- Corresponding Author:
- R. Niero
Post-Graduate Program in Pharmaceutical Sciences, Center for Chemical-Pharmaceutical Research (NIQFAR), University of Vale do Itajaí-UNIVALI, Itajaí, Santa Catarina, Brazil
E-mail: niero@univali.br
| Date of Submission | 04 November 2014 |
| Date of Revision | 22 November 2015 |
| Date of Acceptance | 06 February 2016 |
| Indian J Pharm Sci 2016;78(1):34-40 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Methanol extracts of seven edible fruits found in southern Brazil: Garcinia achachairu, Rubus imperialis, Rubus rosaefolius, Solanum quitoense, Solanum sessiliflorun, Diospyros inconstans and Plinia glomerata, were evaluated for their total phenol content and antioxidant activity in different in vitro free radical scavenging models. In addition, studies were performed on cell viability of extracts of the seeds of G. achachairu against murine melanoma cells. The fruits peel and seeds of G. achachairuwere very promising in terms of total phenol content (data in gallic acid equivalent per gram), as assessed by the Folin-Ciocalteu method, with values of 9.70±3.2 and 8.40±1.1, respectively. On the other hand, antioxidant activity using the 2,2-diphenyl-1-picrylhydrazyl scavenging assay showed that the fruit pulp and peel of P. glomerata presented the best profile, with values of the 16.3±1.8 and 15.9±2.4 μg/ml, respectively. Regarding the cytotoxic effect of methanol extract and guttiferone A from G. achachairu, we have observed that both inhibit the growth of B16F10 tumor cells, with calculated IC50values of 49.6±2.1 mg/ml and 48.6±5.4 mM, respectively.
Keywords
Antioxidant, cytotoxicity, Brazilian fruits, Garcinia achachairu
Medicinal plants are very important for the pharmaceutical industry, considering that they are a resource for the development of drugs such as phytomedicines and phytopharmaceutics, and also as prototypes for the synthesis of new drugs [1,2]. Among the various classes of naturally occurring antioxidants, phenolic compounds are the most widely studied. The plants studied are used in popular folk medicine due to their different therapeutic properties; however, there have been very few studies in the literature regarding their antioxidant effects or other therapeutic properties. In this work, we evaluated the chemical profile by thin layer chromatography and antioxidant effects of methanol extracts of some edible fruits found in southern Brazil as well as the cell viability of extract of the seeds of Garcinia achachairu against murine melanoma cells. The genus Garcinia is known to contain a wide variety of compounds, which show a wide range of biological and pharmacological properties, e.g., antioxidant, antiinflammatory, antimicrobial and cytotoxic activities [3-6]. In Brazil, Garcinia achachairu Rusby is popularly known as “achachairu” and is used in folk medicine to treat rheumatism, inflammation, pain and gastric disorders [7,8].
We investigated the antinociceptive, genotoxic and gastroprotector potential of the methanol extract and of a pure compound using classical models including Guttiferone A [5,6,9]. Solanum sessiliflorun (Solanaceae) is known as “manna”, “manna-cubiu”, and “Indian tomato”. It is originally from Western Amazonia and has been used in folk medicine to control hypercholesterolemia, uric acid, hypertension, and depression, which are attributed to high levels of niacin [10]. Previous studies shown antioxidant activity and antimicrobial effect against Helicobacter pylori [11,12]. R. imperialis is a shrub found in southern Brazil, popularly known as “amora branca” or “amora do mato” and used in folk medicine to treat several diseases such as diabetes, inflammation and pain [13]. Previous studies have shown significant antimicrobial and analgesic properties, and that the triterpene niga-ichigoside F1 isolated from aerial parts is responsible, at least in part, for the antinociceptive action. The probable mechanism of action is related to the dopaminergic, glutamatergic, and cholinergic pathways, besides involving the tachykininergic and oxinitrergic pathways, but it does not involve the participation of the opioid system [14,15]. Rubus rosaefolius is a shrub with white flowers and red berries, also known as “amora vermelha”. In folk medicine, the leaves are used to relieve menstrual cramps and diarrhea, and their fruit, if consumed in large quantities, has laxative action [16]. A study of the aerial parts indicated the presence of pentacyclic triterpenes such as tormentic acid and 28-O-methoxytormentic acid, along with three steroids. Specific assays showed significant antinociceptive effects, mainly against pain of inflammatory origin, with higher inhibition values when compared to clinically used drugs [17]. P. glomerata belongs to the Myrtaceae family and is known as “jabuticaba amarela”. Many of its species have antidiabetic, antimicrobial, antirheumatic, diuretic and digestive system regulation activity [18-20].
The results obtained by our research group revealed relevant antimicrobial activity against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Burkholderia cepacia, including a potent analgesic action that was related to the presence of phenolic compounds [21,22].
Materials and Methods
Gallic acid was acquired from Sigma Chemical Co., St. Louis, MO, USA. Folin-Ciocalteau reagent, ammonium iron (II) sulfate, butanol, methanol, hydrochloric acid (HCl), diethyl ether, ethyl acetate, anhydrous sodium sulfate, acetic acid and acetonitrile were purchased from Merck (Darmstadt, Germany). All reagents were of analytical grade or higher. The fruits of R. imperialis and R. rosaefolius were collected at São Sebastião in Treze de Maio-SC; fruits of G. achachairu in Camboriú-SC; and fruits of P. glomerata, D. inconstans, S. sessiliflorum and S. quitoense were obtained from Epagri (Santa Catarina State Agricultural Research and Rural Extension Agency), Itajaí-SC. All the species were identified at Vale do Itajaí University, and the respective exsiccates were registered (R. imperialis, R. rosaefolius, P. glomerata, D. inconstans, S. sessiliflorum and S. quitoense: VC-Filho 012, 035, 052, 053, 054 and 055; G. achachairu: HBR 52637) and deposited at the Barbosa Rodrigues Herbarium in Itajaí-SC. The fruits were macerated with methanol for seven days and the solvent was changed, to renew the extractor liquid and exhaust the plant drug. After the maceration process the solvent was evaporated in a rotatory evaporator under reduced pressure at 50° and placed in an appropriate desiccator container to obtain constant weight. The extracts obtained were named of crude methanol extract (CME).
Preliminary phytochemical analysis
Aliquot of CME and fractions soluble in chloroform were used to evaluate the chemical composition by thin layer chromatography (TLC). The following solvent systems were used, hexane:ethyl acetate (7:3) and chloroform:methanol (7:3), and they were revealed with the following specific reagents, Sulfuric anisaldehyde for steroids, terpenoids and glycosides compounds. Different standard solutions of steroids, terpenoids and sugars were used for the purposes of comparison.
Gas chromatography-mass spectrometry analysis
Gas chromatography-mass spectrometry analysis (GC-MS) analysis was carried out using a Shimadzu Gas Chromatograph (Model QP-2010S series) equipped with an AOC20i injector and a fused silica capillary column-TRX-1 (30 m × 0.25 mm), film thickness 0.1 μm. The injector temperature was maintained at 300° and the oven temperature started at 80°, maintained for 1 min. then the heat rate was increased by 25°/min to 210°. It was then raised by 15°/min until it reached 290°, finalizing by increasing the heat rate once again by 12°/min to 310° for a holding time of 10 min. Helium gas (99.999%) was used as carrier gas, at a constant flow rate of 0.80 ml/min. Samples of 2 μl of G. achachairu, the most promising material studied, were injected at a split ratio of 40:1. An MS transfer line was maintained at a temperature of 250° coupled with a mass spectrometric detector equipped with the NIST08 software database. The compounds were identified using the database of the NIST08 Library.
Total phenolic content
The total phenolic content (TPC) was determined by the Folin–Ciocalteau spectrophotometric method, using gallic acid (Vetec®, 99% purity) as external standard. For the determination, the extracts were dissolved in ethanol and diluted in distilled water. To 100 ml of each sample was added 1 ml of 10% (v/v) Folin-Ciocalteu. The mixture was incubated for 5 min, and then 1 ml of 10% NaCO2 was added. The mixture was then incubated for 90 min, and the absorbance was read at 750 nm using a UV/Vis Spectrophotometer SP 2000 UV (Bel Photonics). The calculation was performed through the equation obtained by the linear regression of the calibration curve and the result was expressed in mg gallic acid for g of dry extract.
Evaluation of the 2,2-diphenyl-1-picrylhydrazyl radical scavenging capacity
The DPPH radical scavenging activity of methanol extracts and guttiferone A were performed using the standard protocols reported earlier [23]. All the extracts and guttiferone A were diluted in ethanol to make 1-300 mg/ml dilutions. Two milliliter of each dilution was mixed with 1 ml of DPPH solution (0.004% in ethanol) and mixed thoroughly. After homogenization, the mixture was incubated at room temperature for 30 min. Absorbance was measured at 517 nm using a UV/Vis spectrophotometer. All the experiments were performed in triplicate at each concentration. The percentage scavenging of DPPH % by the extracts was calculated according to the following formula: DPPH= [(A-BA)×100]/C, where: A: Absorbance of the samples (with DPPH); BA: Absorbance of the blank samples (without DPPH) and C: Absorbance of the negative control (100% of DPPH). The radical scavenging activity was compared with vitamins C and trolox (analogous to the vitamin E), using the standard curve.
Evaluation of the ABTS radical scavenging capacity
The antioxidant activity of extracts and guttiferone A was assessed by the reducing activity of 2,2’-azinobis( 3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical, a product of the reaction between ABTS (7 mM) and potassium persulfate (2.45 mM) after incubation for 12 h at room temperature, in the dark, with agitation according to the methodology described by Re and Co-workers [24]. All the extracts and guttiferone A were dissolved with ethanol and diluted appropriately with water. One milliliter of test sample was mixed with 3 ml of ABTS solution and incubated at room temperature for 6 min. The ABTS radical reduction was monitored spectrophotometrically at 734 nm. The samples were compared with vitamins C and E and all were analyzed under the same conditions.
Evaluation of the antioxidant activity by the oxygen radical absorption capacity method
The experiment was carried out in a 96-well plate, as described by Poblete et al., with minor modifications [25]. Fifty microliter of a fluorescein solution (80 nM), 50 μl of the tested samples or 50 μl of sodium phosphate buffer 75 mM (pH 7.4) were added to the control wells, which were incubated at 37° for 15 min, then 100 μl of APPH (200 mM) was added. The antioxidant activity was kinetically monitored every 2 min for 80 min in a micro-plate reader at 485 nm and 528 nm. The antioxidant activity was expressed as the area under curve in the presence of the integrated fluorescence over time (ti: Initial time e tf: Final time) using the software program GraphPad Prisma®. The procedures were carried out in triplicate, and the results expressed in oxygen radical absorption capacity (ORAC) and millimolar unit equivalents of trolox/g.ml-1 of extract (mM TE/g.ml-1 of extract). ORAC=(AUC-AUCº)×f×(reference)/ (AUCreference-AUC°), where: AUC=Area under curve in the presence of the tested infusions, integrated between time zero and 80 min; AUCº=Area under curve for the control (blank); AUCreference=Area under curve for the reference compound (Trolox; 25 mM); f=dilution factor, equal to the ratio between the total volume of AAPH-FL solution and the added infusion volume; (reference)=Trolox milimolar concentration. This formula provides ORAC values in terms of the reference mmolar equivalents.
Cell culture
The tumoral cells (B16F10 – murine melanoma) were obtained from the Cell Bank of Rio de Janeiro State, Brazil. The cells were maintained in culture medium containing DMEM (Dulbecco’s Modified Eagle’s Medium), supplemented with 10% fetal bovine serum, 100 U/ml of penicillin, 100 μg/ml of streptomycin, 50 μg/ml of amphotericin B, and10 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) in pH 7.4, at 37° with 5% of CO2. The number of living cells was evaluated by the Trypan blue method [26] and the cell count were performed in a Neubauer chamber.
Cell treatment
The samples were dissolved in DMSO and diluted in culture medium in order to form the concentrations in the experiments (30-100 mg/ml for methanol extracts and 30-100 mM for guttiferone A). The cells (3×104) were incubated with samples in 96-well plates at different times. Cells that did not receive any kind of treatment, as well as those that received DMSO (0.1%) to dissolve the extracts, were used as controls.
Cellular viability assay
Cell viability was analyzed by the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (MTT) as described by Mosmann [27]. After the incubation time, the culture medium was removed and a new medium was added containing the MTT (0.25 μg/μl) followed by incubation for 2 h at 37°. The formazan precipitate was dissolved in (DMSO) and read with an ELISA micro-plate at 540 nm to establish the concentration response curves.
Statistical analysis
The results were expressed as mean±standard deviation of the mean. The arithmetic means was compared using analysis of variance (ANOVA) and Tukey’s test, using the software programs GrahPad Prisma 4.0 and Instat®. Values of 5% probability significance level (P<0.05) were considered.
Results and Discussion
Commonly, in plant extracts that present antioxidant activity, phenolic derivatives are the substance responsible for the effect found, and it is emphasized that their reductive properties depend on the chemical structure, playing an important role in the neutralization or sequestration of free radicals as well as transition metal chelating, acting as a starting point during the propagation stage of the oxidative process [28]. The antioxidant efficiency of bioactive compounds of plant origin depends on structure and concentration. However, the amount of these substances may be influenced by genetic factors, environmental conditions, degree of ripeness, plant variety, among others. With regard to the extractive solvent, methanol, to extract high amount of bioactive compounds, it has been indicated as the most effective [29] As part of the investigations to obtain compounds with biological potential from some plants adapted to our region, methanol extracts from edible fruits of seven species were investigated.
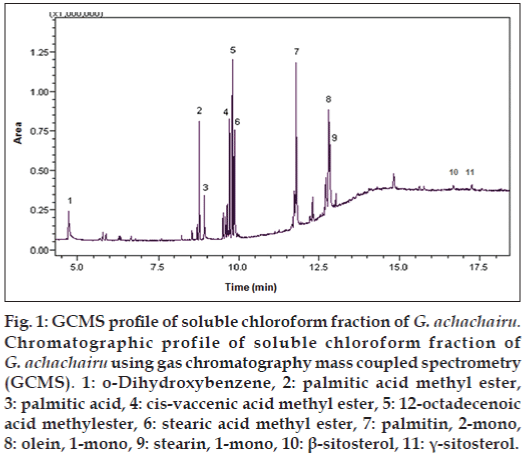
To the best of our knowledge, the TPC, antioxidant properties and preliminary phytochemical analysis by different chromatographic methods are being performed for the first time. Phytochemical analysis of methanol extract evidenced that sugars are the main compounds in all the studied samples. The presence of these compounds was confirmed by Co-TLC using standard samples of fructose, maltose and sucrose. Parallel studies showed the presence of considerable amount of flavonoids only in G. achachairu seeds, P. glomerata and D. inconstans when revealed with ferric chloride. On the other hand, some fatty acids and steroids were observed mainly in the soluble chloroform fraction of G. achachairu seeds, which was confirmed by gas chromatography mass spectrometry (fig. 1). Phenolic compounds are commonly found in plant extracts and attributed to antioxidant action. They act primarily by reducing properties dependent on their chemical structure mainly due to the presence of hydroxyl groups. This property plays an important role in the neutralization or sequestration of free radicals and the chelation of transition metals, acting both in the initiation and propagation step oxidative process. Total phenolic assay using Folin-Ciocalteu reagent is a simple and reproductive method and with this purpose it was used in this work. The results of TPC values per g of extract are shown in Table 1. As we observed, the values decreased in the following order: Fruits, peel and seeds of G. achachairu (9.70 and 8.40 mg GAE/g); D. inconstans seeds (9.30 mg GAE/g), P. glomerata pulp and fruit peel (8.90 and 8.30), S. quitoense fruit peel (8.80), R. imperialis whole fruits (8.10 mg GAE/g); R. rosaefolius whole fruits (5.40 mg GAE/g), and S. sessiliflorum fruit peel (4.20 mg GAE/g). However, the antioxidant activity using the DPPH scavenging assay did not follow the same order, with values of fruit peel and seeds for G. achachairu (92.9±9.1 and 27.0±3.2 μg/ml); D. inconstans seeds (164.4±8.9 μg/ml, P. glomerata pulp and fruit peel (16.3±1.8 and 15.9±2.4 μg/ml), S. quitoense fruit peel (134.6±3.7 μg/ml), R. imperialis whole fruits (24.0±1.2 μg/ml); R. rosaefolius whole fruits (291.7±4.2 μg/ml), and S. sessiliflorum fruit peel (313.3±1.2 μg/ml). Multivariate analysis did not show any correlation between TPC and antioxidant activity by DPPH (r=0.58) and ABTS (r=0.38) assays, which indicates that other compounds may contribute significantly to antioxidant activity [30,31].
| Studied species | Studied part | Total phenols (mg GAE/g) | DPPH (CE50 µg/ml) | ABTS (CE50 µg/ml) | ORAC (mM TE/g/ml) |
|---|---|---|---|---|---|
| G. achachairu | Seeds | 8.4±1.1a | 27.0±3.2a | 23.28±1.43a | 1550.3±3.2a |
| G. achachairu | Fruit peel | 9.7±3.2a | 92.9±9.1d | NT | NT |
| S. quitoense | Fruit peel | 8.8±2.4a | 134.6±3.7c | 99.71±0.1c | NT |
| R. rosaefolius | Whole fruit | 5.4±0.8b | 291.7±4.2e | 135.52±0.2d | NT |
| R. imperialis | Whole fruit | 8.1±2.2a | 24.0±1.2a | NT | NT |
| D. inconstans | Seeds | 9.3±1.6a | 164.4±8.9f | 73.20±0.9b | NT |
| S. sessiliflorun | Fruit peel | 4.2±0.8b | 313.3±1.2g | NT | NT |
| P. glomerata | Pulp and seeds | 8.9±1.4a | 16.3±1.8b | 24.8±0.7a | NT |
| P. glomerata | Fruit peel | 8.3±2.1a | 15.9±2.4b | 23.54±1.2a | 133.4±1.2b |
| Trolox | - | - | 11.9±2.1 | 8.2±0,9 | NT |
| Vitamin C | - | - | 6.8±1.1 | 6.6±1.1 | NT |
| Guttiferone A | - | - | 10.75±2.7h | 8.48±1.46e | 404.0±1.5c |
Values are expressed as mean±SD of three analytical replicates (n=3). Means in the same column with different superscript letters are significantly different (P<0.05), NT: Not tested, DPPH: 2,2-diphenyl-1-picrylhydrazyl, ABTS: 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid), ORAC: oxygen radical absorption capacity, GAE: gallic acid equivalent, SD: standard deviation, G. achachairu: Garcinia achachairu, S. quitoense: Solanum quitoense, R. rosaefolius: Rubus rosaefolius, R. imperialis: Rubus imperialis, D. inconstans: Diospyros inconstans, S. sessiliflorun: Solanum sessiliflorun, P. glomerata: Plinia glomerata.
Table 1: Antioxidant activities and total phenolic content in seven edible fruits
Figure 1: GCMS profile of soluble chloroform fraction of G. achachairu. Chromatographic profile of soluble chloroform fraction of G. achachairu using gas chromatography mass coupled spectrometry (GCMS). 1: o-Dihydroxybenzene, 2: palmitic acid methyl ester, 3: palmitic acid, 4: cis-vaccenic acid methyl ester, 5: 12-octadecenoic acid methylester, 6: stearic acid methyl ester, 7: palmitin, 2-mono, 8: olein, 1-mono, 9: stearin, 1-mono, 10: β-sitosterol, 11: γ-sitosterol.
The most promising results were found for the P. glomerata and G. achachairu seeds, which showed activity that was statistically similar to the positive controls Trolox and vitamin. Celli et al., studied the antioxidant activity of the fruit extract of two varieties of Eugenia uniflora L. (red and purple). For both varieties, the best antioxidant activity was the immature stage (extracts sequestered 75 % DPPH) [32].
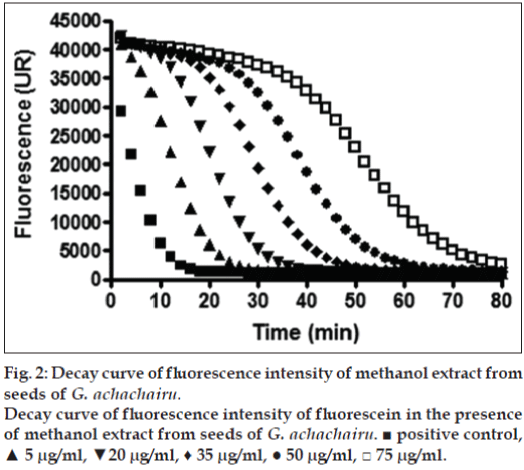
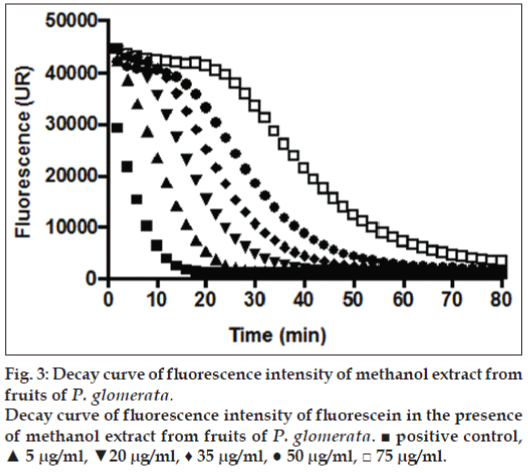
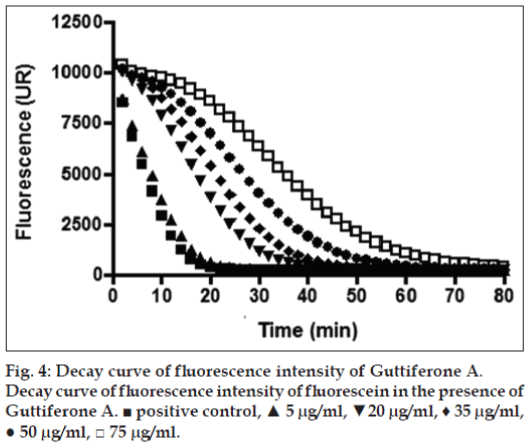
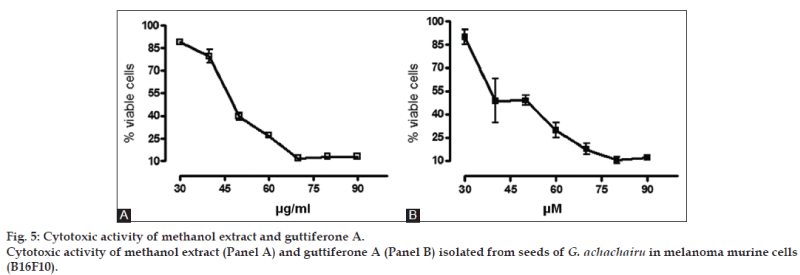
The above mentioned study demonstrates that the concentration of secondary metabolites appears to be greatest during the development of the fruit, since most of these metabolites are responsible for the defense role, whereas in the maturation stage, there is an increase in the concentration of sugars as reported in our results [32]. Considering that the G. achachairu and P. glomerata extracts showed the most promising results, the ORAC assay was used. The ORAC combines time and the percent of inhibition of the action of the radical by the antioxidant, which is the method that measures the radicals generated and uses the area under the curve (AUC) for quantification, instead of the “lag phase” used by other methods [33]. As shown in fig. 2, the AUC of the control (AAPH+fluorescein) was 226.975 while the AUC extract at the highest concentration tested (75 mg/ml) was 2.03×106 (1550.3 mM TE/g.ml-1). Fig. 3 shows the decay of the fluorescence intensity of fluorescein in the presence of radical AAPH and different concentrations of methanol extract from fruit peel of P. glomerata. The AUC of the control (AAPH+fluorescein) was 226.975 while the AUC of the methanol extract at the highest concentration tested was 1.78×106 (133.4 mM TE/g.ml-1). Based on our previous studies on guttiferone A, the main compound isolated from the seeds, which showed a significant antinociceptive and antiulcerogenic effect [5,6], was also evaluated against the ORAC assay (fig. 4). As can be observed, the AUC of the control (AAPH+fluorescein) demonstrated a value of 72.064 and for guttiferone A, this value was the 356.171, which is equivalent to 404 mM TE/g.ml-1 of guttiferone A. Ngouela et al. analyzed the antioxidant activity of guttiferone A using the free radical scavenging activity of DPPH, which showed good radical scavenging activity with 89% inhibition [34]. These data corroborate with the results indicated here and justify, at least in part, the activity verified for the methanol extract of G. achachairu. We also evaluated the cytotoxic effect of methanol extract and guttiferone A isolated from seeds of G. achachairu. It was observed that both inhibited the growth of B16F10 tumor cells with IC50 values of 49.6±2.1 mg/ml and 48.6±5.4 mM, respectively (fig. 5). In conclusion we remark that these fruits, particularly P. glomerata and G. achachairu showed promising results that could be passed on, in order to guide the people who use these plants for this purpose.
Acknowledgements
The authors also are grateful to Dr. Eliseo Soprano and Sir Elias Fratoni for providing the plant material and Dr. Oscar Benigno Iza of Vale do Itajaí University for the botanical support.
Financial support and sponsorship
The authors are grateful to CNPq, UNIVALI and FAPESC-Brazil for their financial support.
Conflicts of interest
There are no conflicts of interest.
References
- Barreiro EJ, Bolzani VS. Biodiversity: Potential source for drug discovery. Quim Nova 2009;32:679-88.
- Cragg GM, Grothaus PG, Newman DJ. New horizons for old drugs and drug leads. J Nat Prod 2014;77:703-23.
- Mbwambo ZH, Kapingu MC, Moshi MJ, Machumi F, Apers S, Cos P, et al. Antiparasitic activity of some xanthones and biflavonoids fromthe root bark of Garcinialivingstonei. J Nat Prod 2006;69:369-72.
- Naldoni FJ, Claudino AL, Cruz JW Jr., Chavasco JK, Faria e Silva PM, Veloso MP, et al. Antimicrobial activity of benzophenones and extracts from the fruits of Garciniabrasiliensis. J Med Food 2009;12:403-7.
- Dal Molin MM, Silva S, Alves DR, Quintão NL, DelleMonache F, CechinelFilho V, et al. Phytochemical analysis and antinociceptive properties of the seeds of Garciniaachachairu. Arch Pharm Res 2012;35:623-31.
- Niero R, Dal Molin MM, Silva S, Damian NS, Maia LO, DelleMonache F, et al.Gastroprotective effects of extracts and guttiferone A isolated from GarciniaachachairuRusby (Clusiaceae) against experimentally induced gastric lesions in mice. NaunynSchmiedebergs Arch Pharmacol 2012;385:1103-9.
- Alves TM, Silva AF, Brandão M, Grandi TS, Smânia E, SmâniaJúnior A, et al. Biological screening of Brazilian medicinal plants. MemInstOswaldo Cruz 2000;95:367-73.
- Barbosa W, Chagas EA, Martins L, Pio R, Tucci ML, Artioli FA. Seeds germination and seedlings early development of achachairu. Rev Bras Frut 2008;30:263-6.
- Terrazas PM, de Souza Marques E, Mariano LN, Cechinel-Filho V, Niero R, Andrade SF, et al.Benzophenoneguttiferone A from GarciniaachachairuRusby (Clusiaceae) presents genotoxic effects in differentcells of mice. PLoS One 2013;8:e76485.
- Pereira MD, Filho-Martins S. Accelerated aging of cubiu (SolanumsessiliflorumDunal) seeds. PesqAgropec Trop 2005;40:251-6.
- Sandoval MAP. In vitro effect of Solanumsessiliflorum “cocona” extract on the growth of Helicobacter pylori. Ciênc Invest 2010;13:30-3.
- Rodrigues E, Mariutti LR, Mercadante AZ. Carotenoids and phenolic compounds from Solanumsessiliflorum, an unexploited Amazonian fruit, and their scavenging capacities against reactive oxygen and nitrogen species. J Agric Food Chem 2013;61:3022-9.
- Cechinel-Filho V, Niero R. Therapeutic potential and chemical composition of plants from the genus Rubus: A mini review of the last 10 years. Nat Prod Commun 2008;3:437-44.
- Ardenghi JV, Kanegusuku M, Niero R, Filho VC, Monache FD, Yunes RA, et al. Analysis of the mechanism of antinociceptive action of niga-ichigoside F1 obtained from Rubusimperialis (Rosaceae). J Pharm Pharmacol 2006;58:1669-75.
- Jorge LI, Markman BE. Histological characterization of the leaves and fruits of Rubusrosaefolius Smith. Rev Inst Adolfo Lutz 1993;53:1-4.
- Kanegusuku M, Sbors D, Bastos ES, de Souza MM, Cechinel-Filho V, Yunes RA, et al. Phytochemical and analgesic activity of extract, fractions and a 19-hydroxyursane-type triterpenoid obtained from Rubusrosaefolius (Rosaceae). Biol Pharm Bull 2007;30:999-1002.
- Oliveira FQ, Gobira B, Guimarães C, Batista J, Barreto M, Souza M. Plants species indicated in odontology. Rev Bras Farmacog 2007,17:466-76.
- Souza-Moreira TM, Severi JA, Santos E, Silva VY, Vilegas W, Salgado HR, et al. Chemical and antidiarrheal studies of Pliniacauliflora. J Med Food 2011;14:1590-6.
- Souza-Moreira TM, Severi JA, Lee K, Preechasuth K, Santos E, Gow NA, et al. Anti-Candida targets and cytotoxicity of casuarinin isolated from Pliniacauliflora leaves in a bioactivity-guided study. Molecules 2013;18:8095-108.
- Serafin C, Nart V, Malheiros A, Cruz AB, DelleMonache F, Gette MA, et al. Evaluation of the antimicrobial effects ofPliniaglomerata (Myrtaceae). Rev Bras Farmacog 2007;17:578-82.
- Fischer LG, Santos D, Serafin C, Malheiros A, DelleMonache F, DelleMonache G, et al. Further antinociceptive properties of extracts and phenolic compounds from Pliniaglomerata (Myrtaceae) leaves.Biol Pharm Bull 2008;31:235-9.
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Food SciTechnol 1995;28:25-30.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cationdecolorization assay. Free RadicBiol Med 1999;26:1231-7.
- Poblete A, Lopez-Alarcon AC, Lissi BE, Campos AM. Oxygen radical antioxidant capacity (ORAC) values of herbal teas obtained employing different methodologies can provide complementary data. J ChilChemSoc 2009;54:153-7.
- Freshney R. Culture of animal cells. In: A Manual of Basic Technique. New York: Alan R. Liss. Inc.; 1987. p. 117.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63.
- Sousa CM, Silva HR, Vieira Junior GM, Ayres MC, Costa CL, Araújo DS, et al. Total phenolic and antioxidant activity of five medicinal plants. Quim Nova 2007;30:351-5.
- Moure A, Cruz JM, Franco D, Dominguez JM, Sineiro J, Dominguez H, et al. Natural antioxidants from residual sources. Food Chem 2001;72:145-71.
- Reynertson KA, Yang H, Jiang B, Basile MJ, Kennelly EJ. Quantitative analysis of antiradical phenolic constituents from fourteen edible Myrtaceaefruits. Food Chem 2008;109:883-90.
- Scalzo J, Politi A, Pellegrini N, Mezzetti B, Battino M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 2005;21:207-13.
- Celli GB, Pereira-Netto AB, Beta T. Comparative analysis of total phenolic content, antioxidant activity, and flavonoids profile of fruits from two varieties of Brazilian cherry (Eugenia uniflora L.) throughout the fruit developmental stages. Food Res Int 2011;44:2442-51.
- Vasconcelos SM, Goulart MO, Moura JB, Manfredine V, Benfato MS, Kubota LT. Reactive oxygen and nitrogen species, antioxidants and markers of oxidative damage in human blood: Main analytical methods for their determination. Quim Nova 2007;30:1323-38.
- Ngouela S, Lenta BN, Noungoue DT, Ngoupayo J, Boyom FF, Tsamo E, et al. Anti-plasmodial and antioxidant activities of constituents of the seed shells of Symphoniaglobulifera Linn f. Phytochemistry 2006;67:302-6.