol at room temperature. The whole solution was filtered through Whatman filter paper No 42 (125 mm). The filtrate was later transferred into a crucible and evaporated into dryness over a water bath and weighed to a constant weight [30].
Total phenolic content was analysed spectrophotometrically using a modified Folin- Ciocalteu colorimetric method [31]. The crude extracts were diluted suitable solvent to attain interpretations within the standard curve ranges of 0.0-100.0 μg of gallic acid/ml. Then the extract was mixed with 1 ml of distilled water in a test tube and 250 μl of Folin- Ciocalteu reagent was added. Then 2.5 ml of 7 % aqueous solution of sodium carbonate (Na2CO3) was added. This mixture was gently shaken and allowed to stand for 6 min. The solution was then made up to 6 ml by adding sufficient amount of distilled water. Samples were allowed to stand for 90 min at room temperature. Then absorbance was measured against the blank at 760 nm using a UV/Vis spectrophotometer. This reagent blank, which was composed of the same reagents but instead of sample using distilled water. The standard values prepared similarly with known concentrations of gallic acid and plot a standard curve. The interpretations of sample absorbance value in standard curve to find out the concentration of phenolic content of the extracts. All values were expressed as mean±SEM for six replications [32,33]. The total phenol content of plant parts was expressed as milligrams of gallic acid equivalents per gram of dry weight (mg of GAE/g DW) were calculated.
TLC analysis of various extracts of S. oleosa
Based upon the preliminary phytochemical tests and quantitative estimation, the ethyl acetate, ethanol and aqueous extracts were subjected to TLC. The semi-solid extract was chromatographed by taking a small quantity of the extract diluted with a drop of the solvent. These extracts were subjected to TLC as per conventional one dimensional ascending method by using standard protocol as per pharmacopoeia was followed. In this method silica gel 60F254, 20× 20 cm, with thickness 0.2-0.3 mm (Merck) pre-coated plate was used. Plate markings were made with soft pencil. Glass capillaries were used to apply the spot on the TLC plate, 1 μl of sample volume applied by using capillary at distance of 1 cm and the 3 tracks and developed in air tight chamber already saturated with same solvent system.
In the twin trough chamber with different solvent systems: chloroform:ethyl acetate:formic acid (5:4:1) solvent system I. In solvent system II, toluene:acetone:formic acid (4.5:4.5:1). In solvent system III, ethyl acetate:methanol:water (100:13.5:10), In solvent system IV, ethyl acetate:formic acid:acetic acid:water (100:11:11:26) used. After pre-saturation with mobile phase, 20 min for development was used. After the run, plates were dried and allowed for visualization [34-36]. After development, initially three spots were visualized in UV chamber (254, 365 nm). In the present study, different visualizing reagents were used such as NH3 vapour, vanillin HCl, 10 % alcoholic KOH, Folin-Ciocalteu. Always freshly prepared reagents were used to detect the better bands on the TLC plates. The movement of the active compound was expressed by its retention factor (Rf), values were calculated for different samples [34-36]. After visualized in UV chamber and spray reagents, the spots were shown in different in colour. The Rf values were measured correctly and carefully and the chromatogram were photographed and presented in the tabulated form.
Results and Discussion
Leaves are paripinnate, w/(2-)3(-4) pairs of pinnae, petiole cross section circular somewhat flattened or slightly grooved above, 2-6(-8) cm long, swollen at base; rachis cross section round to triangular; petiolule swollen, slightly grooved above, 1-3 mm long. Leaflets are elliptical to elliptic-oblong, coriacious, 4.5-18.5 (-25)×2.5-9 cm, dark brown or greyish-green above, lighter-brown to greenish beneath, deep purple when young, veins in 12-15 pairs, looped and joined near the margin [9].
Physicochemical standards such as total ash value (9.06±0.20), acid-insoluble ash value (7.16±0.53), water-soluble ash value (5.73±0.13), sulphated ash value (13.5±1.24), LOD (5.21±0.12), swelling index (19.33±0.23) and crude fibre content (4.13±0.24) were observed as per Indian Pharmacopoeia and WHO guidelines for the Quality control of herbal drugs.
The coarse powder of aerial parts of S. oleosa was subjected to the various systemic physicochemical evolutions as per standard procedure. Extractive values such as ether-soluble extractive (1.2±0.38), alcoholsoluble extractive value (11.15±0.35) and water-soluble extractive (8.76±0.38) were observed. The volatile oil content of the aerial part of the plant was determined by hydro distillation method using Clevenger apparatus. No oil was collected in the graduated tube. All the physicochemical standards values were observed from the mean of six findings (Table 1).
| Experiment | Mean (% w/w)±SEM |
|---|---|
| Total ash value | 9.06±0.20 |
| Water-soluble ash value | 5.73±0.13 |
| Acid-insoluble ash value | 7.16±0.53 |
| Sulphated ash value | 13.5±1.24 |
| Loss on drying | 5.21±0.12 |
| Crude fibre content | 4.13±0.24 |
| Swelling index | 19.33±0.23 |
| Ether-soluble extractive value | 1.2±0.38 |
| Alcohol-soluble extractive value | 11.15±0.35 |
| Water-soluble extractive value | 8.76±0.38 |
Each value is presented as mean±SEM (n=6)
Table 1: Physicochemical standard values of powdered leaves of S. oleosa
Comparative fluorescence analysis was performed for powder and the extracts. The powder was suspended with various chemical reagents, whereas the extracts were dissolved in same solvent. The fluorescence nature was observed and recorded (Tables 2 and 3) by comparing the color development in day light and UV (254 and 366 nm) light.
| Chemical Treatment | Day Light | UV | |
|---|---|---|---|
| 254 nm | 366 nm | ||
| Powder | Green | Brown | Greenish-brown |
| Powder+H2O | Green | Brown | Greenish-brown |
| Powder+1N HCl | Greenish-brown | Brown | Dark brown |
| Powder+5 % NaOH | Green | Brown | Greenish-brown |
| Powder+1N NaOH (alcoholic) | Greenish-yellow | Brownish-red | Greenish-brown |
| Powder+50 % HNO3 | Brown | Dark brown | Brownish |
| Powder+50 % H2SO4 | Brownish-red | Dark brown | Dark brown |
| Powder+ammonia | Light green | Light green | Fluorescent Green |
| Powder+acetic acid | Light green | Light green | Fluorescent Green |
| Powder+I2 Solution | Brown | Yellowish-green | Dark green |
| Powder+FeCl3 | Yellowish-green | Fluorescent green | Dark green |
Table 2: Fluorescence characteristics of powdered leaves of S. oleosa treated with different chemical reagents
| Extracts | Day light | UV observation | |
|---|---|---|---|
| 254 nm | 366 nm | ||
| Petroleum ether | Yellowish-green | Light brown | Dark brown |
| Chloroform | Green | Blackish-green | Black |
| Ethyl acetate | Greenish-brown | Dark brown | Reddish-brown |
| Ethanol | Brown | Dark brown | Reddish-brown |
| Aqueous | Reddish-brown | Reddish-brown | Brownish |
Table 3: Fluorescence characteristics of various crude extracts of leaves of S. oleosa
The aerial parts of the plant were shade dried and made into coarse powder. The coarse powder was extracted by continuous hot percolation method in a Soxhlet apparatus using various solvents in increasing polarity such as petroleum ether, chloroform, ethyl acetate, ethanol and water. Extracts were concentrated by removal of solvent under reduced pressure and finally residue was dried in vacuum. The percent yields were such as petroleum extract 3.26 % w/w, chloroform extract 1.35 % w/w, ethyl acetate extract 1.70 % w/w, ethanol extract 7.73 % w/w and aqueous extract 4.21 % w/w as shown in Table 4.
| Extracts | Colour | Consistency | Percent yield |
|---|---|---|---|
| Petroleum ether | Yellowish-green | Solidify | 3.26 % |
| Chloroform | Green | Solidify | 1.35 % |
| Ethyl acetate | Greenish-brown | Sticky | 1.70 % |
| Ethanol | Brown | Thick viscous | 7.73 % |
| Aqueous | Reddish brown | Solidify | 4.21 % |
Table 4: Extractive values of powdered leaves of S. oleosa
All extracts were subjected to preliminary phytochemical screening to identify the phytoconstituents using qualitative chemical reagents. The phytochemical tests revealed the presence of phytosterols in all the extracts, alkaloids (ethanol, aqueous extract), glycosides (ethanol, aqueous extract), saponins (ethanol, aqueous extract), phenolic compounds (ethyl acetate, ethanol, aqueous extract) and flavonoids (ethyl acetate, ethanol, aqueous extract). Compared with petroleum ether and chloroform extracts, most of the phytoconstituents were present in the ethyl acetate, ethanol and aqueous extracts. The results of preliminary phytochemical were given in Table 5.
| Compounds | Observation | ||||
|---|---|---|---|---|---|
| PET | CF | EA | ET | AQ | |
| Carbohydrates | - | - | - | + | + |
| Glycosides | - | - | - | + | + |
| Proteins and aminoacids | - | - | - | - | - |
| Alkaloids | - | - | - | + | + |
| Saponins | - | - | - | + | + |
| Phenolic compounds and tannins | - | - | + | + | + |
| Flavonoids | - | + | + | + | + |
| Phytosterols | + | + | + | + | + |
| Fixed oils and fats | - | - | - | - | - |
| Gums and mucilage | - | - | - | - | + |
| Volatile oil | - | - | - | - | - |
| Resin | - | - | - | - | - |
PET: petroleum ether extract, CF: chloroform extract, EA: ethyl acetate extract ET: ethanol extract, AQ: aqueous extract
Table 5: Qualitative phytochemical screening of successive extracts of powdered leaves of S. oleosa
Based upon the preliminary phytochemical test, quantitative determination of phytoconstituents were carried out for the powdered plant material of leaves of S. oleosa by various standard methods and found the alkaloid 1.32±0.56 % w/w, total phenol 71.92± 2.92 % w/w, flavonoids 9.69±1.38 % w/w and saponins 1.15±0.21 % w/w were present. The values were recorded from the mean of six repeated observations (Table 6).
| Experiment | Mean±SEM |
|---|---|
| Estimation of total phenols | 71.92±2.92 |
| Estimation of alkaloids | 1.32±0.56 |
| Estimation of total flavonoids | 9.69±1.38 |
| Estimation of saponins | 1.15±0.21 |
Each value is presented as mean±SEM (n=6)
Table 6: Quantitative phytochemical estimation of powdered leaves of S. oleosa
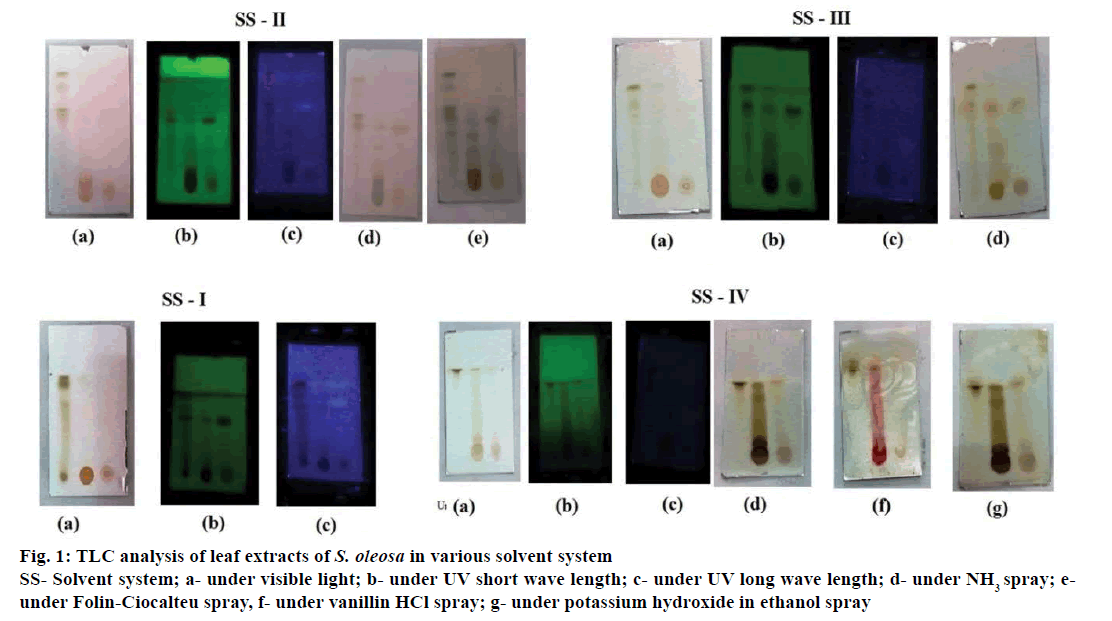
Based upon the preliminary phytochemical studies, ethyl acetate, ethanol and aqueous extracts were chosen for TLC studies. The chromatogram was developed for these three extracts with four different solvent systems. The extracts showed distinct spots with different intensity, spots were identified with the help of various visualizing agent and UV chamber (Figure 1). The resultant spots Rf values were calculated and summarized in the Table 7. The results indicated that the leaves contained an appreciable amount of bioactive compounds. The presence of these phytochemicals especially the phenols and flavonoids might explain the use of S. oleosa in ethnomedicine for the management of various ailments.
Figure 1: TLC analysis of leaf extracts of S. oleosa in various solvent system
SS- Solvent system; a- under visible light; b- under UV short wave length; c- under UV long wave length; d- under NH3 spray; eunder
Folin-Ciocalteu spray, f- under vanillin HCl spray; g- under potassium hydroxide in ethanol spray
| Extracts | Mobile phase | No of spots | Rf values | Intensity |
|---|---|---|---|---|
| Ethyl acetate | SS I | 3 | 0.85, 0.87, 0.91, | #, *, # |
| SS II | 2 | 0.51, 0.56 | *, + | |
| SS III | 2 | 0.84, 0.92 | *, * | |
| SS IV | 1 | 0.92 | * | |
| Ethanol | SS I | 3 | 0.84, 0.86, 0.90 | +, +, + |
| SS II | 2 | 0.51, 0.56 | +, + | |
| SS III | 2 | 0.84, 0.92 | *, + | |
| SS IV | 3 | 0.21, 0.83,0.91 | #, *, * | |
| Aqueous | SS I | 3 | 0.85, 0.86, 0.90 | *, +, + |
| SS II | 2 | 0.51, 0.56 | *, + | |
| SS III | 1 | 0.84 | # | |
| SS IV | 1 | 0.92 | + |
SS I-IV= solvent system I-IV, ‘#’ most intense, ‘*’ moderate intense, ‘+’ less intense
Table 7: Thin layer chromatography analysis of powdered leaves of S. oleosa
The present pharmacognostic data accentuated the awareness of quality and identity of the plant S. oleosa. The qualitative and quantitative parameters showed the important information of the plant. These studies revealed the presence of various important bioactive compounds and proved that the plant leaves can also be of medicinal importance. The plant being a morphologically variable species, these information will also be helpful to differentiate S. oleosa from the closely related other species. These results may help in standardization, identification and in carrying out further research in S. oleosa leaf-based drugs, which are used in traditional and modern pharmacopoeia.
Acknowledgements
The authors thank Mr. V. Chelladurai, Research Officer, Botany, Central Council for Research in Ayurveda and Siddha (Government of India), Tirunelveli, Tamil Nadu for identifying and authenticating the plant material.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Edeoga HO, Ok Wu DE, Mbaebie BO. Phytochemical constituents of some Nigerian Medicinal Plants. Afr J Biotech 2005;4(7):685-88.
- Akinmo-laudn AC, Ibukun EO, Afor E, Obuotor EM, Farombi EO. Phytochemical constituents and antioxidant activity of extracts from leaves of O. gratissimum. Sci Res Essay 2007;2:163-66.
- Rout SP, Choudhary KA, Kar DM, Das L, Jain A. Plants in traditional medicinal system-future source of new drugs. Int J Pharm Pharm Sci 2009;1(1):1-23.
- Saha D, Ramani R, Baboo B. Kusum - Multipurpose Tree, Yet Not Popular. Science Reporter 2010;47(02):20-2.
- Iwasa S. Schleichera oleosa (Lour.) Oken. In: Faridah Hanum I, van der Maesen LJG, editors. Plant Resources of South-east Asia No.11. Auxiliary Plants. Bogor, Indonesia: Prosea Foundation: 1997. p. 27-229.
- Rout SD, Panda T, Mishra N. Ethno-medical plants used to cure different diseases by tribal of Mayurbhanj district of North Orissa. Stud Ethno-Med 2009;3:27-36.
- Mahatma SP, Sahoo HP. An ethano medico botanical study of Bolangi, Orissa, India: native plant remedies against gynaecological diseases. Ethanobot Leafl 2008;12:846-54.
- Varghese S, Narmadha R, Gomathi D, Kalaiselvi M, Devaki K. Phytochemical screening and HPTLC finger printing analysis of Citrullus lanatus (Thunb.) seed. J Acute Dis 2013;122-26.
- https://forskning.ku.dk/find-en-forsker/?pure=files%2F34317454%2FSchleichera_oleosa_.pdf.
- Khandekar U, Bobade A, Ghongade R. Evaluation of antioxidant activity, in vitro antimicrobial activity and phytoconstituents of Schleichera oleosa (lour.) Oken. IJBPR 2015;6(2):137-43.
- Ghongade R. Phytochemical analysis of citrus fruit. Int J Pharma Bio Sci 2013;4(2B):1162-67.
- Kumar M, Mondal P, Borah S, Mahato K. Physico-chemical evaluation, preliminary phytochemical investigation, fluorescence and TLC analysis of leaves of the plant Lasia spinosa (lour) Thwaites. Int J Pharm Sci 2013;5(2):306-10.
- Mohan GK, Sachin YS, Manohar VP, Dipak LR, Sanjay JS. Pharmacognostical investigation and physicochemical analysis of Celastrus paniculatus willd. Leaves. Asian Pac J Trop Biomed 2012;2(3):S1232-S36.
- Wallis TE. Text book of Pharmacognosy. London: J. & A. Churchill Ltd.; 1985. p. 572-75.
- Udayan D, Nair SN, Padinchareveetil SK, Palayullaparambil AKT, Juliet S, Ravindran R, et al. Evaluation of Phytochemical constituents, Proximate and Fluorescence analysis of ethanolic extract and its fractions of Clerodendrum philippinum 1 Schauer found in Wayanad region of Kerala, India. Res J Chem Sci 2014;4(9):1-6.
- Chase CR. Pratt R. Fluorescence of powdered vegetable drugs with particular reference to development of a system of identification. J Am Pharm Assoc 1949;38(6):324-31.
- Ahmad A, Husain A, Mujeeb M, Khan SA, Alhadrami HAA, Bhandari A. Quantification of total phenol, flavonoid content and pharmacognostical evaluation including HPTLC fingerprinting for the standardization of Piper nigrum Linn fruits. Asian Pac J Trop Biomed 2015;5(2):101-07.
- Sharma D, Bhatia V, Patil S, Sharma PC. Antimicrobial activity of selected cryptogams from solan region. IJBPR 2013;4(6):448-54.
- Association of Official Analytical Chemists. Wet digestion for nonvolatile metals. In: AOAC official methods of analysis.16th ed. Virginia: Association of Official Analytical Chemists; 1998.
- Khandelwal KR. Practical pharmacognosy, techniques and experiments. 17th ed. Pune: Nirali Prakashan Publishers; 2007. p. 149-56.
- Anonymous. Indian Pharmacopoeia. Vol. I. Controller of Publications, 7th ed. New Delhi: Government of India; 2014. p. 98-9.
- Mukherjee PK. Quality control of herbal drugs. New Delhi; Business Horizons; 2002. p. 160-219.
- Okerele O. Who guidelines for the assessment of Herbal Medicines. Fitoterapia 1992;63(2):99-110.
- Indian Herbal Pharmacopoeia. Controller of Publications. 7th ed. New Delhi: Government of India; 2004. p. 493-97.
- Trease GE, Evans WC. Pharmacognosy. 11th ed. London: Bailliere Tindall Ltd.; 1978. p. 60-75.
- Harborne JB. Phytochemical methods– A guide to modern techniques of plant analysis. 2nd ed. London: Cassell and Collier Macmillan Publishers Ltd.; 1984, p. 9-15.
- Raman N. Phytochemical Techniques. New Delhi: New India Publishing Agency; 2006. p. 19-24.
- Harborne JB. Phytochemical methods. 1st ed. London: Chapman and Hall Ltd.; 1973. p. 49-188.
- Obdoni BO, Ochuko PO. Phytochemical studies and comparative efficacy of the crude extracts of some Homostatic plants in Edo and Delta States of Nigeria. Global J Pure Appl Sci 2001;8b:203-08.
- Boham BA, Kocipai-Abyazan R. Flavonoids and condensed tannins from leaves of Hawaiian Vaccinium vaticulatum and V. calycinium. Pac Sci 1974;48:458-63.
- Dewanto V, Xianzhong Wu, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem2002;50:3010-14.
- Medini F, Fellah H, Souri RK, Abdelly C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J Taibah Univ Sci 2014;8:216-24.
- Kumari A, Sharma RV. Estimation of total phenol, falconoid contents and DPPH free radical scavenging activity of Oxalis corniculata linn. IJBPR 2015;6(3):178-81.
- Sandhya M, Nidhi R, Sudhanshu, Menghani E. Determination of natural compounds in dasmool extracts by thin layer chromatography and high pressure liquid chromatography. Int J Res Ayu Pharm 2012;3(6):814-17.
- Harborne JB. Phytochemical methods: A Guide to Modern Techniques of Plant Analysis. 3rd ed. London: Chapman and Hall Ltd.; 1998. p. 40-96.
- Bladt S. Plant drug analysis: A Thin Layer Chromatography Atlas. Heidelberg, Germany: Springer-Verlag Berlin Heidelberg; 1996. p. 350-64.