- *Corresponding Author:
- Poornima Kannappan
Department of Biochemistry, Karpagam Academy of Higher Education, Coimbatore, Tamil Nadu 641021, India
E-mail: poorniovya@gmail.com
| Date of Received | 14 October 2021 |
| Date of Revision | 28 April 2022 |
| Date of Acceptance | 21 November 2022 |
| Indian J Pharm Sci 2022;84(6):1358-1368 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Colorectal cancer is a major global health concern in terms of incidence and mortality. Treatment strategies constitute conventional surgery, radiation, chemotherapy and innovative measures like targeted and immunotherapy. All these treatment options are associated with considerable side effects which demand an alternative regime with enhanced therapeutic efficacy, specificity and less toxicity. Phytomedicine or herbal therapeutics is an emerging trend, as medicinal alternative and botanicals constitute an immense treasure of bioactive principles in the form of secondary metabolites with significant pharmacological attributes. Phytochemicals like curcumin, resveratrol, quercetin, lycopene, organosulphur compounds etc., are few among isolated herbal constituent proved to be effective against colorectal carcinogenesis in both in vitro and in vivo trials. These bioactive ingredients exert apoptotic, antimetastatic, antiangiogeneic and antiproliferiative properties by targeting various signaling pathways like Wnt/β-catenin, nuclear factor-kappa B, phosphoinositol 3 kinase/protein kinase B, epidermal growth factor receptor, vascular endothelial growth factor, epithelial-mesenchymal transition, resulting in up regulation and down regulation of associated target genes and proteins. Consolidation of the information in the present review highlights the significance of phytomedicine or herbal medicine in oncology therapeutics as conventional and innovative treatment options are associated with serious health hazards.
Keywords
Colorectal cancer, anticancer, phytotherapeutics, bioactive compounds, chemoprevention, signaling pathways
Colorectal cancer is a gastrointestinal malignancy affecting parts of large intestine and rectum and occupies third position with respect to incidence and ranks fourth among overall cancer related mortality worldwide[1]. As per estimates of American Cancer Society based on National Centre for Health Statistics data about 147 950 people will be diagnosed with colorectal cancer and 53 200 patients will die of the malignancy in 2020 of which 17 930 case registries and 3649 individuals in both sexes will be below age 50 y[2]. Colorectal cancer initiates as small outgrowths called polyps in inner lining of colon or rectum which gradually transforms into a malignant growth of colonic epithelium as a result of cumulative genetic and epigenetic mutations[3]. About 60 % of colorectal malignancy is of sporadic phenotype without any family background, 30 % is familial and 10 % belongs to hereditary phenotype with inherited genetic mutations[4]. Colorectal cancer is designated into three genetic variants; Chromosomal Instability (CIN) leading to deletion of wild allele of tumor suppressor genes (Adenomatous Polyposis Coli (APC), p53 etc), Microsatellite Instability (MSI) due to incorporation incorrect bases during Deoxyribonucleic Acid (DNA) replication as result of mutation in DNA (Mismatch Repair genes (MMR): MutL Homolog (MLH)-1, MLH2, Mismatch Repair Endonuclease (PMS2) etc.,) and aberrant DNA methylation with hyper methylation at Genetic phenotype of colorectal malignancy (CpG) island sequence. p53, APC, p27, Loss Of Heterozygosity (LOH) 18q, MSI, deletion 5 qallele constitute tumour suppressor genes and Kirsten Rat Sarcoma (K-RAS) virus oncogene homolog, Transforming Growth Factor(TGF)-β 1, Erb-B2, TGF-α, Epidermal Growth Factor Receptor (EGFR) (Erb-B1) are oncogenes mainly associated with colorectal cancer. Molecular signaling pathways like Wnt/APC/β-catenin, TGF-β/Signaling molecules (Smad), Nuclear Factor Kappa B (NF- κB), Phosphoinositide 3-Kinase (PI3K)/Protein kinase B (AKT)/Glycogen Synthase Kinase-3β (GSK-3β) exhibit alterations in gene expression leading to dysfunctional or truncated proteins contributing to tumorigenic responses like cell proliferation, loss of apoptosis and angiogenesis.
Medicinal plants usually serve as a repository of secondary metabolites which harbor numerous bioactive ingredients with potential therapeutic activity. An approximate of 35 000 plant species has been screened for anticancer activity by National Cancer Institute (NCI)[5]. Tumour biology is multifactorial and hence cancer drugs both natural and synthetic must be developed to target multiple signaling pathways in carcinogenesis like angiogenesis, metastasis, cell cycle arrest etc.,[6]. Search for herbal anticancer drug dates back to 1950’s with the discovery of vinca alkaloids from Catheranthus roseus (Madagascar Periwinkle) by Canadian scientists Charles Beer and Robert Noble. Vinca alkaloids are microtubule destabilizing agents that bind to β-tubulin near guanosine triphosphate binding site. Vincristine, Vinblastine, Vindesine and Vinorelbine are four major groups of vinca alkaloids which are usually employed in the treatment of leukaemias, Hodgkin’s lymphomas, lung, testicular and breast cancer. Harringtonine and Homoharringtonine are two major class of anti-leukemic constituents isolated from Cephalotaxus harringtonia which inhibit translation of protein via interacting with ribosomal A-site[7]. Another tubulin binding plant derived drug includes taxanes (paclitaxel and docetaxel) from Taxus brevifolia and Taxus baccata respectively which employed in treatment of ovarian, lung, breast, head and neck cancers. Apart from these other major phytoconstituent based cancer drugs in chemotherapeutics includes endophyllotoxins (etoposide and teniposide) from Podophyllum peltatum inhibiting topoisomerase II and camptothecins derivates (irrinotecan and camptothecin) from Camptotheca acuminate which induce breaks in DNA by interfering with topoisomerase I. Irrinotecan is approved for the treatment of colorectal cancer[8-11]. According to World Health Organization (WHO), an approximate of 80 % of global population depends on traditional medicine of which phytotherapy is one which employs the use of medicinal herbs and their formulations. Phytomedicine in addition to culinary effects also exerts mental, physical and emotional wellbeing[12].
Phytotherapeutics is a rapidly emerging and developing research area in medicine. Significance of the review lies in the fact that finding an herbal alternative for conventional cytotoxic chemotherapeutic drugs is a rapidly developing and demanding prerequisite in oncology therapeutics. Herbal medicine can be employed as cancer chemotherapeutic agents or used in combination with synthetic chemo drugs to relive the possible side effects and improve the clinical outcome as complementary and alternative medicine. In designing a potential phytochemical drug its molecular mechanism of action (target signaling pathway) in the tumour microenvironment is the main thrust area to be analyzed and explored. Specific molecular mechanism of action of anticancer phytochemicals is a major thrust area of research still now. However a variety of complex mechanism of action of anticancer herbs in tumour biology had already described, such as activation of antioxidant enzymes thereby reducing the level reactive oxygen or nitrogen species, inhibition of neoplastic cellular transformation, modulation of cellular signaling pathways and preventing the metabolic transformation of a procarcinogen to active form[13]. Even though various herbs exert diverse neoplastic properties by modulating varied signaling pathways, proteins, receptors, transcription factors and metabolites, but some of them share similarity in anticancer properties as the lead constituent responsible for the medicinal attribute of herbs include phytochemicals like alkaloids, flavanoids, polyphenols, terpenoids, glycosides etc.,[13].
Flavone apigenin found in celery, chamomile and parsley promote apoptosis in neoplastic cells. Curcumin of Curcuma longa, Epigallocatechin Gallate (EGCG), bilberry anthocyanin’s, quercetin act through NF-kB pathway and found to slow down the growth of metastatic cancer cells[13]. Carotenoid crocetin present in Crocus sativus and Gardenia jasminoides act on GATA binding protein 4, prevent cardiac myopathy and serve as potential target for gene therapy[14]. A vast array of phytochemicals has been evaluated for anticancer activity by both in vitro and in vivo trials. Findings suggest that all of them share similarity in anticancer property. They exert diverse and wide range of complex mechanisms on different molecular signaling pathways, membrane receptors like kinases mediating signal transduction, tumour activating or tumour suppressor proteins, micro Ribonucleic acid (mRNA), transcription factors, cyclins and caspases[15].
Present review provides a basic overview of colorectal cancer and experimental evidence of isolated phytoconstituent in colorectal cancer with insights on mechanism of action of bioactive compounds in specific signaling pathways involved in tumour initiation cascade. In this context the information in the article will highlight therapeutic efficacy of phytoconstituent mentioned and this will provide fundamental information for further exploration of respective phytoconstituent in colorectal therapeutics.
Risk Factors and Genetics
Factors which contributes as risk for sporadic colorectal cancer include obesity, lack of physical activity, diabetes, consumption of processed and red meat, reduced level of fibers and high fat content in diet, excessive alcohol consumption, smoking, inflammatory bowel diseases like ulcerative colitis, Crohn’s disease and age[11]. Experiments have demonstrated that diet rich in vegetables, fruits and spices considerably reduces the risk of colorectal cancer. Phytochemicals in dietary constituents like curcumin, resveratrol, apple polysaccharides, quercetin etc., have proven chemo preventive effect in colon malignancy[12]. Genetic predispositions are responsible for inherited colorectal cancer, for example hereditary syndromes like familial adenomatous polyposis coli and hereditary nonpolyposis colorectal cancer can considerably increases individual risk in developing colon neoplasm[16].
Molecular genetics involved in colorectal cancer is heterogeneous. Based on genetic instability and altered genetic expression associated with tumourogenesis, colorectal cancer is designated into three genetic variants; CIN leading to deletion of wild allele of tumour suppressor genes (APC, p53 etc.); MSI due to incorporation of incorrect bases during DNA replication as a result of mutation in DNA (MMR: MLH1, MLH2, PMS2 etc.,) and aberrant DNA methylation with hyper methylation at CpG island sequence. p53, APC, p27, LOH 18q, MSI, deletion 5 q allele constitute tumour suppressor genes and K-RAS, TGF-beta 1, Erb-B2, TGF-α, EGFR (Erb-B1) are oncogenes mainly associated with colorectal cancer[17]. Molecular signaling pathways like Wnt/APC/β-catenin, TGF-β/Smad, NF-κB, PI3K/AKT/GSK-3β exhibit alterations in gene expression leading to dysfunctional or truncated proteins contributing to tumour responses like cell proliferation, loss of apoptosis and angiogenesis[18].
Diagnosis and Management
Prevention of any type of malignancy involves early diagnosis and implementation of appropriate treatment strategies. Diagnostic tools employed for colorectal cancer detection comprise flexible sigmoidoscopy, fecal occult blood test, colonoscopy, computed tomography colonoscopy and magnetic resonance imaging. More sensitive and non-invasive screening strategies involve fecal immune chemical test and detecting molecular markers (DNA, RNA and protein biomarkers) in blood and fecal samples[19]. Usually recommended standard and conventional treatment options for colorectal cancer include surgery, radiation and chemotherapy using cytotoxic drugs[20]. Choosing a specific treatment option usually depends on disease stage, patient’s age, immunity etc. Colorectal cancer is usually designated into 5 stages; stage 0, I, II, III and IV. In case of stage 0 tumour cells are removed by colonoscopy itself. For stage I, II and III surgical resection is practiced. In stage IV with distant metastasis chemotherapy is required after surgery[21]. Recent therapy is particularly concentrated in designing drugs specifically targeting the cancer cells thereby reducing cytotoxicity. One such approach is to design targeted drugs that target proteins in tumour cells. Two variants of targeted therapy include monoclonal antibodies like cetuximab and panitumumab targeting EGFR and bevacizumab targeting Vascular Endothelial Growth Factor (VEGF). Chemical drugs targeting tumour protein kinases, like gefetinib targeting EGFR and vemurafenib targeting BRAF are some variants of targeted therapy in oncology therapeutics[9]. Drugs involved in targeting immune checkpoint inhibitors like ipilimumab constitute another advanced therapeutic implementation designated as immunotherapy in cancer research[22]. Acute side effects of all the above mentioned anticancer drugs demands an alternative strategy based on natural or herbal products to combat cancer.
Herbal Therapeutics and Colorectal Cancer
According to NCI about 69 % of cancer drugs approved during 1980s and 2002 belong to eithernatural products or developed from it[7]. There are reports of plant derived bioactive compounds being effective in chemoprevention in vitro and clinical phases[23]. Literature based on experimental and clinical studies shows that dietary phytochemicals present in like carrots, apricots, broccoli, garlic, grape, pomegranate, turmeric, soybean, green tea are significantly effective in colon cancer therapeutics[6]. Signaling pathways mainly targeted by phytochemicals include PI3K/AKT, VEGF, NFkB, Mitogen-Activated Protein Kinase (MAPK) and main target include B-Cell Lymphoma-2 (BCL-2), BCL-2-Associated X Protein (BAX), B-cell lymphoma-extra-large (BCL-xl), BCL-2 Associated Agonist of Cell Death (Bad), Caspase 3, Caspase 7, Caspase 8, Caspase 9, BRAF, K-RAS, EGFR etc.,[12]. Phytochemicals with experimental evidence on colorectal therapeutics are listed below with experimental evidence on their mechanism of action.
Curcumin:
Curcumin is common phenolic dietary phytochemical found in turmeric (Curcuma longa). Curcumin has got excellent antioxidant, anti-inflammatory, chemo preventive, antimetastatic and antiangiogenic properties. Numerous in vitro trials proved therapeutic efficacy of curcumin. Curcumin inhibit proliferation of cells in HCT-116 and HT29 cell lines by arresting cell division at Growth 2 (G2)/Mitotic (M) phase and p53 up regulation respectively. Expression of Cycloxygenase-2 (COX-2) and NF-kB has got a significant role in colon cancer by initiating inflammatory pathway. Curcumin treatment can down regulate the expression of COX-2 and NFkB and induce apoptosis in HCT-116 and HT29 cell lines[24,25]. Evidences shows that Insulin like Growth Factor-1 (IGF-1) and survivin may lead to apoptotic inhibition and cell proliferation. Curcumin treatment mediate lowering of IGF-1, TNF-α and survivin expression, up regulation of p53 and consequent induction of apoptosis[26].
In mouse models curcumin administration (300 mg/kg Body Wight (BW)) delayed or reduced the incidence of adenoma in a period of 15 w treatment[27]. In Sprague-Dawely rat models curcumin treatment exhibit a decrease in expression of K-RAS and β-catenin genes. There is also a consequent decrease in expression of COX-2 and survivin in colon tissue[28].
Resveratrol:
Resveratrol is a phytoalexin found to be rich in grapes, peanuts and berries, proved to have anticancer properties like inhibition of cell division and metastasis. In HCT-116 cell lines resveratrol effect growth inhibition by mediating Phosphatase and Tensin homolog deleted on chromosome 10 (PTEN)/ PI3K/AKT and Wnt/β-catenin pathways[29]. Recent studies indicate that resveratrol decreases the expression of proinflammatory cytokines like TNF-α and IL-1β and enzymes like nitric oxide synthase and COX-2 there by modulating NF-kB inflammatory pathway, a major risk factor for colon malignancy[30].
In azoxymethane treated mouse models administration of resveratrol (200 mg/kg BW) for 100 d reduced the Aberrant Crypt Foci (ACF) formation and increased expression of BAX[31]. In APC knocked out and K-RAS loci activated genetically modified mouse a daily diet of resveratrol (300 ppm) results in 60 % inhibition in tumour formation, decrease in tumour volume and also a down regulation in expression of K-RAS[32]. 3'-hydroxypterostilbene a metabolite of resveratrol in xenograft mice model found to inhibit tumour progression by inhibiting VEGF, COX-2, cyclin D1 and promoting apoptosis[33]. A combination of resveratrol and grape seed extract exhibit chemo preventive effect in in vitro and in vivo colon carcinogenic models by suppression of Wnt/β- catenin pathway, cyclin D1, increased apoptosis and elevation of p53 and BAX/BCL-2 ratio[27].
Quercetin:
Quercetin is a flavanoids found in fruits, onion, beverages etc., and proved to be an effective anticancer agent in colon carcinogenesis. Treatment of HT29 cells with quercetin results in arrest of cell cycle at G1 phase and associated increase in expression of p53, AMPK and p21. In SW480 colon cancer cell lines quercetin treatment (160 μmol/l) down regulated β-catenin/TCF expression. Quercetin was also found to inhibit NF-kB, EGFR phosphorylation, COX-2 expression in colon cancer cell lines[13]. Quercetin derivative inhibit growth of SW480, HCT-116 and DLD-1 cell lines by modulating Wnt/ β-catenin signaling. In CT26 cell lines quercetin prevent Epithelial Mesenchymal Transition (EMT) by inhibiting N-cadherin, β-catenin and up regulating E-cadherin expression[33]. Lung metastasis of colorectal cancer was found be considerably reduced in in vivo models[34].
EGCG:
EGCG is a phytochemical rich in green tea. Studies proved that EGCG enhanced drug sensitivity. In HCT-116 and DLD1 cell lines treated with 50 μM of EGCG along with 5-Flurouracil (5-FU) increases sensitivity to 5-FU and apoptotic efficiency of cells. GRP78 expression was down regulated by EGCG treatment, which results in activation of NF-kB pathway and elevated expression of micro RNA (miR)-155-5p which suppress MDR1 expression and thereby prevent efflux of 5-FU. 5-FU accumulation contributes to increase in Bad, Caspase 3 activation, BCL-2 down regulation and apoptotic signaling[35]. In Caco-2 and Lovo cells EGCG increase the efficacy of chemo drugs like raphasatine in arresting cell division at G0/G1 phase. EGCG in vitro studies proved to be involved in inhibiting tumour pathways Wnt/β-catenin and PI3K/AKT[33].
Genistein:
Genistein is a flavone present in soybeans and is highly valued as an anticancer agent. In HCT-116 and HT29 cell lines Genistein suppresses inflammatory cytokines TNF-α, Interleukin (IL)-1β and IL-6 and down regulates NF-kB pathway. In in vivo models genistein exhibit anti-colorectal cancer effect by inhibiting expression of Proliferative Cell Nuclear Antigen (PCNA) and enhancing Nuclear Related Factor-2 (NRF-2)[33]. In HT29 colon cancer cell line genistein at 200 μM/l results in EMT reversal and down regulation of N-cadherin and inhibition of EMT marker expressions like FOXC1, FOXC2, Snail/Slug, TWST1 , ZEB1 and ZEB2. There is also an associated increase of E-cadherin level, BAX/ BCL-2 and caspase 3 and decrease in NF-kB, notch-1 and p-NF-kB expression[36]. Genistein reveal a very good potency as chemo preventive agent in colorectal cancer.
Organosulphur compounds:
Organosulphur compounds include sulphuric containing organic moieties with potential anticancer activity. Few organosulphur compounds with anticancer potential include allicin present in garlic (Allium sativum), allyl propyl disulfide (Allium cepa), asparagusic acid (Asparagus spp.) and sulforaphane in cruciferous vegetables like broccoli, cabbage etc.,[37]. Garlic is proved to be an effective chemo preventive agent in colorectal carcinogenesis even though the exact mechanism remains unclear. Garlic initiate anticancer response by inducing expression of NRF-2, trigger apoptosis and prevent angiogenesis[38]. In in vivo models garlic also exhibit colon carcinoma progression by attenuation of NF-kB[39]. Onion and Asparagus extract proved to have apoptotic and anti-proliferative effect in in vitro models[37]. Sulforaphane in cruciferous vegetables results in cellular apoptosis in Caco-2 and HCT-116 cells by up regulation of BAX, Cytochrome C, Caspase 3, -8, -9 and down regulation of antiapoptotic proteins[33].
Carotenoids:
Carotenoids are pigments present in algae, bacteria, fungi and plants. Major class of carotenoids with anti-colorectal cancer activity includes lycopene, β-carotene and lutein. In general carotenoids are proved to be antioxidant, anti-inflammatory and anticancer us activity[37]. Experimental evidence of lycopene, β-carotene and lutein in colorectal cancer is summarized in Table 1[40-44]. Apart from the phytochemicals listed above some other pharmacologically significant bioactive compounds effective in colorectal malignancy is listed in Table 2[45-51,33].
| Carotenoid | Source | Activity | References |
|---|---|---|---|
| Lycopene | Tomatos, watermelon, papaya, pink grapes, guava etc. | Inhibit the expression of NF-kB, COX-2, TNF-α, IL-1, IL-6 in SW480 cell lines. | [40] |
| β-carotene | Carrot, spinach, apricot, sweet potatoes. | Elevation in expression of BAX and p53 in in vitro studies. Mediate apoptosis by modulation of MAPK/ERK and PI3K/AKT pathway in cell lines. | [41,42] |
| Lutein | Green leafy vegetables, yellow fruits, egg yolk | Decrease in expression of K-RAS and AKT tumours in animal models. Reduction in Aberrant crypt foci in mice. | [43,44] |
Table 1: Carotenoids (Lycopene, Β-Carotene and Lutein) having Anticancer Attributes
| Phytochemical | Source | Activity | References |
|---|---|---|---|
| Garcinol | Garcinia indica | Induces apoptosis in in vitro colorectal models | [45] |
| Thymoquinone | Nigella sativa | Promote apoptosis in colorectal cell lines | [46] |
| Fiestin | Apples, strawberries, grapes, onion, cucumber | Anti-carcinogenic effect in HCT-116 cell lines Exert modulatory effect on protein kinase | [47] |
| Apigenin | Parsely, celery, chammoline, Moringa peregrina | Induce cytotoxicity in HCT-116 cell lines Reduction of Aberrant Crypt Foci (ACF) in azoxymethane treated Sprague-Dawley rats | [48,49] |
| Gingerol | Ginger | Inhibition of leukotriene A (4) hydrolase protein in HCT-116 cell lines | [50] |
| Vitamin D | Mushrooms | Intake of 1000-2000 IU/d of Vitamin D reduces the risk of colorectal malignancy in a meta analytic finding | [51] |
| Kaempferol | Apples, grapes, tomatoes, pine, ginkgo | Down regulation of PI3K/AKT pathway, increased expression of caspase 9,-3,-7 and -8, decrease in Bcl-2 | [33] |
Table 2: Phytochemicals with Therapeutic Potency in Colorectal Cancer
As mentioned, phytochemicals work by modulating specific pathways and programming expressions of corresponding target genes and proteins. Molecular mechanism involved in chemoprevention is unique for specific phytochemical and also depends on the type of malignancy. Detailed molecular mechanisms of some of the signaling pathways targeted by phytochemicals are briefly narrated.
Molecular Pathways Targeted by Phytochemicals in Chemoprevention
Apoptotic induction by phytochemicals:
Most of the phytoconstituent effect anticancer activity by inducing apoptotic response in tumour cells. Apoptosis is a kind of programmed cell death induced by increased activity of apoptotic proteins BAX, Bad, cytochrome c which activates apoptotic protein activating factor-1 which subsequently activates procaspase 9 to active casspases 9 and subsequent action of caspases involved in cellular degradation. There is also an associated reduction in anti-apoptotic proteins BCL-2, BCL-xl etc.,[52]. In certain aspects TNF-α, an inflammatory cytokine mediates apoptosis through Fas-ligand-Fas-receptor interaction leading to activation of Fas-associated death domain protein, caspase 8 and -10[47].
Anti-inflammatory mechanism by regulation of COX-2, TNF-α expressions and NF-kB pathway:
NF-κB-light-chain-enhancer of activated B cells signaling pathway plays a significant role in colorectal tumour initiation and progression and is directly correlated with inflammatory responses in colonic mucosa. Inflammatory process results in elevated levels of prostaglandins, (IL-6, IL-1β), COX-2 and TNF-α. These inflammatory markers contributes to the activation of NF-kB signaling and there by initiating cellular proliferation mediated by protein expressions like Ikappa B kinase, inhibitory protein kinase of kappa B[53].
DNA epigenetics modifications, DNA methylation, histone modifications and miRNA expression related alterations:
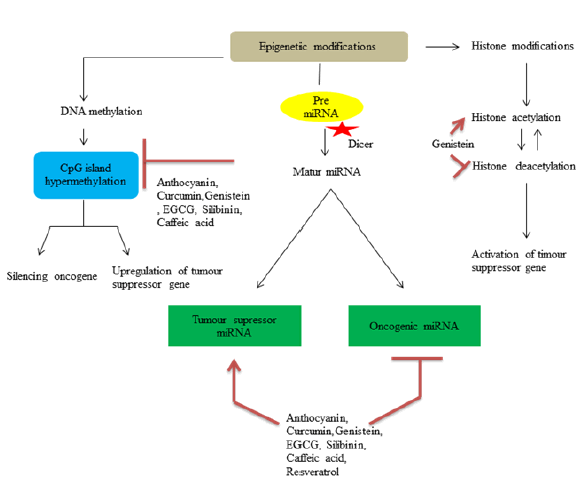
Alterations in DNA methylation like hyper methylation of cystosine in 5th position of pyrimidine ring serves CpG Island hyper methylation phenotype, which leads to activation of oncogenes[54]. These altered DNA methylation serves as therapeutic target for phytochemicals. Histones are DNA binding alkaline proteins which are responsible for compaction of DNA within the genome. Methylation, phosphorylation, acetylation and ubiquitination include types of histone modification which can lead to expression or suppression of specific genes[33]. For example one of the mechanism by which genistein inhibit colon cancer cells is by activating expression of dickkopf-1 related protein 1 through histone acetylation and suppressing Histone Deacetylase (HDAC) and reduction in protein level HDAC1[55,56]. MiRNAs are non-coding RNAs involved in post translational regulation of messenger RNA (mRNA) expression or targeted gene. Increase or decrease in specific miRNA may contribute to regulate tumour induction process by modulating corresponding target mRNA and hence miRNA serves as potential target for chemoprevention[47]. Resveratrol treatment in APCCKO/K-rasmut mouse model increase expression of miR-96 which brings down expression of K-RAS in colon tissues[32]. Diagrammatic illustration of DNA epigenetic changes are shown in fig. 1.
Wnt/β-catenin pathway:
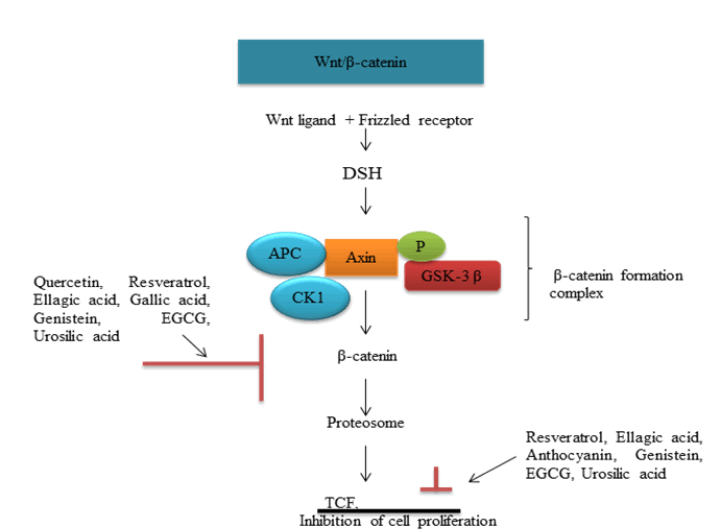
Wnt signaling plays an important role in carcinogenic process. Binding of Wnt protein with its frizzled receptor in cell surface activates Disheveled (DSH) proteins. DSH inhibits protein complex axin-GSK3- APC involved in proteolytic cleavage of β-catenin. Stabilized β-catenin will be translocated to nucleus and initiates cell proliferation (fig. 2)[57]. Wnt ligand, axin, GSK3, APC etc., serves as potential target in therapeutic intervention. Genistein exhibit preventive effect in in vitro and in vivo colorectal models by an elevation of APC and associated reduction in β-catenin stabilizing targets like PCNA, cyclin D[58].
PI3K/AKT signaling:
PI3K are enzymes involved in cellular proliferation and cell growth. PTEN is one among the target gene involved in activation of PI3K, leading to the formation of phosphatidylinositol (3,4,5)-triphosphate and phosphatidylinositol (3,4,5)-diphosphate and consequent inhibition of AKT leading to carcinogenesis[59]. It is evident from literature that anthocyanins usually exhibit chemo preventive effect by blocking PI3K[33].
Signal Transducer and Activator of Transcription 3 (STAT3) pathway:
STAT3 belongs to protein family comprising seven proteins (STAT 1, 2, 3, 4, 5a, 5b and 6) that regulate transcription of genes involved in cell proliferation, angiogenesis, apoptosis and metastasis. Activation of STAT3 is mediated by cytokines and growth factor receptors like EGFR. STAT3 up regulation inhibit apoptosis and promote cellular proliferation and angiogenesis[60].
Inhibition of angiogenesis and cell cycle arrest:
Angiogenesis is a fundamental process responsible for tumour progression and metastasis involving regeneration of new blood vessels from existing ones. VEGF by binding to specific receptor stimulates protein kinase G leading to whole process of angiogenesis. Therefore molecules targeting VEGF receptor can be effectively employed as chemo preventive agents[61].
In addition to these mechanisms phytochemicals induce cell cycle arrest by modulating expression of Cyclins, CDks, p53, p21 etc. In HT-29 and COLO cell lines curcumin induce G2/M and G0/G1 cell cycle arrest respectively. Resveratrol induce cell cycle arrest at G1/S phase in HCT-116 and Caco2 (Colon cancer) cell lines. Quercetin another potential phytochemical is capable of G0/G1 arrest in HT-29 cells[62].
Understanding of detailed cellular mechanism of action of herbs and modulation of various regulatory factors by specific phytochemicals requires elucidation and interpretation of various phytoconstituent and their possible targets at molecular level. Studies have shown that compared to individual phytoconstituent, herbal preparations exhibit superior pharmacological response and hence herbal preparations and extracts are used alone or in combination with conventional chemo drugs to improve the anticancer potency[63]. In depth information on mechanism of action of phytochemicals is accomplished by metabolomics analysis in the present scenario[64].
Metabolic alteration is a characteristic feature of malignant cells as a result of genetic and epigenetic mutations. Level of metabolisms like glucose, amino acid, pyruvate etc., directly pinpoint alterations in enzyme activities, signaling pathways, anabolic and catabolic reactions in neoplastic cells[65]. Metabolic profiling will help in the understanding the dominant compound related to anticancer activity of potential herb and also help in authentication and standardization of herbal medicine and phytoconstituent[64].
Modulation of Cancer Metabolism by Herbs
Phytoconstituent and herbal extracts were found to inhibit metabolic transporters and enzymes of signaling pathways like PI3K/AKT, rat sarcoma virus/rapidly accelerated fibrosarcoma/MEK/ERK/ MAPK thereby preventing tumor progression[63]. Studies have shown that resveratrol exposure results in elevated expression of pyruvate dehydrogenase phosphatases mRNA in Caco2 cells[66]. Huzhangoside (triterpenoid glycoside) from Anemone found to inhibit pyruvate dehydrogenase kinase and promote apoptosis in colorectal adenocarcinoma. Terminalia arjuna is an indigenous medicinal plant with a promising reputation in cancer treatment as well. As per in vivo and in vitro studies aqueous bark extract of Terminalia arjuna induce cell membrane damage and mitochondrial dysfunction in cancer cells. It also protects the heart tissue from doxorubicin toxicity[67]. A detailed analysis of mechanism of action of herbs in tumour microenvironment includes interpretation of nature of metabolite, enzyme activity and alteration of DNA, RNA and protein levels[68].
Phytochemicals in Current Chemotherapeutics and Clinical Trials in Colorectal Cancer
Phytochemicals currently practiced in colorectal chemotherapeutics include camptothecin and podophyllotoxin. Camptothecin is a quinolone alkaloid which induces cytotoxicity by complexing with DNA topoisomerase I there by blocking relegation of DNA and results in DNA double strand breaks. Irinotecan is a camptothecin semi-synthetic derivative approved by food and drug administration for colorectal malignancy. Podophyllotoxin derivative etoposide which induce DNA cleavage by interfering with topoisomerase II are employed as drugs in colorectal malignancy[15]. Phytochemicals now in clinical trial phase in colorectal carcinoma is described in Table 3.
| Phytochemical | Clinical trial | Effect | Reference |
|---|---|---|---|
| Berberine | Phase 2/3 trial of berberine hydrochloride (300 mg/twice/d) in metastatic colorectal cancer patient (NCT03281096)* | Reduced the occurrence of new adenoma | [15] |
| EPCG | Phase 1 trial of 450 mg/PO/d of TeavigTM (Highly purified and refined green tea extract with 94 % EGCG) in colorectal patients (NCT03072992)* | Improved curative resections | |
| Lycopene | Phase 2 trial of 20 mg/PO/d in colorectal patients along with panitumumab (NCT03167268)* | Reduction in skin toxicity |
Table 3: Phytochemicals in Clinical Trial Phase in Colorectal Cancer
Conclusion and Perspectives
Present review comprehensively highlights the significance of medicinal and dietary phytochemicals as an effective therapeutic alternative for colorectal cancer. Even though surgery, radiation and chemotherapy remains to be clinically practiced treatment options for colorectal malignancy, enhanced side effects and lack of target specificity of chemo drugs, there remains a demand for an alternative approach with target specificity and less side effects.
Medicinal herbs are rich in pharmacologically significant constituents in the form of secondary metabolites like alkaloids, flavanoids, terpenoids, saponins, anthocyanins, phenolic acids, tannins etc. Each category of secondary metabolite harbors diverse array of bioactive ingredients such as quercetin, resveratrol, genistein, epigallocatechingallate, lycopene, allicin, apigenin etc. As discussed in the review these active principles exhibit in vitro and in vivo evidences of anti-colorectal activity. They exhibit anti-carcinogenic potential by targeting specific signaling pathway and associated genes. Most of these compounds are dietary constituents and hence a diet rich in fruits and vegetables considerably reduces the risk of colorectal cancer. Future research in medicinal plants demands the following research aspects: Intensive studies on mechanism of action of phytochemicals, their target signaling pathway and associated genes and proteins, pharmacokinetic challenges like bioavailability, metabolism of phytochemicals and phytochemical-drug interaction, toxicological studies and dosage standardization, effective phase I, II and III clinical trials, proper identification, characterization and stringent measures to avoid adulteration of phytochemicals.
Acknowledgments:
We, the authors are sincerely thankful to our Chancellor, Chief Executive Officer, Vice- Chancellor and Registrar of Karpagam Academy of Higher Education for providing facilities and encouragement.
Conflict of interests:
The authors declared no conflict of interests.
References
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66(4):683-91.
[Crossref] [Google Scholar] [PubMed]
- Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020;70(3):145-64.
- Mundade R, Imperiale TF, Prabhu L, Loehrer PJ, Lu T. Genetic pathways, prevention and treatment of sporadic colorectal cancer. Oncoscience 2014;1(6):400.
[Crossref] [Google Scholar] [PubMed]
- Kheirelseid EA, Miller N, Kerin MJ. Molecular biology of colorectal cancer: Review of the literature. Am J Mol Biol 2013;3(2):72-80.
- Pawar SR, Jangam SS, Waghmare SA. Anti-cancer herble drugs: An overview. J Drug Deliv Ther 2018;8(4):48-58.
- Salehi B, Zucca P, Sharifi-Rad M, Pezzani R, Rajabi S, Setzer WN, et al. Phytotherapeutics in cancer invasion and metastasis. Phytother Res 2018;32(8):1425-49.
[Crossref] [Google Scholar] [PubMed]
- Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol 2005;100(1-2):72-9.
[Crossref] [Google Scholar] [PubMed]
- Benarba B, Pandiella A. Colorectal cancer and medicinal plants: Principle findings from recent studies. Biomed Pharmacother 2018;107:408-23.
[Crossref] [Google Scholar] [PubMed]
- Efferth T, Saeed ME, Mirghani E, Alim A, Yassin Z, Saeed E, et al. Integration of phytochemicals and phytotherapy into cancer precision medicine. Oncotarget 2017;8(30):50284.
[Crossref] [Google Scholar] [PubMed]
- Amin A, Gali-Muhtasib H, Ocker M, Schneider-Stock R. Overview of major classes of plant-derived anticancer drugs. Int J Biomed Sci 2009;5(1):1-11.
[Google Scholar] [PubMed]
- Kuruppu AI, Paranagama P, Goonasekara CL. Medicinal plants commonly used against cancer in traditional medicine formulae in Sri Lanka. Saudi Pharm J 2019;27(4):565-73.
[Crossref] [Google Scholar] [PubMed]
- Aiello P, Sharghi M, Mansourkhani SM, Ardekan AP, Jouybari L, Daraei N, et al. Medicinal plants in the prevention and treatment of colon cancer. Oxid Med Cell Longev 2019;2019:2075614.
[Crossref] [Google Scholar] [PubMed]
- Singh S, Sharma B, Kanwar SS, Kumar A. Lead phytochemicals for anticancer drug development. Front Plant Sci 2016;7:1667.
[Crossref] [Google Scholar] [PubMed]
- Cai J, Yi FF, Bian ZY, Shen DF, Yang L, Yan L, et al. Crocetin protects against cardiac hypertrophy by blocking MEK-ERK1/2 signalling pathway. J Cell Mol Med 2009;13(5):909-25.
[Crossref] [Google Scholar] [PubMed]
- Choudhari AS, Mandave PC, Deshpande M, Ranjekar P, Prakash O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front Pharmacol 2020;10:1614.
[Crossref] [Google Scholar] [PubMed]
- Li YH, Niu YB, Sun Y, Zhang F, Liu CX, Fan L, Mei QB. Role of phytochemicals in colorectal cancer prevention. World J Gastroenterol 2015;21(31):9262.
[Crossref] [Google Scholar] [PubMed]
- Munteanu I, Mastalier B. Genetics of colorectal cancer. J Med Life 2014;7(4):507-11.
- De Rosa M, Pace U, Rega D, Costabile V, Duraturo F, Izzo P, et al. Genetics, diagnosis and management of colorectal cancer. Oncol Rep 2015;34(3):1087-96.
[Crossref] [Google Scholar] [PubMed]
- Kolligs FT. Diagnostics and epidemiology of colorectal cancer. Visc Med 2016;32(3):158-64.
[Crossref] [Google Scholar] [PubMed]
- Karpuz M, Silindir-Gunay M, Ozer AY. Current and future approaches for effective cancer imaging and treatment. Cancer Biother Radiopharm 2018;33(2):39-51.
[Crossref] [Google Scholar] [PubMed]
- Granados-Romero JJ, Valderrama-Treviño AI, Contreras-Flores EH, Barrera-Mera B, Herrera Enríquez M, Uriarte-Ruíz K, et al. Colorectal cancer: A review. Int J Res Med Sci 2017;5(11):4667.
- Huang L, Ding X, Zhang L. Immunotherapy for colorectal cancer. Am J Dig Dis 2015;2:11-28.
- Jacobs EC. Potential therapeutic effects of phytochemicals and medicinal herbs for cancer prevention and treatment. Arch General Int Med 2018;2(3):44-8.
- Pricci M, Girardi B, Giorgio F, Losurdo G, Ierardi E, Di Leo A. Curcumin and colorectal cancer: From basic to clinical evidences. Int J Mol Sci 2020;21(7):2364.
[Crossref] [Google Scholar] [PubMed]
- Bush JA, Cheung Jr KJ, Li G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp Cell Res 2001;271(2):305-14.
[Crossref] [Google Scholar] [PubMed]
- He ZY, Shi CB, Wen H, Li FL, Wang BL, Wang J. Up regulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest 2011;29(3):208-13.
[Crossref] [Google Scholar] [PubMed]
- Perkins S, Verschoyle RD, Hill K, Parveen I, Threadgill MD, Sharma RA, et al. Chemo preventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev 2002;11(6):535-40.
[Google Scholar] [PubMed]
- Abdel-Rahman M, Ahmed HH, Salem FE, Shalby AB, Lokman MS. Curcuma longa and colon cancer: Evidence and mechanisms. World J Med Sci 2013;8(3):279-95.
- Panaro MA, Carofiglio V, Acquafredda A, Cavallo P, Cianciulli A. Anti-inflammatory effects of resveratrol occur via inhibition of lipopolysaccharide-induced NF-κB activation in Caco-2 and SW480 human colon cancer cells. Br J Nutr 2012;108(9):1623-32.
[Crossref] [Google Scholar] [PubMed]
- Honari M, Shafabakhsh R, Reiter RJ, Mirzaei H, Asemi Z. Resveratrol is a promising agent for colorectal cancer prevention and treatment: Focus on molecular mechanisms. Cancer Cell Int 2019;19(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Tessitore L, Davit A, Sarotto I, Caderni G. Resveratrol depresses the growth of colorectal aberrant crypt foci by affecting bax and p21 CIP expression. Carcinogenesis 2000;21(8):1619-22.
[Crossref] [Google Scholar] [PubMed]
- Saud SM, Li W, Morris NL, Matter MS, Colburn NH, Kim YS, et al. Resveratrol prevents tumorigenesis in mouse model of Kras activated sporadic colorectal cancer by suppressing oncogenic KRAS expression. Carcinogenesis 2014;35(12):2778-86.
[Crossref] [Google Scholar] [PubMed]
- Afrin S, Giampieri F, Gasparrini M, Forbes-Hernández TY, Cianciosi D, Reboredo-Rodriguez P, et al. Dietary phytochemicals in colorectal cancer prevention and treatment: A focus on the molecular mechanisms involved. Biotechnol Adv 2020;38:107322.
[Crossref] [Google Scholar] [PubMed]
- Tang SM, Deng XT, Zhou J, Li QP, Ge XX, Miao L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed Pharmacother 2020;121:109604.
[Crossref] [Google Scholar] [PubMed]
- La X, Zhang L, Li Z, Li H, Yang Y. (−)-Epigallocatechin Gallate (EGCG) enhances the sensitivity of colorectal cancer cells to 5-FU by inhibiting GRP78/NF-κB/miR-155-5p/MDR1 pathway. J Agric Food Chem 2019;67(9):2510-8.
[Crossref] [Google Scholar] [PubMed]
- Zhou P, Wang C, Hu Z, Chen W, Qi W, Li A. Genistein induces apoptosis of colon cancer cells by reversal of epithelial-to-mesenchymal via a Notch1/NF-κB/slug/E-cadherin pathway. BMC Cancer 2017;17(1):813-20.
[Crossref] [Google Scholar] [PubMed]
- Al-Ishaq RK, Overy AJ, Büsselberg D. Phytochemicals and gastrointestinal cancer: Cellular mechanisms and effects to change cancer progression. Biomolecules 2020;10(1):105.
[Crossref] [Google Scholar] [PubMed]
- Zhou X, Qian H, Zhang D, Zeng L. Garlic intake and the risk of colorectal cancer: A meta-analysis. Medicine 2020;99(1):e18575.
[Crossref] [Google Scholar] [PubMed]
- Zhou Y, Zhuang W, Hu W, Liu GJ, Wu TX, Wu XT. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology 2011;141(1):80-9.
[Crossref] [Google Scholar] [PubMed]
- Cha JH, Kim WK, Ha AW, Kim MH, Chang MJ. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr Res Pract 2017;11(2):90-6.
[Crossref] [Google Scholar] [PubMed]
- Cha JH, Kim WK, Ha AW, Kim MH, Chang MJ. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr Res Pract 2017;11(2):90-6.
[Crossref] [Google Scholar] [PubMed]
- Purup S, Larsen E, Christensen LP. Differential effects of falcarinol and related aliphatic C17-polyacetylenes on intestinal cell proliferation. J Agric Food Chem 2009;57(18):8290-6.
[Crossref] [Google Scholar] [PubMed]
- Pan MH, Ho CT. Chemo preventive effects of natural dietary compounds on cancer development. Chem Soc Rev 2008;37(11):2558-74.
[Crossref] [Google Scholar] [PubMed]
- Femia AP, Tarquini E, Salvadori M, Ferri S, Giannini A, Dolara P, et al. K-ras mutations and mucin profile in preneoplastic lesions and colon tumors induced in rats by 1, 2-dimethylhydrazine. Int J Cancer 2008;122(1):117-23.
[Crossref] [Google Scholar] [PubMed]
- Gali-Muhtasib HU, Younes IH, Karchesy JJ, El-Sabban ME. Plant tannins inhibit the induction of aberrant crypt foci and colonic tumors by 1, 2-dimethylhydrazine in mice. Nutr Cancer 2001;39(1):108-16.
[Crossref] [Google Scholar] [PubMed]
- Saadat N, Gupta SV. Potential role of garcinol as an anticancer agent. J Oncol 2012;2012:647206.
[Crossref] [Google Scholar] [PubMed]
- Kooti W, Servatyari K, Behzadifar M, Asadi-Samani M, Sadeghi F, Nouri B, et al. Effective medicinal plant in cancer treatment, part 2: Review study. J Evid Based Complementary Altern Med 2017;22(4):982-95.
[Crossref] [Google Scholar] [PubMed]
- Wang H, Oo Khor T, Shu L, Su ZY, Fuentes F, Lee JH, et al. Plants cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med Chem 2012;12(10):1281-305.
[Crossref] [Google Scholar] [PubMed]
- El-Alfy TS, Ezzat SM, Hegazy AK, Amer AM, Kamel GM. Isolation of biologically active constituents from Moringa peregrina (Forssk.) Fiori. (Family: Moringaceae) growing in Egypt. Pharmacog Mag 2011;7(26):109-15.
[Crossref] [Google Scholar] [PubMed]
- Leonardi T, Vanamala J, Taddeo SS, Davidson LA, Murphy ME, Patil BS, et al. Apigenin and naringenin suppress colon carcinogenesis through the aberrant crypt stage in azoxymethane-treated rats. Exp Biol Med 2010;235(6):710-7.
[Crossref] [Google Scholar] [PubMed]
- Jeong CH, Bode AM, Pugliese A, Cho YY, Kim HG, Shim JH, et al. [6]-Gingerol suppresses colon cancer growth by targeting leukotriene A4 hydrolase. Cancer Res 2009;69(13):5584-91.
[Crossref] [Google Scholar] [PubMed]
- Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Optimal vitamin D status for colorectal cancer prevention: A quantitative meta-analysis. Am J Prev Med 2007;32(3):210-6.
[Crossref] [Google Scholar] [PubMed]
- Dejean LM, Martinez-Caballero S, Manon S, Kinnally KW. Regulation of the mitochondrial apoptosis-induced channel, MAC, by BCL-2 family proteins. Biochim Biophys Acta 2006;1762(2):191-201.
[Crossref] [Google Scholar] [PubMed]
- Yin TF, Wang M, Qing Y, Lin YM, Wu D. Research progress on chemo preventive effects of phytochemicals on colorectal cancer and their mechanisms. World J Gastroenterol 2016;22(31):7058-68.
[Crossref] [Google Scholar] [PubMed]
- Daura-Oller E, Cabre M, Montero MA, Paternain JL, Romeu A. Specific gene hypomethylation and cancer: New insights into coding region feature trends. Bioinformation 2009;3(8):340-3.
- Wang H, Li Q, Chen H. Genistein affects histone modifications on Dickkopf-related protein 1 (DKK1) gene in SW480 human colon cancer cell line. PloS One 2012;7(7):e40955.
[Crossref] [Google Scholar] [PubMed]
- Groh IA, Chen C, Lüske C, Cartus AT, Esselen M. Plant polyphenols and oxidative metabolites of the herbal alkenylbenzene methyleugenol suppress histone deacetylase activity in human colon carcinoma cells. J Nutr Metab 2013;2013.
[Crossref] [Google Scholar] [PubMed]
- Taketo MM. Shutting down Wnt signal–activated cancer. Nat Genet 2004;36(4):320-2.
[Crossref] [Google Scholar] [PubMed]
- Du Q, Wang Y, Liu C, Wang H, Fan H, Li Y, et al. Chemo preventive activity of GEN-27, a genistein derivative, in colitis-associated cancer is mediated by p65-CDX2-β-catenin axis. Oncotarget. 2016 Apr 4;7(14):17870.
[Crossref] [Google Scholar] [PubMed]
- Gupta VK, Singh R, Sharma B. Phytochemicals mediated signalling pathways and their implications in cancer chemotherapy: Challenges and opportunities in phytochemicals based drug development: A review. Biochem Comp 2017;5(1):1-5.
- Johnston PA, Grandis JR. STAT3 signaling: Anticancer strategies and challenges. Mol Interv 2011;11(1):18.
[Crossref] [Google Scholar] [PubMed]
- Browning DD, Kwon IK, Wang R. cGMP-dependent protein kinases as potential targets for colon cancer prevention and treatment. Future Med Chem 2010;2(1):65-80.
[Crossref] [Google Scholar] [PubMed]
- Ahmed K, Zaidi SF, Cui ZG, Zhou D, Saeed SA, Inadera H. Potential proapoptotic phytochemical agents for the treatment and prevention of colorectal cancer. Oncol Lett 2019;18(1):487-98.
[Crossref] [Google Scholar] [PubMed]
- Fendt SM. Is there a therapeutic window for metabolism-based cancer therapies? Front Endocrinol 2017:150.
[Crossref] [Google Scholar] [PubMed]
- Oyenihi OR, Oyenihi AB, Erhabor JO, Matsabisa MG, Oguntibeju OO. Unravelling the anticancer mechanisms of traditional herbal medicines with metabolomics. Mol 2021;26(21):6541.
[Crossref] [Google Scholar] [PubMed]
- Wang XX, Yu PC, Li J. High-Throughput metabolomics for identification of metabolic pathways and deciphering the effect mechanism of dioscin on rectal cancer from cell metabolic profiles coupled with chemometrics analysis. Front Pharmacol 2020;11:68.
[Crossref] [Google Scholar] [PubMed]
- Saunier E, Antonio S, Regazzetti A, Auzeil N, Laprévote O, Shay JW, et al. Resveratrol reverses the Warburg effect by targeting the pyruvate dehydrogenase complex in colon cancer cells. Sci Rep 2017;7(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Biswas M, Karan T, Bhattacharya S, Kumar RS, Ghosh A, Haldar P. Acute and sub-chronic toxicity study of Terminalia arjuna leaf in swiss albino mice. Pharmacologyonline 2011;1:366-71.