- *Corresponding Author:
- Ramakrishnan Padmini
Department of Biochemistry, Vels Institute of Science, Technology and Advanced Studies, Pallavaram, Chennai, Tamil Nadu 600117, India
E-mail: padmini.sls@velsuniv.ac.in
| Date of Received | 17 February 2021 |
| Date of Revision | 21 April 2022 |
| Date of Acceptance | 19 October 2022 |
| Indian J Pharm Sci 2022;84(5):1309-1322 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This research aimed to explore the antioxidant potency of leaf extracts of Syringodium isoetifolium seagrass. In this study, seagrass leaves were collected from the local tribe’s source, dried and extracted with water, alcohol, hydro alcohols, acetone and n-hexane. Phytochemical investigation on extracts was performed using chemical and gas chromatography-mass spectrometric techniques. It reveals that the extracts are enriched with pharmacologically active compounds such as phenol, polyphenol, alkaloid, glycoside, saponin, flavonoids, dicarboxylic acids and other constituents. Later, the crude extracts were estimated for antioxidant activity using different in vitro methods such as 2,2-diphenyl-1-picryl-hydrazyl-hydrate, total antioxidant, nitric oxide, superoxide anion and hydroxyl radical scavenging activities. The result demonstrated that the extracts exhibited higher radical scavenging activity as compared to the ascorbic acid. Among the different extracts, the hydroalcoholic extract had shown an excellent antioxidant activity due to the existence of more potent phytochemicals.
Keywords
Syringodium isoetifolium, leaf extracts, phytochemical screening, antioxidant activity
The ecosystem is the structural and functional component of ecology where the living things interact with each other and the environment[1]. There are many ecosystems existing in the galaxy[2]. Marine ecosystems are the main ecosystems and exist in sea waters[3]. In which, seagrasses are a vital part of the marine ecosystem, found in shallow, sheltered, softbottomed marine waters[4]. They were found all along with the coastal areas of India[5,6]. These are belonging to the families of Cymodoceaceae, Zoseraceae, Hydrocharitaceae and Posidoniaceae[7]. Around the earth, 52 species of seagrasses were found so far in which 14 species were identified in the west and east coastal part of India[8]. The biomass of seagrass has been utilized frequently as food and drug by coastal indigenous people[9]. Seagrasses are well documented for the presence of potent diverse secondary metabolites. There is an immediate need to quantitatively survey the traditional knowledge of seagrasses in areas where they are abundant and serve as an important resource to coastal communities. In folk medicine, seagrasses have been employed for many therapeutic purposes such as skin diseases, fever, wounds, stomach problems, muscle pains and as a remedy against different kinds of rays[10]. They also provide different pharmacological activities like antioxidant[11], anti-microbial[12], antiviral[ 13], against stomach problems[14], anti-diabetic[15], wounds[16], tranquillizer[17] and anticancer[18] activities etc. Human pollution has contributed most to seagrass declines around the world. There is a necessity to take some initiation to preserve the seagrass and ensure the existence of seagrass to the poor people in future. For many decades, herbal medicines have been used in developing countries as the primary source of medical treatments. The root of Cymodocea sp. is eaten as food commonly known as sea sugarcane. Some of Cymodocea sp. are used against malaria, cough and also used as tranquilizers for babies. Halophila ovalis was used by the fishing communities of Cuddalore and Nagapattinam districts of Tamil Nadu, South India as medicine to treat various skin diseases, burns and boils. Cymodocea sp. are being used as a tranquilizer for babies, as soothing helps during pregnancy and against cough and malaria. Halophila stipulacea (Forssk.) Asch., Cymodocea serrulata (R. Br.) Asch and Magnus and Halodule pinifolia (Miki) Hartog possessed effective antimicrobial effects against seven human pathogens[19,20]. From the past few decades, there has been an upsurge in the search for antioxidant drugs derived from plants.
In the review of the above observation and continuation of our interest to identify the therapeutic activity of Syringodium isoetifolium (S. isoetifolium), antioxidant activity was evaluated. The result suggested that all the extracts of S. isoetifolium were shown excellent activity like standard drugs and it is an important milestone in the development of a naturally derived antioxidant drug for various oxidative stress-related diseases.
Materials and Methods
Collection of seagrass:
An excellent quality of S. isoetifolium from the family of Cymodoceaceae was collected from Devipattinam, Ramanathapuram District, Tamilnadu state, India. The collected materials of the plants were authenticated by Dr M. U. Sharief, Scientist E and head, Botanical Survey of India, Southern Regional Centre, T. N. A. U Campus, Coimbatore 641003, India. The Voucher specimen number of the S. isoetifolium is BSI/SRC/5/23/2021/ Tech/372.
Preparation of seagrass extracts:
Collected seagrasses were washed thoroughly with sterile seawater to remove the extraneous dirt, dried in a shade, pulverized and prepared extracts with different solvents of increasing polarity such as distilled water, alcohol, hydro alcohol (30:70), acetone and n-hexane. After drying, the seagrass were sliced into small pieces and then mashed until it becomes a fine powder. The powdered seagrass was immersed in 450 ml of cold solvents in a closed container with gentle shaking for 24 h. These seagrass extracts were collected, filtered and the solvent was eliminated at 60° in the rotary evaporator (model: rotavapor R-210 from Buchi)[21,22]. The solid seagrass extracts have persevered in a dark container (fig. 1). The yield was calculated using the following formula
Percentage Yield=(W1×100)/W2
Here W1-Weight of extract after removing the solvent; W2-Dry weight of the sample.
These plant extracts were utilized for the identification of different phytochemicals present in S. isoetifolium.
Quality control of S. isoetifolium:
Determination of ash value: 3 gm of S. isoetifolium powder was incinerated in a Silica crucible over the burner. The charred material was heated in muffle furnace for 6 h at 600°.The ash formed was white and free from carbon. It was cooled and weighed on the ash less filter paper[23].
Powder fineness and sieve size: Place 100 g of the powder being examined upon the appropriate sieve having a close fitting receiving pan and cover. Shake the sieve in a rotary horizontal direction and vertically by tapping on the hard surface for not less than 20 min. Weigh accurately the amount remaining on the sieve and in the receiving pan[24].
Phytochemical analysis:
The collected dried extracts of S. isoetifolium were analyzed for the presence of different phytochemicals by chemical and Gas Chromatography-Mass Spectrometry (GC-MS) techniques. In the chemical method, phytochemicals were identified through qualitative and quantitative ways using appropriate reagents. Whereas, GC-MS compounds were recognized through their mass value and distinctive mass fragmentation patterns[25].
Chemical method:
Chemicals: All chemical substances were purchased with the highest purity (≥98.0 %) and utilized for the identification of different phytochemicals present in the leaf extract of S. isoetifolium. The chemicals such as Ferric chloride (FeCl3), Olive oil, Folin-Ciocalteu reagent, Aluminium chloride (AlCl3), Ammonia, Sulfuric acid (H2SO4), Hydrochloric acid (HCl), Acetic anhydride, Chloroform, Mayer reagent, Ethanol, Ferricyanide, Glacial acetic acid, Sodium hydroxide (NaOH), Ammonium hydroxide (NH4OH), Gallic acid, Sodium carbonate (Na2CO3), Ammonium chloride (NH4Cl), Methanol, Potassium acetate (CH3COOK), Folin-Denis reagent, 2,2-Diphenyl-1-Picryl-Hydrazyl- Hydrate (DPPH), Sodium phosphate, Ammonium molybdate, Sodium nitroprusside, Phosphate buffer, Sulfanilic acid reagent, 1,10-phenanthroline, Butylated Hydroxyl Toluene (BHT) etc., were all purchased from Merck, United States of America (USA).
Qualitative phytochemicals screening:
Chemical tests were performed with different reagents using standard procedures to know the phytochemicals present in the leaf extracts of S. isoetifolium[26].
Quantitative analysis of phytochemicals:
Total Phenolic Content (TPC): Approximately 1 ml of seagrass extract or Gallic acid standard phenolic compound was mixed to 9 ml of distilled water in a volumetric flask. About 1 ml of Folin-Ciocalteu’s phenol reagent was added and blended gently. After a few minutes, nearly 10 ml of 7 % Na2CO3 solution was admixed into the above solution. The solution was diluted to 25 ml of purified water. The concoction was kept aside for 1.5 h at 23°, after which the absorbance was examined at 750 nm. The TPC was calculated from the extrapolation of calibration curve which was made from the different concentrations of seagrass/gallic acid solutions (20 to 80 μg/ml). The TPC was expressed as milligrams of Gallic acid equivalents per gram in a dried seagrass sample[27].
Total Flavonoids Content (TFC): The TFC was conducted according to Hossain et al.[28] using the colorimetric method. About 0.5 ml of seagrass extracts were mixed with 1.5 ml of methyl alcohol, 0.1 ml 10 % AlCl3, 0.1 ml 1 M CH3COOK and 2.8 ml of water. The above mixture remained at 20°-25° for 30 min and absorbance was measured at 510 nm. The calibration curve was made using quercetin solutions at the concentration of 20 to 80 μg/ml in methyl alcohol. TFC was disclosed as mg of QE/g of dry weight[28].
Total Saponins Content (TSC): The TSC in different concentrations of plant extracts were estimated by colorimetric methods. 0.25 ml of seagrass extract admixed with 1 ml of reagent mixture (H2SO4/glacial acetic acid 1:1 v/v). The content was heated at 60° for 30 min in a water bath and kept aside for some time. The absorbance of seagrass extract was calculated at a wavelength of 527 nm using a spectrophotometer. The TSC was expressed as 100 g-1 of oleanolic acid equivalents[29].
Total Tannin Content (TTC): The TTC was performed according to Medini et al.[30] method. 0.2 ml of seagrass extract admixed with 0.5 ml of Folin-Denis reagent, 1 ml of Na2CO3 solution and 7.5 ml of water. The content of the concoction was mixed well, kept aside for 30 min and the absorbance was measured at 760 nm. TTC was expressed as milligrams of tannic acid equivalents per gram of dried sample. The results were evaluated by correlation coefficient (R2) and linear regression and using Microsoft excel[30].
GC-MS analysis:
GC-MS analysis of aqueous, ethanol and hydro alcoholic extracts of S. isoetifolium was done at SRM institute of science and technology, Kattankulathur, Chennai using Agilent 7890B GC connected to 5977A mass selective detector, furnished with Electron Ionization (EI) and a fused phenyl methyl silox column HP-5 (30 m×250 μm×0.25 μm film thickness) was used. The oven temperature was modified from -60°-325° for 55 min. Highly pure helium gas was used as carrier for this study. The flow rate of carrier gas was fixed 1 ml/min, for sample injection of 1 μl and the ionization voltage of MS-analysis was controlled by EI procedure at 70 eV[31].
Antioxidant activity:
Preparation of sample: S. isoetifolium leaves were airdried at room temperature and pulverized into powder for extraction. The powder (20 g) was macerated in 1000 ml of hydro alcohol (30:70 %), shake vigorously for about 1 h using a rotatory shaker. The mixture was kept aside for 24 h and then the extracts were filtered with Whatman filter paper (No.1). The filtrate was drie concentrations (20, 40, 60 and 80 μg/ml) for in vitro antioxidant activity[31].
DPPH radical-scavenging activity:
The activity was done according to Gopi et al.,[32]. Take a 2 ml DPPH methyl alcohol solution (25 μg/ ml) and mix with 0.5 ml seagrass extracts at various concentrations. The above content was stirred and allowed to rest at 30°-35° for 30 min. The absorbance was calculated at 517 nm.
Percentage radical scavenging activity=100-(Ac-As)/ Ac×100
Where AC=Absorbance in control; AS=Absorbance of extracts.
Total Antioxidant Capacity (TAC):
The phosphomolybdate technique has been employed regularly to assess the TAC of seagrass extracts[33]. In the existence of seagrass extracts, a green-colored phosphomolybdenum V complex formed, which shows absorbance at 695 nm. 0.3 ml of seagrass extracts was mixed with 3 ml of reagent (28 M sodium phosphate, 0.6 M H2SO4 and 4 M ammonium molybdate) in a tube. It was incubated at 95° for 1.5 h. The absorbance was measured at 695 nm. Methyl alcohol (0.3 ml) is used as a blank[31]. The TAC was calculated according to the following equation Percentage inhibition=A0-A1/A0×100
Where A0=Absorbance of the control and A1=Absorbance of the extracts.
Superoxide anion scavenging activity:
It was measured according to Pavithra et al.[34]. In this experiment, the superoxide anion was produced in 3 ml of Tris-HCl buffer (100 mM, pH 7.4) containing 0.75 ml of Nicotinamide Adenine Dinucleotide (NADH) (936 μM) solution, 0.75 ml of nitro blue tetrazolium chloride (300 μM) solution and 0.3 ml of various concentrations of the extracts. The reaction started with the addition of 0.75 ml of phenazine methosulfate (120 μM) to the mixture. After 5 min at room temperature, the absorbance was measured at 560 nm. The activity was calculated according to the following formula
Percentage inhibition=A0-A1/A0×100
Where A0=Absorbance of the control and A1=Absorbance of the seagrass extracts.
Nitric Oxide (NO) scavenging activity:
The NO scavenging activity was determined according to Ali et al.,[35]. Sodium nitroprusside in an aqueous solution produces NO, which reacts with oxygen to generate Nitrite ions (NO2-). Take 2 ml of 10 mM sodium nitroprusside in 0.5 ml phosphate buffer saline (pH 7.4) was mixed with 0.5 ml of seagrass extracts at different concentrations and the mixture was incubated at 25° for 2.5 h. After incubation, 0.5 ml was taken out and added into a 1.0 ml sulfanilic acid reagent (33 % of sulfanilic acid in 20 % glacial acetic acid) and incubated at 30°-35° for 5 min. Finally, 1.0 ml naphthyl ethylenediamine dihydrochloride (0.1 % w/v) was added and incubated 30°-35° for 30 min. The absorbance was measured at 540 nm using a spectrophotometer. The scavenging activity was measured by using the following equation
Percentage inhibition=A0-A1/A0×100
Where A0=Absorbance of the control and A1=Absorbance of the extracts.
Hydroxyl radical scavenging activity:
Hydroxyl radicals scavenging activity was calculated by Fenton reaction according to Sowndhararajan et al.[36]. The hydrogen peroxide was added to the reaction mixture containing 90 μl of 1 mM 1, 10-phenanthroline, 60 μl of 1.0 mM FeCl3, 2.4 ml of 0.2 M phosphate buffer (pH 7.8), 150 μl of 0.17 M H2O2 and 1.5 ml of seagrass extracts at different concentrations. After incubation at 30°-35° for 5 min, the absorbance was measured at 560 nm using a spectrophotometer. The radical (OH.) scavenging activity was calculated using the following formula
Percentage inhibition=A0-A1/A0×100
Where A0=Absorbance of the control and A1=Absorbance of the extracts.
Statistical method:
All the scavenging activities were done in triplicates. The quantity of seagrass required to inhibit radicals concentration by 50 % was graphically calculated by a linear regression method using GraphPad Instat software (version 3). The result is shown as graphically/mean±standard deviation.
Results and Discussion
An excellent quality of leaf of S. isoetifolium from the family of Cymodoceaceae was collected from Devipattinam, Ramanathapuram District, Tamilnadu state, India. Collected seagrasses were washed thoroughly with sterile seawater to remove the extraneous dirt, dried in a shade, pulverized into fine powder. It was used to study the quality control test such as ash value and degree of coarseness or fineness of the powder. The ash content of the seagrass was determined using a dry ashing method. It shows leaf of S. isoetifolium contained ash value of 29.42 %±0.35 %. The degree of coarseness or fineness of a powder is differentiated by nominal aperture size of the mesh of the sieve through which the powder is able to pass, expressed in μm. The particles of the powder which pass through a No. 355 sieve and not more than 40 % through a No.180 sieve. Therefore, the coarseness or fineness of the leaf powder is moderately fine. The results of this study are in line with the comparative proximate composition values for different species of the seagrasses. The presence of phytochemicals was identified through the chemical methods and GCMS technique. The phytochemicals were confirmed in the chemical method through the qualitative and quantitative tests using different reagents (Table 1). Several phytochemicals such as tannin, saponin, steroids, terpenoids, triterpenoids, anthraquinone, flavonoids, polyphenol, glycosides, alkaloids and coumarins were present in Aqueous Extracts of S. isoetifolium (AESI), Ethanol Extracts of S. isoetifolium (EESI) and Hydro Alcohol Extracts of S. isoetifolium (HAESI), Acetone Extracts of S. isoetifolium (ACESI) and n-Hexane Extracts of S. isoetifolium (HESI) (Table 2). The phytochemical analysis of the S. isoetifolium was examined and summarized in Table 1-Table 8 and fig. 1-fig. 7. This was highly supported by the previously published studies on seagrass[37,38]. Among the different extracts, the hydroalcoholic extract had shown the highest yield of phytochemicals such as TPC (192.44±13.47 mg GAE/g), TFC (106.54±7.45 mg quercetin/g), TSC (52.61±3.68 mg Quillaja/g) and TTC (80.65±5.64 mg tannic acid/g). Moreover, the quantitative analysis data for S. isoetifolium in the present study was found to be consistent with those reported in the past[39-42].
| Name of Sample | Total phenol (Milligrams GAE equivalents per gram) | Flavonoids (Milligrams of quercetin equivalents per gram) | Saponin (Milligrams of Quillaja saponin equivalents per gram) | Tannin (Milligrams of tannic acid equivalents per gram) |
|---|---|---|---|---|
| S. isoetifolium | 192.44±13.47 | 106.54±7.45 | 52.61±3.68 | 80.65±5.64 |
Table 1: Quantitative Analysis Of Phenol, Flavonoids, Saponin And Tannin Content Present In The Leaf Extracts Of S. isoetifolium
| S. No | Phytochemicals | Aqueous extract | Ethanol extract | Hydro-alcoholic extract (30:70 %) | Acetone | Chloroform |
|---|---|---|---|---|---|---|
| 1 | Tannin | ++ | ++ | ++ | - | + |
| 2 | Saponin | ++ | ++ | ++ | - | - |
| 3 | Flavonoids | + | ++ | ++ | - | + |
| 4 | Steroids | + | ++ | + | - | - |
| 5 | Terpenoids | + | + | + | + | - |
| 6 | Triterpenoids | + | + | + | + | - |
| 7 | Alkaloids | + | ++ | + | - | + |
| 8 | Anthroquinone | + | + | + | - | - |
| 9 | Polyphenol | ++ | ++ | ++ | - | - |
| 10 | Glycoside | + | + | + | + | - |
| 11 | Coumarins | + | + | + | + | - |
| 12 | Emodins | - | - | - | - | - |
| 13 | Anthocyanins | - | - | - | - | - |
| 14 | Carbohydrate | + | + | + | + | + |
| 15 | Carboxylic acid | + | + | + | + | + |
Note: (“+” indicates the presence of the compounds; “-” indicates an absence of the compounds and “++” indicates the high concentration)
Table 2: Qualitative Phytochemical Analysis of Leaf Extracts of S. isoetifolium
| Peak | R. Time | Area % | Height % | MW | MF | Name of the compounds |
|---|---|---|---|---|---|---|
| AESI-A | ||||||
| 1 | 4.226 | 89.303 | 52862499 | 92 | C7H8 | Toluene |
| 2 | 28.607 | 0.029 | 99898 | 296 | C21H44 | Heptadecane, 2,6,10,,15-teetramethyl phthalic acid |
| 3 | 32.255 | 0.033 | 116198 | 306 | C18H26O4 | Hex-3-yl isobutyl ester |
| 4 | 33.05 | 0.016 | 83883 | 324 | C23H48 | Heptadecane, 9-hexyl- |
| 5 | 33.243 | 0.045 | 134057 | 270 | C17H34O2 | hexadeconic acid methyl ester |
| 6 | 34.164 | 0.033 | 85822 | 278 | C16H22O4 | Dibytylphthalate |
| 7 | 34.58 | 0.157 | 141928 | 256 | C16H32O2 | n-Hexadeconic acid |
| 8 | 36.482 | 0.07 | 355706 | 294 | C19H34O2 | 11,14-Octadecadienoic acid methyl ester |
| 9 | 36.578 | 0.225 | 873038 | 296 | C19H36O2 | 10-Octadecenoic acid methyl ester |
| 10 | 37.039 | 0.026 | 112149 | 298 | C19H38O2 | Heptadecanoic acid, 16-methyl-, methyl ester. |
| 11 | 38.183 | 0.278 | 118728 | 356 | C21H40O4 | 9-Octa decenoic acid (z)-2-hydroxy-1-(hydroxy methyl) ethyl ester |
| 12 | 40.716 | 0.074 | 93685 | 456 | C27H52O5 | Dodecanoic acid, 1-hydroxymethyl)-1,-2 ethanediyl ester |
| 13 | 44.119 | 0.241 | 844372 | 390 | C24H38O4 | Phthalic acid, di (2-propyl pentyl) ester |
| 14 | 44.527 | 0.172 | 277878 | 456 | C27H52O5 | Dodecanoic acid, 1-(hydroxy methyl)-1,2-ethanediyl ester. |
| EESI-B | ||||||
| 1 | 4.137 | 56.942 | 44162888 | 92 | C7H8 | Toluene |
| 2 | 5.021 | 0.719 | 876023 | 269 | C16H28ClN | Benzyltri-n-propyl ammnonium chloride |
| 3 | 5.482 | 0.483 | 732237 | 129 | C7H15NO | N,N dimethyl pivalamide |
| 4 | 26.104 | 0.448 | 787201 | 200 | C12H24O2 | Dodecanoic acid |
| 5 | 30.398 | 0.506 | 1002310 | 228 | C14H28O2 | Tetra decanoic acid |
| 6 | 31.111 | 0.192 | 385376 | 196 | C12H20O2 | 9,10-dimethyl tricyclo {4.2.1.1.(2,5)}decane-9-10-diol. |
| 7 | 31.386 | 0.041 | 350738 | 338 | C22H42O2 | Phytol, acetate |
| 8 | 31.579 | 0.058 | 381084 | 268 | C18H36O | 2-pentadecanone, 6,10,14-trimethyl |
| 9 | 32.195 | 0.082 | 238835 | 278 | C16H22O4 | Dibutyl phthalate |
| 10 | 33.02 | 0.011 | 77071 | 366 | C26H54 | Octa decane, 3-ethyl-5-(2-ethyl butyl)- |
| 11 | 33.117 | 0.046 | 329843 | 256 | C17H36O | 1-Hexadecanol,2-mehtyl- |
| 12 | 33.183 | 0.066 | 490890 | 270 | C17H34O2 | Hexadecanoic acid, methyl ester |
| 13 | 34.067 | 0.79 | 1738912 | 254 | C16H30O2 | Palmtoleic acid |
| 14 | 34.543 | 9.237 | 18383784 | 256 | C16H32O2 | n-Hexadecanoic acid |
| 15 | 36.036 | 0.082 | 143713 | 268 | C17H32O2 | Cis-10-heptadecenoic acid |
| 16 | 36.289 | 0.065 | 237470 | 270 | C17H34O2 | Heptadecanoic acid |
| 17 | 36.43 | 0.068 | 505440 | 294 | C19H34O2 | Methyl 9-cis, 11-trans-octa decadienoate |
| 18 | 36.534 | 0.221 | 1492702 | 296 | C19H36O2 | Trand 13-octa decenoic acid, methyl ester |
| 19 | 36.831 | 0.189 | 1026914 | 296 | C20H40O | Phytol |
| 20 | 36.994 | 0.045 | 220832 | 298 | C19H38O2 | Heptadecanoic acid, 16-methyl ester |
| 21 | 37.871 | 19.654 | 27064423 | 282 | C18H34O2 | Cis-vaccenic acid |
| 22 | 38.168 | 2.877 | 13812305 | 284 | C18H36O2 | Octadecanoic acid |
| 23 | 38.502 | 0.64 | 1200659 | 280 | C18H32O2 | 9,12,Octadecadienoic acid |
| 24 | 38.914 | 0.063 | 1491659 | 310 | C20H38O2 | Ethanol z-(9,12-octa decadienyloxy)-(z,z) |
| 25 | 39.193 | 0.711 | 1895472 | 280 | C18H32O2 | 9,12-octadeca dienoic acid (z,z)- |
| 26 | 41.028 | 0.996 | 2585848 | 310 | C20H38O2 | Cis-11-eicosenoic acid |
| 27 | 41.4 | 0.419` | 1535543 | 312 | C20H40O2 | Eicosenoic acid |
| 28 | 42.224 | 0.024 | 147908 | 356 | C21H40O4 | 9-octa decenoic acid, (z)-,2-hydroxy-1-(hydroxymethyl) ethyl ester |
| 29 | 43.829 | 0.138 | 379780 | 330 | C19H38O4 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester. |
| 30 | 44.081 | 0.35 | 2336750 | 390 | C24H38O4 | Phthalic acid, di(2-propyl pentyl) ester |
| 31 | 44.26 | 2.605 | 8047321 | 338 | C22H42O2 | Erucic acid |
| 32 | 44.564 | 0.387 | 1530826 | 340 | C22H44O2 | Docosanoic acid |
| 33 | 46.555 | 0.74 | 1494775 | 356 | C21H40O4 | 9-octa decenoic acid (z)-,2-hydroxy-1-(hydroxy methyl ethyl ester) |
| 34 | 47.662 | 0.086 | 229287 | 368 | C24H48O4 | Tetra cosanoic acid |
| HAESI-C | ||||||
| 1 | 4.152 | 35.927 | 20959817 | 92 | C7H8 | Toluene |
| 2 | 32.3 | 0.044 | 86703 | 320 | C19H28O4 | Phthalic acid, 5-methyl hex-2-yl isobutyl ester |
| 3 | 33.273 | 0.013 | 57102 | 270 | C17H34O2 | Hexadecanoic acid, methyl ester |
| 4 | 34.179 | 0.049 | 103427 | 376 | C23H36O4 | Phthalic acid, butyl undecyl ester |
| 5 | 34.432 | 0.764 | 1043999 | 256 | C16H32O2 | N-hexadecanoic acid |
| 6 | 36.519 | 0.026 | 132412 | 294 | C19H34O2 | Methyl 10-trans, 12-cis-octadecadienoate. |
| 7 | 36.608 | 0.073 | 335007 | 296 | C19H36O2 | Trans-13-octa decenoic acid, methyl ester |
| 8 | 36.898 | 0.019 | 83247 | 296 | C20H40O | Phytol |
| 9 | 37.069 | 0.023 | 77335 | 298 | C19H38O2 | Hepta decanoic acid, 16-methyl-,methyl ester |
| 10 | 37.782 | 0.156 | 218002 | 282 | C18H34O2 | Cis-vaccenic acid |
| 11 | 38.116 | 0.139 | 210895 | 284 | C18H36O2 | Octa decanoic acid |
| 12 | 44.148 | 0.116 | 338489 | 390 | C24H38O4 | Diisooctyl phthalate |
| 13 | 49.646 | 43.343 | 3289601 | 456 | C27H52O5 | Dodecanoic acid, 1-(hydroxy methyl)-1,2-ethane diyl ester |
| 14 | 54.719 | 2.134 | 446409 | 436 | C26H44O5 | Ethyl iso-allocholate |
Table 3: phytochemical screening leaf extracts of S. isoetifolium using GC-MS techniques
| Samples (percentage inhibition) | Concentrations (µg/ml) | IC50 value (µg/ml) | |||
|---|---|---|---|---|---|
| 20 µg/ml | 40 µg/ml | 60 µg/ml | 80 µg/ml | ||
| S. isoetifolium | 23.64±1.65 | 44.55±3.11 | 62.73±4.39 | 79.10±5.53 | 47.28 |
| Ascorbic acid (Std) | 25.46±1.78 | 47.28±3.30 | 78.64±5.50 | 89.55±6.26 | 40.84 |
Note: Values are expressed as Mean±SD for triplicate
Table 4: DPPH Radical Scavenging Activity of Leaf Extracts of S. isoetifolium and Ascorbic Acid at Different Concentrations
| Samples (Percentage inhibition) | Concentrations (µg/ml) | IC50 value (µg/ml) | |||
|---|---|---|---|---|---|
| 20 µg/ml | 40 µg/ml | 60 µg/ml | 80 µg/ml | ||
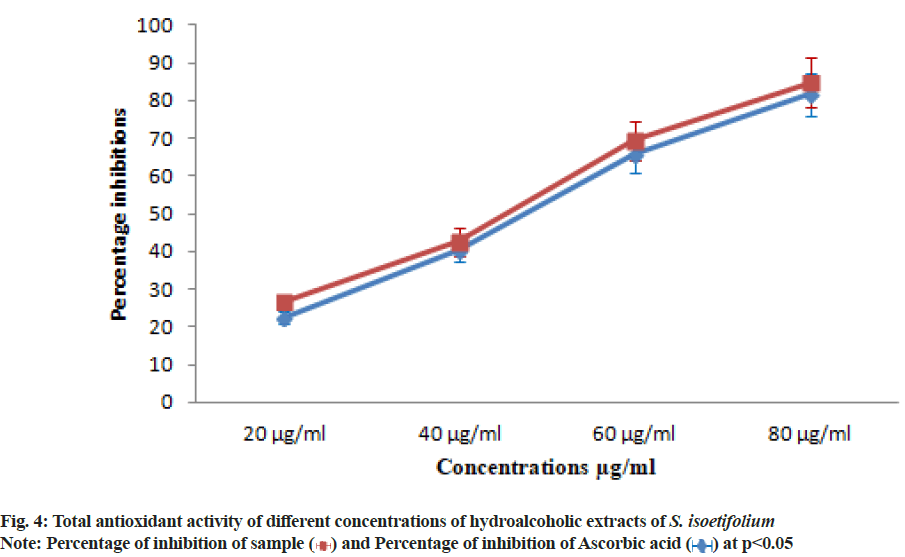
| S. isoetifolium | 22.50±1.57 | 40.31±2.82 | 65.62±4.59 | 81.56±5.70 | 47.53 |
| Ascorbic acid (Std) | 26.56±1.85 | 42.50±2.97 | 69.37±4.85 | 84.68±5.92 | 44.25 |
Table 5: Total Antioxidant Activity of Leaf Extracts of S. isoetifolium and Ascorbic acid at different Concentrations
| Samples (Percentage inhibition) | Concentrations (µg/ml) | IC50 value (µg/ml) | |||
|---|---|---|---|---|---|
| 20 µg/ml | 40 µg/ml | 60 µg/ml | 80 µg/ml | ||
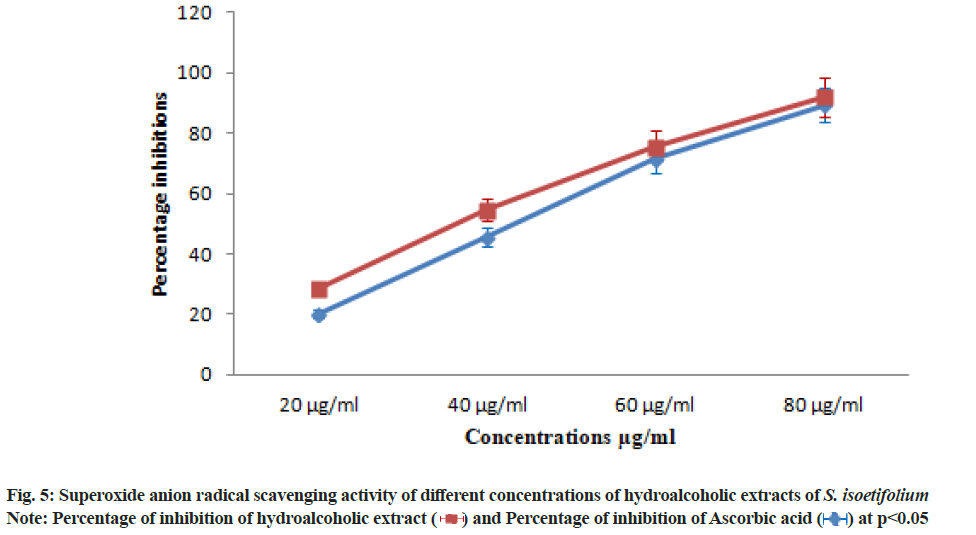
| S. isoetifolium | 20.35±1.42 | 45.71±3.19 | 71.42±4.99 | 89.28±6.24 | 44.24 |
| Ascorbic acid (Std) | 28.57±1.99 | 54.64±3.82 | 75.71±5.29 | 92.14±6.44 | 37.94 |
Table 6: Superoxide anion Radical Scavenging activity of S. isoetifolium and Ascorbic acid at different concentrations
| Samples (Percentage inhibition) | Concentrations (µg/ml) | IC50 value (µg/ml) | |||
|---|---|---|---|---|---|
| 20 µg/ml | 40 µg/ml | 60 µg/ml | 80 µg/ml | ||
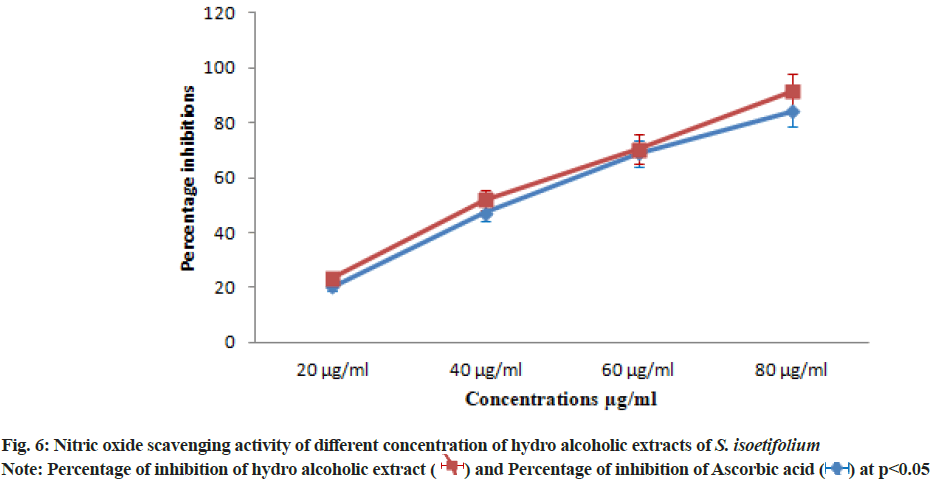
| S. isoetifolium | 20.47±1.43 | 47.14±3.29 | 69.04±4.83 | 84.28±5.89 | 45.09 |
| Ascorbic acid (Std) | 23.33±1.63 | 51.90±3.63 | 70.47±4.93 | 91.42±6.39 | 41.67 |
Table 7: Nitric Oxide Scavenging Activity of S. isoetifolium and Ascorbic Acid at Different Concentrations
| Samples (Percentage inhibition) | Concentrations (µg/ml) | IC50 value (µg/ml) | |||
|---|---|---|---|---|---|
| 20 µg/ml | 40 µg/ml | 60 µg/ml | 80 µg/ml | ||
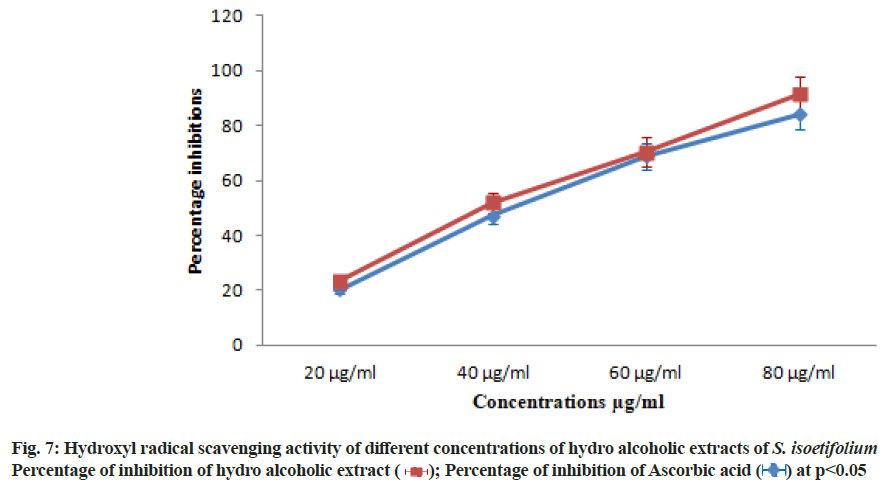
| S. isoetifolium | 21.66±1.51 | 43.33±3.03 | 68.75±4.81 | 81.25±5.68 | 46.32 |
| Ascorbic acid (Std) | 25.41±1.77 | 53.75±3.76 | 72.91±5.10 | 93.33±6.53 | 39.81 |
Table 8: Hydroxyl Radical Scavenging Activity of S. isoetifolium and Ascorbic Acid at Different Concentrations
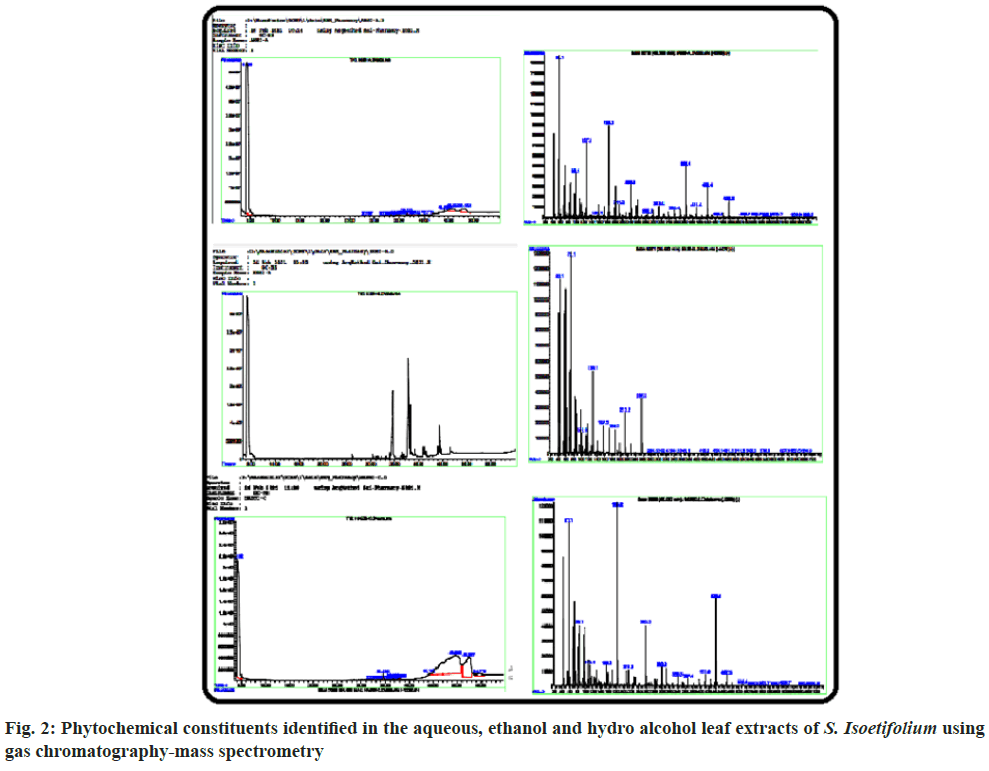
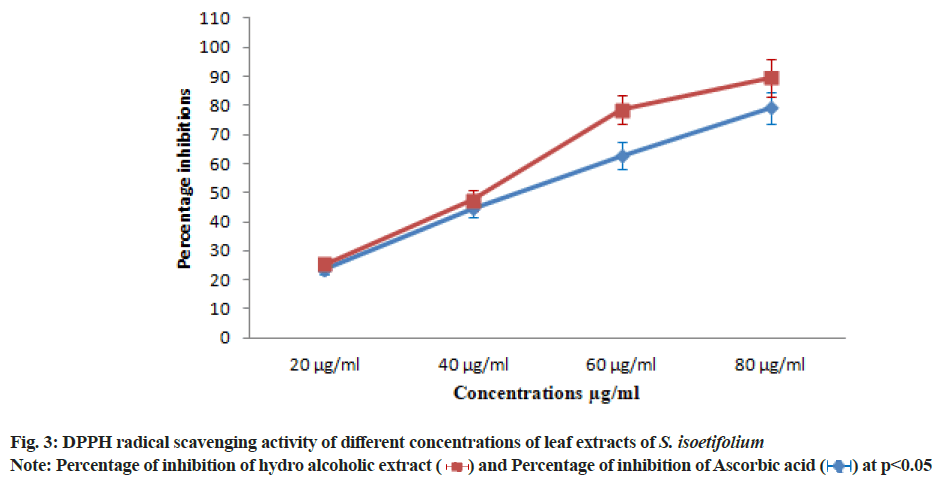
In GC-MS, the existence of different phytochemicals was identified through their Molecular Weight (MW) and Molecular Formula (MF) etc. There were 63 compounds identified in this method from the HAESI extracts (fig. 2 and Table 3). The prevailing compounds found in the mentioned extracts are Hexadecanoic acid methyl ester, Hexadecanoic acid, Ethyl iso-allocholate etc., Phytochemicals present in the plant extract significantly contributes to biological activities. Therefore, it is necessary to identify the different phytochemicals of the plant extract. Earlier reports show that seagrasses have antioxidant activity due to the presence of polyphenols, flavonoids and other phytochemicals[43-45]. Flavonoids are naturally occurring biological compounds that can act as potent antioxidants and can prevent cardiovascular disease by preventing the oxidation of Low-Density Lipoprotein (LDL)[46]. In the review of the above observation and continuation of our interest to identify the therapeutic activity of S. isoetifolium, antioxidant activity was evaluated. Antioxidant activity is a complex procedure and is influenced by different mechanisms, which cannot be identified through a single method. Therefore, it is needed to perform different ways to measure antioxidant activity. Different aliquots of hydroalcoholic extract of S. isoetifolium and ascorbic acid were prepared for in vitro antioxidant activity d for in vitro antioxidant activity such as DPPH, TAC, Superoxide radical scavenging activity, NO scavenging activity and Hydroxyl radical scavenging activities. Minimum Inhibitory Concentration (MIC) is also estimated by calculating the IC50 value. The DPPH radical scavenging activities increased with the increasing concentration (20, 40, 60 and 80 μg/ml) of HAESI extract. The radical scavenging activities of 23.64±1.65, 44.55±3.11, 62.73±4.39, 79.10±5.53 μg/ml respectively at various concentrations (20, 40, 60 and 80 μg/ml) was observed and IC50 was found to be 47.28 μg/ml (Table 4 and fig. 3). IC50 values of the scavenging assay were calculated by plotting the percentage of inhibition against the concentration HAESI extracts. Similarly, the radical scavenging activities of ascorbic acid were performed and the results were between 25.46±1.78 to 89.55±6.26 μg/ ml with increasing concentrations. The IC50 value of the standard drug was found to be 40.84 μg/ml. The DPPH radical scavenging activities of HAESI extracts are competitive when compared to the standard at the given concentrations.
The hydroalcoholic extract showed the significant DPPH scavenging activity with the lowest IC50 value due to the presence of polyphenols compounds (Table 4). DPPH free radical scavenging activity reported in our study of S. isoetifolium is much higher than result reported from other seagrasses[47,48]. The TAC was increased with the increasing concentration (20, 40, 60 and 80 μg/ ml) of HAESI extracts. The total antioxidant activities 22.50±1.57, 40.31±2.82, 65.62±4.59, 81.56±5.70 μg/ml respectively at different concentrations were observed and IC50 was found to be 47.53 μg/ml (Table 5 and fig. 4). Similarly, the total antioxidant radical scavenging activities of ascorbic acid were performed and the result was between 26.56±1.85 to 84.68±5.92 μg/ml with increasing concentration. The IC50 value of the standard drug was found to be 44.25 μg/ml. Superoxide radical is believed to be a chief source of reactive oxygen species in living things. The superoxide radical scavenging activity of different concentrations (20, 40 60 and 80 μg/ml) of HAESI extracts was evaluated with the same dose of ascorbic acid. The HAESI extract showed superoxide anion radical scavenging activities were 20.35±1.42, 45.71±3.19, 71.42±4.99, 89.28±6.24 μg/ml respectively at various concentrations and IC50 was found to be 44.24 μg/ml (Table 6 and fig. 5). In the same way, the superoxide radical scavenging activities of ascorbic acid was performed with the ascorbic acid and the result was between 28.57±1.99 to 92.14±6.44 with increasing concentrations. The IC50 value of the standard drug was found to be 37.94 μg/ml. In another method, NO scavenging activity of HAESI extracts was evaluated with the same doses of ascorbic acid ranging from 20-80 μg/ml. The HAESI extracts showed NO radical scavenging activities 20.47±1.43, 47.14±3.29, 69.04±4.83, 84.28±5.89 μg/ml respectively at different concentrations and IC50 was found to be 45.09 μg/ml (Table 7 and fig. 6). Similarly, the radical NO scavenging activity of ascorbic acid was performed and the result was between 23.33±1.63 to 91.42±6.39 with increasing concentrations. The IC50 value of the standard drug was found to be 41.67 μg/ml.
Athiperumalsami et al.[49] have reported highest antioxidant activity in the methanolic extract of Halophila ovalis than Halodule pinifolia tested by the NO scavenging method. At last, hydroxyl radical scavenging potential of HAESI extracts to inhibit hydroxylradicals (OH) was assessed at various concentrations 20-80 μg/ml. The HAESI extracts showed hydroxyl radical scavenging activities 21.66±1.51, 43.33±3.03, 68.75±4.81, 81.25±5.68 μg/ml respectively at different concentrations and IC50 was found to be 46.32 μg/ml (Table 8 and fig. 7). In the same way, the hydroxyl radical scavenging activity of ascorbic acid was performed and the result was between 25.41±1.77 to 93.33±6.53 μg/ml with increasing concentrations. The IC50 value of the standard drug was found to be 39.81 μg/ml. In agreement with previous studies there was a significant correlation between our antioxidant value and the values in the study of Duan et al.[50]. The result demonstrated that the hydroalcoholic extracts exhibited scavenging and/or reducing effects on DPPH, phosphomolybdate, superoxide, NO, hydroxyl radicals present in the reagents. The radical scavenging activity of hydroalcoholic extract of S. isoetifolium is due to the presence of polyphenols, flavonoids and other phytochemicals. At all concentrations of hydroalcoholic extract from 20 to 80 μg/ml, displayed an excellent antioxidant effect. However, the lowest radical scavenging activity was observed in the lower concentrations of hydroalcoholic extracts. Whereas the high concentration of hydroalcoholic extract exhibits excellent antioxidant activity as similar to that of standard drug. The order of radical scavenging activity of hydroalcoholic extract of S. isoetifolium was found to be 80 μg/ml?60 μg/ml?40 μg/ml?20 μg/ml. Similar results were also obtained in studies conducted by other scientists who were involved in the determination of antioxidant activity in the seagrass at various concentrations[51-53]. All the extracts were less effective than the standard ascorbic acid radical scavenging activity and IC50 values. This present finding corroborate well with earlier reports in other species of seagrass.
In conclusion, the seagrass leaf extracts of S. isoetifolium were screened for phytochemicals by using chemical and GC-MS techniques. It is observed that the presence of phenol, polyphenol, alkaloid, glycoside, saponin, flavonoids, dicarboxylic acids and other constituents in the qualitative analysis. Whereas, quantitative determination shows the presence of TPC (192.44±13.47 mg GAE/g), TFC (106.54±7.45 mg quercetin/g), TSC (52.61±3.68 mg Quillaja/g) and TTC (80.65±5.64 mg tannic acid/g). The GC-MS analysis of the seagrass extract of hydro alcohol revealed the presence of 62 compounds that could contribute to the medicinal property of the plant leaves. Here, the compounds were used to identify the different phytochemicals present in the hydroalcoholic extract from the MF and MW of compounds. In addition to that, the HAESI extracts of S. isoetifolium proved to be the most effective radical scavenger activity in the DPPH, total antioxidant, NO, superoxide anion, hydroxyl radicals etc., the higher radical scavenging activity of HAESI extracts due to the presence of phenol, polyphenols and other compounds. They react with the various species of radicals such as hydroxyl, peroxide, superoxide and NO etc., and reduce their existence. Therefore, extracts of S. isoetifolium can be recommended for various oxidative stress-related diseases. The above result encourages us to isolate and confirm the specific phytoconstituent responsible for the observed antioxidant activity and their mode of action.
Acknowledgements:
The Authors are thankful to the management of VISTA, Chennai and GIET School of Pharmacy, Rajahmundry for offering their kind support to carry out this study.
Conflict of interests:
The authors declared no conflict of interest.
References
- Kavitha D, Kalaivani P, Vanitha V. A review on phytochemicals and biological activities of seagrass. J Crit Rev 2020;7(7):257-64.
- La Notte A, D’Amato D, Mäkinen H, Paracchini ML, Liquete C, Egoh B, et al. Ecosystem services classification: A systems ecology perspective of the cascade framework. Ecol Indicat 2017;74:392-402.
- Ani CJ, Robson B. Responses of Marine ecosystems to climate change impacts and their treatment in biogeochemical ecosystem models. Mar Pollut Bull 2021;166:112223.
- McKenzie LJ, Yoshida RL. Over a decade monitoring Fiji's seagrass condition demonstrates resilience to anthropogenic pressures and extreme climate events. Mar Pollut Bull 2020;160:111636.
- Short F, Carruthers T, Dennison W, Waycott M. Global seagrass distribution and diversity: A bioregional model. J Exp Mar Biol Ecol 2007;350(1-2):3-20.
- Balaji V. Acoustic survey of seagrass beds in northern Palk Bay, India. Indian J Geo Mar Sci 2018;47(8):1607-15.
- Larkum AW, Davey PA, Kuo J, Ralph PJ, Raven JA. Carbon-concentrating mechanisms in seagrasses. J Exp Bot 2017;68(14):3773-84.
[Crossref] [Google Scholar] [PubMed]
- Dilipan E, Sivakumar K, Thangaradjou T. Distribution and biology of seagrass resources of Lakshadweep group of Islands, India. Indian J Geo Mar Sci 2011;40(5):624-34.
- Newmaster AF, Berg KJ, Ragupathy S, Palanisamy M, Sambandan K, Newmaster SG. Local knowledge and conservation of seagrasses in the Tamil Nadu State of India. J Ethnobiol Ethnomed 2011;7(1):37.
[Crossref] [Google Scholar] [PubMed]
- Kannan RR, Arumugam R, Iyapparaj P, Thangaradjou T, Anantharaman P. In vitro antibacterial, cytotoxicity and haemolytic activities and phytochemical analysis of seagrasses from the Gulf of Mannar, South India. Food Chem 2013;136(3-4):1484-9.
[Crossref] [Google Scholar] [PubMed]
- Kannan RR, Arumugam R, Thangaradjou T, Anantharaman P. Phytochemical constituents, antioxidant properties and p-coumaric acid analysis in some seagrasses. Food Res Int 2013;54(1):1229-36.
- Zidorn C. Secondary metabolites of seagrasses (Alismatales and Potamogetonales; Alismatidae): Chemical diversity, bioactivity, and ecological function. Phytochemistry 2016;124:5-28.
[Crossref] [Google Scholar] [PubMed]
- Martinez MJ, Del Olmo LM, Benito PB. Natural marine antiviral products. Stud Nat Prod Chem 2008;35:101-34.
- Huang Y, Xiao X, Xu C, Perianen YD, Hu J, Holmer M. Seagrass beds acting as a trap of microplastics-Emerging hotspot in the coastal region?. Environ Pollut 2020;257:113450.
[Crossref] [Google Scholar] [PubMed]
- Ranjbar G, Mikhailidis DP, Sahebkar A. Effects of newer antidiabetic drugs on nonalcoholic fatty liver and steatohepatitis: Think out of the box. Metabolism 2019;101:154001.
[Crossref] [Google Scholar] [PubMed]
- Premarathna AD, Ranahewa TH, Wijesekera SK, Harishchandra DL, Karunathilake KJ, Waduge RN, et al. Preliminary screening of the aqueous extracts of twenty-three different seaweed species in Sri Lanka with in vitro and in vivo assays. Heliyon 2020;6(6):e03918.
[Crossref] [Google Scholar] [PubMed]
- Unsworth RK, McKenzie LJ, Nordlund LM, Cullen-Unsworth LC. A changing climate for seagrass conservation? Curr Biol 2018;28(21):R1229-32.
[Crossref] [Google Scholar] [PubMed]
- Shailaja VL, Christina VS, Mohanapriya CD, Sneha P, Sundaram RL, Magesh R, et al. A natural anticancer pigment, Pheophytin a, from a seagrass acts as a high affinity human mitochondrial translocator protein (TSPO) ligand, in silico, to reduce mitochondrial membrane Potential (? ?mit) in adenocarcinomic A549 cells. Phytomedicine 2019;61:152858.
[Crossref] [Google Scholar] [PubMed]
- Chanthini AB, Balasubramani G, Ramkumar R, Sowmiya R, Balakumaran MD, Kalaichelvan PT, et al. Structural characterization, antioxidant and in vitro cytotoxic properties of seagrass, Cymodocea serrulata (R. Br.) Asch. and Magnus mediated silver nanoparticles. Journal of Photochem Photobiol B 2015;153:145-52.
[Crossref] [Google Scholar] [PubMed]
- Rengasamy KR, Sadeer NB, Zengin G, Mahomoodally MF, Cziáky Z, Jeko J, et al. Biopharmaceutical potential, chemical profile and in silico study of the seagrass–Syringodium isoetifolium (Asch.) Dandy. South Afr J Bot 2019;127:167-75.
- Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: A comprehensive review. Chin Med 2018;13(1):1-26.
[Crossref] [Google Scholar] [PubMed]
- Casal-Porras I, Jiménez-Ramos R, ZubÃÂa E, Brun FG. Importance of the chemical defenses and sugars in the feeding preference of Paracentrotus lividus over two sympatric template seagrass species. Estuar Coast Shelf Sci 2021;259:107466.
[Crossref] [Google Scholar]
- Tangon E, Alivio ER, Pajiji JA, Ajik KO. Phytochemical screening and proximate composition of the seagrass Halodule pinifolia of the coastal waters of Carmen, Agusan Del Norte, Philippines. Int J Mod Pharm 2021;5(2):75-80.
- Lisowski A, Matkowski P, Dabrowska M, Piatek M, Swietochowski A, Klonowski J, et al. Particle size distribution and physicochemical properties of pellets made of straw, hay, and their blends. Waste Biomass Valoriz 2020;11(1):63-75.
- Pergent G, Boudouresque CF, Dumay O, Pergent-Martini C, Wyllie-Echeverria S. Competition between the invasive macrophyte Caulerpa taxifolia and the seagrass Posidonia oceanica: Contrasting strategies. BMC Ecol 2008;8(20):1-3.
[Crossref] [Google Scholar] [PubMed]
- Mandal S, Patra A, Samanta A, Roy S, Mandal A, Mahapatra TD, et al. Analysis of phytochemical profile of Terminalia arjuna bark extract with antioxidative and antimicrobial properties. Asian Pac J Trop Biomed 2013;3(12):960-6.
[Crossref] [Google Scholar] [PubMed]
- Zidorn C. Secondary metabolites of seagrasses (Alismatales and Potamogetonales; Alismatidae): Chemical diversity, bioactivity, and ecological function. Phytochemistry 2016;124:5-28.
[Crossref] [Google Scholar] [PubMed]
- Hossain MA, Shah MD, Gnanaraj C, Iqbal M. In vitro total phenolics, flavonoids contents and antioxidant activity of essential oil, various organic extracts from the leaves of tropical medicinal plant Tetrastigma from Sabah. Asian Pac J Trop Med 2011;4(9):717-21.
[Crossref] [Google Scholar] [PubMed]
- Kolodziej B, Seczyk L, Sugier D, Kedzia B, Chernetskyy M, Gevrenova R, et al. Determination of the yield, saponin content and profile, antimicrobial and antioxidant activities of three Gypsophila species. Industrial Crops Prod 2019;138:111422.
- Medini F, Fellah H, Ksouri R, Abdelly C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J Taibah Univ Sci 2014;8(3):216-24.
- Isobe K, Koba K, Ueda S, Senoo K, Harayama S, Suwa Y. A simple and rapid GC/MS method for the simultaneous determination of gaseous metabolites. J Microbiol Methods. 2011;84(1):46-51.
[Crossref] [Google Scholar] [PubMed]
- Gopi C, Sastry VG, Dhanaraju MD. Synthesis and spectroscopic characterisation of novel bioactive molecule of 3-(2-substituted)-1H-indol-3-yl)-1-(thiophen-2yl) prop-2-en-1-one chalcone derivatives as effective anti-oxidant and anti-microbial agents. Beni Suef Univ J Basic Appl Sci 2016;5(3):236-43.
- Rahman M, Islam M, Biswas M, Khurshid Alam AH. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes 2015;8(1):1-9.
- Pavithra K, Vadivukkarasi S. Evaluation of free radical scavenging activity of various extracts of leaves from Kedrostis foetidissima (Jacq.) Cogn. Food Sci Hum Wellness 2015;4(1):42-6.
- Ali BM, Boothapandi M, Nasar AS. Nitric oxide, DPPH and hydrogen peroxide radical scavenging activity of TEMPO terminated polyurethane dendrimers: Data supporting antioxidant activity of radical dendrimers. Data Brief 2020;28:104972.
[Crossref] [Google Scholar] [PubMed]
- Sowndhararajan K, Kang SC. Free radical scavenging activity from different extracts of leaves of Bauhinia vahlii Wight and Arn. Saudi J Biol Sci 2013;20(4):319-25.
[Crossref] [Google Scholar] [PubMed]
- Dawes CJ. Seasonal proximate constituents and caloric values in seagrasses and algae on the west coast of Florida. J Coastal Res 1986:25-32.
- Rengasamy RR, Radjassegarin A, Perumal A. Seagrasses as potential source of medicinal food ingredients: Nutritional analysis and multivariate approach. Biomed Prev Nutr 2013;3(4):375-80.
- Kannan Rengasamy RR, Rajasekaran A, Micheline GD, Perumal A. Antioxidant activity of seagrasses of the Mandapam coast, India. Pharm Biol 2012;50(2):182-7.
[Crossref] [Google Scholar] [PubMed]
- Kar K, Sahoo SS, Kar BA, Naik SK, Panda PC. Antioxidant activity of Halophila ovalis and Halophila beccarii (Hydrocharitaceae): Two important seagrass species of Chilika lagoon, India. Asian J Pharm Clin Res 2019;12(3):1-5.
- Yuvaraj N, Kanmani P, Satishkumar R, Paari A, Pattukumar V, Arul V. Seagrass as a potential source of natural antioxidant and anti-inflammatory agents. Pharm Biol 2012;50(4):458-67.
[Crossref] [Google Scholar] [PubMed]
- Bharathi NP, Jayalakshmi M, Amudha P, Vanitha V. Phytochemical screening and in vitro antioxidant activity of the seagrass Cymodocea serrulata. Indian J Mar Sci 2019;48(8):1216-21.
- Wong-Paz JE, Contreras-Esquivel JC, RodrÃÂguez-Herrera R, Carrillo-Inungaray ML, López LI, Nevárez-Moorillón GV, et al. Total phenolic content, in vitro antioxidant activity and chemical composition of plant extracts from semiarid Mexican region. Asian Pac J Trop Med 2015;8(2):104-11.
[Crossref] [Google Scholar] [PubMed]
- Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med 2012;12(1):221.
[Crossref] [Google Scholar] [PubMed]
- Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019;8(4):96.
[Crossref] [Google Scholar] [PubMed]
- Lekshmi S, Saramma AV. Antioxidant activity of Synechococcus sp. Nägeli isolated from cochin estuary, India. Indian J Geomar Sci 2018;47(11):2213-6.
- Santos F, Monteiro JP, Duarte D, Melo T, Lopes D, da Costa E, et al. Unraveling the lipidome and antioxidant activity of native Bifurcaria bifurcata and invasive Sargassum muticum seaweeds: A lipid perspective on how systemic intrusion may present an opportunity. Antioxidants 2020;9(7):642.
[Crossref] [Google Scholar] [PubMed]
- Kannan RR, Arumugam R, Anantharaman P. In vitro antioxidant activities of ethanol extract from Enhalus acoroides (LF) Royle. Asian Pac J Trop Med 2010;3(11):898-901.
- Athiperumalsami T, Rajeswari VD, Poorna SH, Kumar V, Jesudass LL. Antioxidant activity of seagrasses and seaweeds. Bot Mar 2010;53:251-7.
- Duan XJ, Zhang WW, Li XM, Wang BG. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem 2006;95(1):37-43.
- Nakbi H, Dallel W, Hammami S, Mighri Z. Phytochemical profile and antioxidant properties of leaves extracts from Posidonia oceanica (L.) Delile and their allelopathic potential on terrestrial plant species. Bull Chem Soc Ethiopia 2020;34(3):437-47.
- Santoso J, Anwariyah S, Rumiantin RO, Putri AP, Ukhty N, Yoshie-Stark Y. Phenol content, antioxidant activity and fibers profile of four tropical seagrasses from Indonesia. J Coastal Dev 2012;15(2):189-96.
- Cornara L, Pastorino G, Borghesi B, Salis A, Clericuzio M, Marchetti C, et al. Posidonia oceanica (L.) delile ethanolic extract modulates cell activities with skin health applications. Mar Drugs 2018;16(1):21.
[Crossref] [Google Scholar] [PubMed]
 and Percentage of inhibition of Ascorbic acid
and Percentage of inhibition of Ascorbic acid  at p?0.05
at p?0.05