- Corresponding Author:
- V. Ciddi
University College of Pharmaceutical Sciences, Kakatiya University, Warangal-506 009, India
E-mail: ciddiveeresham@yahoo.co.in
| Date of Submission | 18 September 2013 |
| Date of Revision | 17 January 2014 |
| Date of Acceptance | 20 January 2014 |
| Indian J Pharm Sci 2014;76(2):97-106 |
Abstract
Diabetes is a disease, which has assumed vital public health importance because of the complications associated with it. Various mechanisms including polyol pathway along with a complex integrating paradigm have been implicated in glucose-mediated complications. Though polyol pathway was established as a major mechanism, precise pathogenesis of these complications is not yet completely elucidated. Thus research focus was shifted towards key enzyme, aldose reductase in the pathway. Even though various compounds with aldose reductase inhibitory activity were synthesised, a very few compounds are under clinical use. However, studies on these compounds were always under conflicting results and an attempt has been made to review various natural substances with aldose reductase inhibitory activity and their role in management of diabetic complications.
Keywords
Polyol pathway, aldose reductase, diabetes, oxidative stress
Diabetes mellitus (DM) is a chronic metabolic disorder of impaired metabolism of carbohydrates, fats and proteins, characterised by hyperglycaemia resulting from decreased utilisation of carbohydrate and excessive glycogenolysis and gluconeogenesis from amino acids and fatty acids[1]. According to recent reports, incidence of DM is about 6.4% globally affecting 285 million adults in 2010 and will increase to 7.7%, affecting 439 million adults by 2030[2]. People with DM are at higher risk of developing serious complications including heart attacks, blindness, kidney failure and neuropathy. Large prospective clinical studies show a strong relationship between glycaemia and diabetic micro vascular complications in DM[3,4].
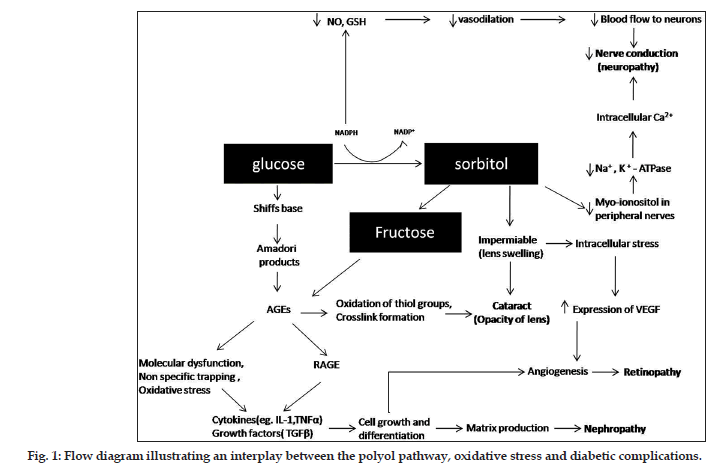
Four molecular mechanisms are being extensively studied for their role in causing diabetic complications; increase in the flux of glucose through polyol pathway, increased intracellular formation of advanced glycation end-products (AGEs), activation of protein kinase C (PKC) and increased flux through the hexosamine pathway[5]. Among these, polyol pathway play an important role in the development of complications in DM[6]. Aldose reductase (AR), which is the first enzyme in the polyol pathway, is a cytosolic, monomeric oxidoreductase enzyme that catalyses the NADPHdependent reduction of glucose[7]. In the polyol pathway, sorbitol is oxidised to fructose by the enzyme sorbitol dehydrogenase, with NAD+ reduced to NADH. Hyperglycaemia-induced polyol flux leads to increase in sorbitol-induced osmotic stress, decreased Na+/K+-ATPase activity, an increase in cytosolic NADH/NAD+ ratio and a decrease in cytosolic NADPH. As NADPH is required for regenerating reduced glutathione (GSH), this could induce or exacerbate intracellular oxidative stress leading to changes in respiration, membrane metabolism and oxidative resistance[8,9]. Since it is not possible to demonstrate a derived factor of aldose reductase inhibitor (ARI) activity that has more prognostic value for diabetic complications, a critical review of the literature on various ARIs from plants and their actual mechanism by which they produce a beneficial effect on progression of diabetic complications has been studied.
Even though, a large variety of compounds have been synthesised with potent in vitro ARI activity, very few compounds are clinically available because of undesirable side effects and poor pharmacokinetics[10]. The failure of these compounds has increased the need for search of newer molecules from natural sources. Till date numerous plant extracts and their phytoconstituents have been reported to have AR activity, but a specific review on the efficacy of these ARIs in the management of various diabetic complications has not yet been published. The present review aims at critical appraisal of the available literature on various ARIs from plants for their role in the management of diabetic complications.
Diabetic Nephropathy
Diabetic nephropathy or diabetic kidney disease is one of the most important complications and affects 20-30 % of patients with DM. It is a progressive condition culminating into a kidney failure. Diabetic nephropathy has been classically defined by the presence of proteinuria greater than 0.5 g/24 h. The onset of diabetic nephropathy was found to be 17 years after the diagnosis of DM[11]. The pathogenic role of AR in diabetic nephropathy is a significant increase of the enzyme in the glomerulus[12]. Hyperactivation of AR in renal cells leads to the generation of AGEs. These AGEs along with the generation of reactive oxygen species (ROS) results in the expression and activation of transcription factors like nuclear factor NF-?B and PKC, which are implicated in the pathogenesis of diabetic nephropathy (fig. 1). AGEs also contribute to the release of proinflammatory cytokines, expression of growth factors and adhesion molecules[13,14]. This data suggest that inhibition of AR in kidney contributes to the protective effect on diabetic kidney.
Flavonoids like quercetin and myricetin, which were reported to have potent ARI activity[15] were found to show protective effect as well as prophylactic role on the diabetic kidney by decreasing the oxidative stress[16,17]. In different studies, rosmarinic acid isolated from Origanum vulgare, nepetrin and nepetin isolated from Rosmarinus officinalis showed potent ARI activity. The aqueous extract of leaves of R. officinalis was found to alleviate the nephrotoxicity induced by CCl4 in albino rats, which was attributed to the antioxidative activity of one or more of its constituents. However, administration of rosmarinic acid alone was reported to inhibit glomerular hypertrophy and reduce glomerulosclerosis significantly in diabetic rats[18-21].
Haraguchi et al.[22] investigated the ARI activity of isoquircetrin and other flavonoids isolated from Polygonun hydropiper. In another study by Li et al.,[23] isoquercetrin was found to display a strong scavenging ability for nitrite and nitric oxide (NO) and exhibited a protective effect on the kidneys of mice. Moreover, isoquercetrin can increase the superoxide dismutase or catalase activities and reduce the malondialdehyde, protein carbonyl and NO levels in the livers and kidneys of mice.
The dihydroflavonol astilbin isolated from the leaves of Englhardtia chrysolepis has been reported to show ARI activity, whereas astilbin isolated from the rhizome of Smilax china L significantly ameliorates diabetic nephropathy by reducing renal production of transforming growth factor (TGF)-β1 and connective tissue growth factor[24,25]. The ARI activity of components isolated from active fractions of Chrysanthemum indicum have been examined and among the tested compounds, luteolin was found to show potent ARI activity, which was found to prevent morphological destruction of kidney in diabetic rats caused by increased oxidative stress induced by polyol pathway. One of the mechanisms of the renoprotective effect of luteolin was also related to increasing heme oxygenase-1 expression and elevating antioxidant status in diabetic nephropathy[26,27].
Murata et al.[28] reported the inhibitory activity of various phytoconstituents isolated from green tea, which showed varying degrees of ARI effect with (-)-epigallocatechin having no activity. These catechins composing epigallocatechin, epicatechin gallate and epicatechin were found to normalise the morphological alterations in diabetic rats, indicating that catechin has renoprotective effects on diabetic nephropathy[29]. Yamabe et al.[30] reported the beneficial effect of (-)-epigallocatechin 3-O-gallate on diabetic nephropathy via suppressing hyperglycaemia, AGEs, their related oxidative stress and cytokine activations, and thereby altering the pathological states due to its multifocal mechanisms.
Phytochemical analysis of Salacia chinensis led to the isolation of mangiferin, which was found to be a potent ARI[31]. Mangiferin improved the renal function in diabetic rats, which was manifested by its decreasing effect on 24 h urinary albumin excretion. This beneficial effect was related to mangiferin’s inhibitory effect on over expression of TGF-β1, AGE and extra cellular matrix accumulation, polyol pathway activation, ROS generation and mesangial cells proliferation[32].
Goodarzi et al.[33] investigated the ARI effect of naringin in streptozotocin (STZ)-induced diabetic rats. It was found that the inhibitory effect of naringin was comparable to that of quercetin. Besides this, naringin prevented the pathological alterations due to its insulin-sensitising, antiinflammatory, antidyslipidaemic and antioxidant activity. The mechanism for such an outcome is modulation of PPARγ, and NF-kB protein expression by naringin in kidney tissue indicating that naringin is an effective therapeutic strategy for the treatment of DM and its associated complications[34].
Curcumin, a potent inhibitor of AR, was reported to show its efficacy in diabetic nephropathy. It reverses the alterations in the activities of kidney cellular enzymes associated with DM, reverses the decrease in polyunsaturated fatty acids to saturated fatty acids ratio and ATPases activity of renal membranes, lowers nephromegaly in diabetic animals. Curcumin also acts through prevention of nuclear translocation of NF-kB, which is responsible for mesangial expansion[35-37].
Nine isoflavonoids isolated from Belamcanda chinensis showed varying degrees of ARI activity. Even though there are no reports on the protective effect of the plant on diabetic nephropathy, apocynin, one of the constituent of the plant with NADPH oxidase inhibitory activity was found to block the effect of increased glucose concentration to activate PKC-induced NADPH oxidase, and ?bronectin secretion by peritoneal cells. Apocynin blocks the increase in PKC, O-2 generation and proliferation during incubation of mesangial cells with glycated albumin[38,39].
Ellagic acid isolated from Myrciaria dubia and caffeic acid isolated from Origanum vulgare were found to inhibit AR in vitro and was also found to significantly diminish renal activity of AR and sorbitol dehydrogenase, as well as suppressed renal AR mRNA expression along with inhibition of IL-6, IL-1β, tumour necrosis factor alpha (TNF-α) and monocyte chemoattractant protein 1 in vivo suggesting the combined effect of ARI and antiinflammatory activities of ellagic acid for its beneficial role in diabetic nephropathy[40,41].
Kato et al.[42] investigated the ARI activity of various phytoconstituents in rhizome of Zingiber officinalis Roscoe and was found to posses good inhibitory activity. However, the protective effect of the plant on diabetic kidney was characterised by the inhibition of structural distortions that developed by increased free radicals after a short period of hyperglycaemia. Besides this, the plant was found to protect the kidney against the endothelial dysfunction resulting from membranous glycation[43]. Chethan et al.[44] reported the AR inhibiting activity of ferulic acid and other constituents isolated from Finger millet (Eleusine coracana) were found to show renal protective effects through improved glycemic control and renal structural changes, which are involved in the inhibition of oxidative stress, inflammation and the expression of TGF-β1 and type IV collagen[45].
Butein isolated from bark of Rhus verniciflua was found to be a potent inhibitor of human recombinant aldose reductase (HRAR) but the nephroprotective activity of the compound was attributed to its antioxidant ability and also the renal concentrating ability[46,47]. Berberine isolated from Coptis japonica was found to possess potent ARI activity, also showed the renoprotective effects, which was related to inhibition of glycosylation and improvement of antioxidation leading to upregulation of renal nephrin and podocin expressions[48,49] (Table 1).
Even though inhibition of AR plays a considerable role in the development of diabetic nephropathy, experimental studies with ARI in animal models in various studies have produced remarkably contradictory results. Treatment with tolrestat reduced the progression of urinary albumin excretion when compared with diabetic control rats, whereas other studies have failed to show this protective effect. Some studies have reported the normalisation of tissue sorbitol without much effect on urinary albumin excretion. Similarly glomerular filtration rate was found to be normalised in some studies while others have failed to show this effect on glomerular filtration rate[50]. Apart from these, various studies on ARIs have shown their efficacy in diabetic nephropathy with mechanisms that include decrease in oxidative stress-induced damage and antiinflammatory effect, which infers that even some of the potent ARIs like quercetin were found to reduce the oxidative damage that may be caused by activation of AR or by any other mechanisms. Thus, in this situation, it can be concluded that, a multiple therapy, which aims at different causative factors and various mechanisms rather than AR alone would be more beneficial in the therapy and prevention of diabetic nephropathy.
| Compound | Mechanism of action | Reference |
|---|---|---|
| Quercetin | Reducing the oxidative stress | 16 |
| Myricetin | Reducing the oxidative stress | 17 |
| Rosmarinic acid | Reducing the oxidative stress | 20 |
| Astilbin | Inhibition of inflammatory mediators | 25 |
| Luteloin | Reducing the oxidative stress | 27 |
| Catechin | Reducing the oxidative stress | 29 |
| ( )?Epigallocatechin Decreased AGEs induced oxidative | 30 | |
| 3?O?Gallate | stress, antiinflammatory property | |
| Mangiferin | Decreased AGEs induced oxidative | 32 |
| stress and antiinflammatory | ||
| property | ||
| Naringin | Antioxidant and antiinflammatory | 34 |
| property | ||
| Curcumin | Cholesterol lowering ability | 36, 37 |
| Apocynin | Decreased PKC. Decreased | 39 |
| oxidative stress | ||
| Berberine | Decreased AGEs and decresed | 49 |
| oxidative stress | ||
| Ellagic acid | Decreased AR, glycative and | 41 |
| inflammatory mediators | ||
| Ferulic acid | Antioxidant and antiinflammatory | 45 |
| property | ||
| Caffeic acid | Decreased AR, glycative and | 41 |
| inflammatory mediators | ||
Table 1: Phytoconstituents With Ari Property Studied In Diabetic Nephropathy.
Diabetic Neuropathy
Neuropathy is a common complication of both type 1 and type 2 DM with a prevalence of about 8% in newly diagnosed individuals to 50% in patients with long standing disease. There are many different diabetic neuropathies involving different nerve types, which are mainly characterised by diffuse or focal damage to peripheral somatic or autonomic nerve fibres resulting from DM. Diabetic neuropathy can be classified into diffuse and focal neuropathies with diffuse neuropathy being more common, chronic and progressive, whereas focal neuropathies are less common and acute in nature. However, all these neuropathies are thought to occur from hyperglycaemia-induced damage to nerve cells and from neuronal ischemia resulting from hyperglycaemia-induced changes in the four pathways described above[51].
| Compound | Mechanism of action | Reference |
|---|---|---|
| Quercetin | Central analgesic activity | 53 |
| Rutin | Metal chelating property | 54 |
| Baicalein | Antioxidative, | 55 |
| antiinflammatory and | ||
| inhibition of sorbitol pathway | ||
| Chlorogenic acid | Antioxidative and | 56 |
| antiinflammatory properties | ||
| Epigallocatechin?gallate | Antioxidant property | 57 |
| Ellagic acid | Antioxidant property | 58 |
| Naringin | Antioxidant property | 59 |
| Curcumin | Antioxidative and | 61 |
| antiinflammatory properties | ||
| Puerarin | Dilation of blood vessels | 60 |
| (no exact mechanism) | ||
| Baicalin | Inhibition of AR | 62 |
| Eugenol | No mediated vasodilatation | 63 |
| AR=Aldose reductase |
Table 2: Phytoconstituents With Ari Property Studied In Diabetic Neuropathy.
Increase in sorbitol concentrations by polyol pathway leads to cellular injury and decrease of myo-ionositol in the peripheral nerves and thereby leading to decrease in Na+/K+-ATPase activity, which is essential for nerve conduction[52]. Moreover, decreased NADPH results in decreased NO and reduced GSH production resulting in decreased vasodilatation and increased ROS production and oxidative damage (fig. 1). Thus ARIs are likely to contribute to the beneficial effect on development of diabetic neuropathy. A detailed review of reports on ARIs from medicinal plants showing their effect on neuropathy and their mechanism has been described (Table 2).
A potent natural ARI quercetin was found to increase nociceptive threshold indicating an antinociceptive activity of quercetin in diabetic rats[53]. However, quercetin affecting the physiological and biochemical alterations leading to diabetic neuropathy was not yet reported. Another ARI rutin was found to show a protective effect against diabetic neuropathy by its metal chelating property. It was proposed to sequester the transition metal preventing the fenton reaction, which may contribute to the development of diabetic neuropathy[54]. The protective effect of baicalein was attributed to various mechanisms like inhibition of PKC, oxidative stress and LOX pathways but does not attributed to sorbitol pathway, even though the compound was reported to be a potent ARI[55]. A phenolic ARI chlorogenic acid was found to show antihyperalgesic activity due to its antioxidant and antiinflammatory properties[56]. Similarly the beneficial effect of a potent ARI epigallocatechin-gallate against diabetic neuropathy was due to the inhibition of oxidative stress in diabetic rats[57].
Ellagic acid, which is a potent ARI along with antioxidant property, was found to show a protective effect against diabetic neuropathy by its antioxidant property. It was found to significantly decrease malondialdehyde (MDA) and nitrate levels in diabetic rats when compared with control group[58]. Likewise, a potent antioxidant, naringin with an ARI property was found to exhibit neuroprotective effect in diabetic rats by downregulation of free radical and cytokine regulated TNF-α[59]. Similarly an isoflavonoid, puerarin, was found to increase the conductive velocity of the nerves, which is due to the dilation of blood vessels leading to improvement of microcirculation and reduced blood viscosity. However, the mechanism by which these alterations occurred were yet to be studied[60]. Chronic treatment with curcumin, a potent ARI and antioxidant led to inhibition of NO and TNF-α and thereby antihyperalgesic activity in diabetic rats[61].
Baicalin was found to show a protective effect and relieve clinical symptoms of diabetic neuropathy by direct inhibition of AR in diabetic rats[62]. Similarly eugenol, a potent ARI was found to improve diabetic neuropathy by augmentation of NO and endotheliumderived hyperpolarising factor (EDHF)-mediated vasorelaxation[63].
Aremisia dracunculus, a plant with various potent ARIs, exhibited a beneficial effect of diabetic neuropathy by decreasing the sciatic nerve and spinal cord 12/15-lipoxygenase activation and oxidative nitrosative stress, but has failed to ameliorate hyperglycaemia or reduce sciatic nerve sorbitol pathway[64,65]. Likewise the curative and preventive property of trigonella in diabetic neuropathy was due to improvement in glucose intolerance, antiinflammatory and antioxidant property, even though the plant was found to possess a potent ARI activity[66,67].
Administration of a Momordica charantia fraction with potent ARI activity in diabetic rats led to a slight increase in myelinated fibre area. Even though, the mechanism for this beneficial effect of M. charantia on the structural abnormalities of peripheral nerves in experimental DM was not established, however, the antioxidative property of the plant for the prevention of functional abnormalities in STZ-diabetic rats[68] was reported.
Apart from the above mentioned, plants such as Olea europaea, with no reported AR activity, was found to be effective against diabetic neuropathy and was found to attenuate thermal hyperalgesia in diabetic rats. The plant was found to show the effect by preventing the glucose-induced neuronal apoptosis[69].
Diabetic Cataract
Cataract is a condition where the crystalline lens of the eye loses its transparency. DM has been associated with an increase in cataract among adults[70]. Cataract in DM is mainly caused by swelling of crystalline lens due to osmotic changes caused by increased sorbitol concentration. The other mechanism is the cross linking of lens proteins due to nonenzymatic glycosylation. These glycated products generally called as AGEs readily accumulate in the lens and cause oxidation of thiol groups, cross link formation and aggregation of the crystalline proteins producing high molecular weight insoluble proteins, which are responsible for opacification[71] (fig. 1). Thus the polyol pathway plays a momentous role in the formation of cataract in DM.
Even though various classes of drugs such as antioxidants, vitamins, Nonsteroidal antiinflammatory drugs (NSAIDs) and others, have been developed, which aim to interact with the altered lens metabolism in cataract; ARIs remain to be the most studied class of drugs. The role of these drugs in the prevention of diabetic cataract is now well established and various natural and synthetic ARIs are under active research. A good number of synthetic compounds have been found to exhibit anticataract potential in different animal models and clinical trials. Never the less, even various natural compounds were found to possess good anticataract activity. Thus a brief review of various herbal ARIs and their mechanism in inhibiting the diabetic cataract has been compiled.
| Plant | Type of cataract | Mechanism of action | Reference |
|---|---|---|---|
| Pterocarpus | Diabetic | Antihyperglycemic | 67 |
| marsupium | affect | ||
| Trigonella | Diabetic | Antihyperglycemic | 67 |
| foenum-graecum | affect | ||
| Green tea | Diabetic | Antihyperglycemic | 72 |
| affect | |||
| Adhatodavasica | Diabetic | Inhibition of AR; | 73 |
| antioxidant activity | |||
| Cassia fistula | Diabetic | Inhibition of AR; | 73 |
| Ocimum | Diabetic | Decrease in polyol | 74 |
| sanctum | accumilation | ||
| Silybum | Diabetic | Antioxidant property | 75 |
| marianum | |||
| Hydrocotyl | Sorbitol induced | Antioxidant property, | 76 |
| bonariensis | reduced apoptosis | ||
| Curcuma longa | Diabetic | Inhibition of AR; | 77 |
| antioxidant activity | |||
| Aralia elata | Diabetic | Inhibition of AR; | 80 |
| antioxidant activity | |||
| Brickelliaarguta | Diabetic | Inhibition of AR | 79 |
| Emblica | Diabetic | Inhibition of AR | 78 |
| officinalis |
Table 3: Plants With Ari Property Studied In Diabetic Cataract.
To date numerous studies were conducted to evaluate the anticataract activity of different medicinal plants and phytoconstituents. Enlisting a few of them, green tea was found to inhibit AR, glycated protein and along with its hypoglycaemic affect, it was found to show anticataract activity in diabetic rats[72]. Similarly Adhatoda vasica exhibited anticataract activity by inhibiting lens AR. Administration of byakangelicin to diabetic rats led to of suppression of sorbitol leading to increase in Na+/K+-ATPase and thereby exhibiting a protective effect on diabetic cataract. However, the mechanism was not proposed. Trigonella foenum-graceum and Pterocarpus marsupium were found to exhibit anticataract activity by means of antihyperglycaemic effect with trigonella exhibiting ARI activity, while there is no ARI activity reported for the later[67,73] (Table 3).
Cassia fistula was found to show anticataract activity by significantly inhibiting AR activity in rat lens[73]. Ocimum sanctum and Silybum marianum significantly decreased polyol accumulation and increased the GSH levels, respectively, leading to a protective effect on diabetic cataract[74,75]. Leaves extract of Hydrocotyl bonariensis reduced the lens protein precipitation and lens peroxidation and thereby increasing lens antioxidant status and delayed the formation of diabetic cataract. It also led to the reduction in lens apoptosis and epithelial proliferation[76]. Treatment of diabetic rats with turmeric or curcumin reversed the diabetic changes with respect to lipid peroxidation, reduced GSH. It also led to the changes in osmotic stress by modification of polyol enzymes and insolubilisation of lens proteins was also prevented[77]. Tannoid principles from Emblica officinalis and flavonoids from Brickellia arguta inhibited lens AR activity and thereby inhibited the polyol pathway induced oxidative stress leading to a protective effect on diabetic cataract in rats[78,79]. Aralia elata extract inhibited lens AR and along with antioxidant activity it also showed a preventive effect on cataractogenesis in xylose containing lens organ cultures and in vivo in STZ-induced diabetic rats[80]. Similarly flavonoids from Emilia sochifolia modulated lens opacification by reducing the oxidative stress in selenite-induced cataract[81].
| Compound | Type of cataract | Mechanism of action | Reference |
|---|---|---|---|
| Quercetin | Diabetic | Inhibition of oxidative damage; by inhibiting lens AR | 87 |
| Myricetin | Diabetic | Inhibittion of lens AR | 88 |
| Quercitrin | Diabetic | Inhibittion of lens AR | 88 |
| Rutin | Selenite | Prevention of depletion of GSH, Inhibition of lipid peroxidation | 82 |
| Fisetin | Radiation | Decreased ROS; modulation of activation of NF??b and MAPK | 83 |
| Ellagic acid | Selenite | Inhibition of lipid | 84 |
| peroxidation | |||
| Puerarin | Diabetic | Unknown mechanism | 90 |
| Puerariafuran | Diabetic | Inhibition of AR, Inhibition of oxidative damage | 89 |
| Genistein | Diabetic | Increased expression of connexin (Cx) 43 | 85 |
Table 4: Phytoconstituents With Ari Propertystudied In Diabetic Cataract.
Apart from the above-mentioned plants, various phytoconstituents were also found to inhibit AR with a significant protectant role in diabetic cataract[82-85]. Quercetin, which is a well-known ARI when studied in a cataract model, and its metabolite 3′-O-methyl quercetin inhibited oxidative damage in the lens[86]. Other flavonoids such as quercetrin, myricetin and puerariafuran showed the protective effect on diabetic cataract by inhibiting the lens AR[87-89]. However, puerarin was found to protect against diabetic cataract by exhibiting an anti effect on lens epithelial cells[90] (Table 4).
Diabetic Retinopathy
Diabetic retinopathy (DR) is the most common ocular complication in DM and is an important cause of preventable blindness. DR is broadly classified as nonproliferative DR involving intraretinal microvascular changes and proliferative DR involving the formation of new vessels or fibrous tissue or both on the retina. DR primarily effects the microvascular circulation of the retina. The factors leading to these changes are thickening of basement membrane of the capillary wall, increased platelet stickiness and changes in RBCs resulting in sluggish microvascular circulation and biochemical changes in the form of activation of polyol pathway resulting in tissue damage. Since the retinal ganglionic cells and endothelial cells are endowed with AR enzyme, these cells are more prone to damage caused by the activation of polyol pathway leading to DR[91] (fig. 1).
Even though a good glycaemic control seems to be the preferred option for the management of DR, knowledge and modulation of the pathways like polyol pathway through which hyperglycaemia causes diabetic complications showed promising results[92]. ARIs were able to prevent or reduce the changes in fructose fed STZ rats and normalise the retinal blood flow[93]. In contrast to the above study, a 5-year treatment with sorbinil did not show any effect on the retinal pathology in diabetic dogs[94]. In view of the contradicting results pertaining to the efficacy of ARIs in DR, a comprehensive review of the available literature on the ARIs from natural sources and their role in showing a beneficial effect in DR is essential in understanding the importance of ARIs.
Among the various ARIs from natural sources, few plants like Ocimum sanctum, Tinospora cordifolia, Azardiracta indica, Ganoderma lucidum were tested for their efficacy in retinopathy in various animal models. However, some plants like Ocimum sanctum were found to protect from DR only when given in combination with Vitamin E[95]. Tinospora cordifolia was found to inhibit over expression of angiogenic and inflammatory mediators and thereby prevent retinal oxidative stress exhibiting a protective effect on DR[96]. Similarly, Ganoderma lucidum was found to be effective in DR by enhancing the capability of antioxidation in diabetic rats and reducing the damage of retina from oxidation[97].
Phytoconstituents like baicalein, curcumin and hesperetin were effective in DR with baicalein ameliorating inflammatory process and thereby inhibiting vascular abnormality and neuronal loss in retinal tissues, whereas curcumin and hesperitin were found to decrease the levels of various mediators like vascular endothelial growth factor[98-100]. Other substances like quercetin and rosmarinic acid were found to show their effect on DR by prevention of angiogenesis, which can be related to the antioxidative properties of the compounds[101,102].
Conclusion
As stated earlier, many theories have been proposed and studied to explain mechanisms leading to diabetic complications, which includes glucose metabolism through polyol pathway where AR plays a vital role and excessive oxidative stress. While ARIs are the promising targets for the treatment of diabetic complications, most of the developed ARIs show poor or only a partial amelioration and some show unacceptable toxicities[103]. This can be explained by the fact that diverse complications may not share the same and single mechanism. Since it is highly improbable for any single mechanism to explain the pathogenesis associated with diabetic complications, drugs targeted to a specific mechanism often produce unintended effects, which is one of the drawback for ARIs.
As mentioned in the above reports, various plant extracts and their phytoconstituents, which showed ARI activity, exhibited their beneficial effect on various complications. However, majority of the ARIs acted by inhibiting oxidative stress or inflammatory changes that occur during DM. As a matter of fact, polyol pathway leads to oxidative stress and inhibition of AR leads to the decrease of oxidative stress. But the protective effect of the above-mentioned ARIs in diabetic complications is due to the ARI activity, thereby decreasing the polyol pathwayinduced oxidative stress and/or due to the antioxidant property of the compound. Combined with this clinical efficacy of the compounds with either ARI activity or antioxidant property alone seems to be uncertain with various reasons, which are beyond the scope of this review. Thus, a molecule with both ARI activity and antioxidative properties could be more effective than a compound with either ARI or antioxidant property alone.
References
- Pontiroli AE, Calderara A, Pozza G. Secondary failure of oral hypoglycaemic agents: Frequency, possible causes, and management. Diabetes Metab Rev 1994;10:31-43.
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res ClinPract 2010;87:4-14.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-86.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53.
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813-20.
- Gabbay KH. The sorbitol pathway and the complications of diabetes. N Engl J Med 1973;288:831-6.
- Wilson DK, Bohren KM, Gabbay KH, Quiocho FA. An unlikely sugar substrate site in the 1.65 A structure of the human aldose reductaseholo enzyme implicated in diabetic complications. Science 1992;257:81-4.
- Garcia FS, Virág L, Jagtap P, Szabó E, Mabley JG, Liaudet L, et al. Diabetic endothelial dysfunction: The role of poly(ADP-ribose)polymerase activation. Nat Med 2001;7:108-13.
- Lee AY, Chung SS. Contributions of polyol pathway to oxidative stress in diabetic cataract. Faseb J 1999;13:23-30.
- Rosenstock PJ. Aldose reductase inhibitors and diabetic complications. Am J Med 1987;83:298-306.
- Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type I diabetes. Am J Med 1985;78:785-94.
- Kasajima H, Yamagisi S, Sugai S, Yagihashi N, Yagihashi S. Enhanced in situ expression of aldose reductase in peripheral nerve and renalglomeruli in diabetic patients. Virchows Arch 2001;439:46-54.
- Kapor-Drezgic J, Zhou X, Babazono T, Dlugosz JA, Hohman T, Whiteside C. Effect of high glucose on mesangial cell protein kinase C-delta and -epsilon is polyol pathway-dependent. J Am SocNephrol 1999;10:1193-203.
- Oates PJ, Mylari BL. Aldose reductase inhibitors: Therapeutic implications for diabetic complications. Expert OpinInvestig Drugs 1999;8:2095-119.
- Varma SD, Mikuni I, Kinoshita JH. Flavonoids as inhibitors of lens aldose reductase. Science 1975;188:1215-6.
- Anjaneyulu M, Chopra K. Quercetin, an antioxidant bioflavonoid, attenuates diabetic nephropathy in rats. ClinExpPharmacolPhysiol 2004;31:244-8.
- Ozcan F, Ozmen A, Akkaya B, Aliciguzel Y, Aslan M. Beneficial effect of myricetin on renal functions in streptozotocin-induced diabetes. ClinExp Med 2012;12:265-72.
- Tomás-Barberán FA, López-Gómez C, Villar A, Tomás-Lorente F. Inhibition of lens aldose reductase by Labiatae flavonoids. Planta Med
- 1986;3:239-40.
- Koukoulitsa C, Zika C, Geromichalos GD, Demopoulos VJ, Skaltsa H. Evaluation of aldose reductase inhibition and docking studies of some secondary metabolites, isolated from Origanumvulgare L. ssp. hirtum. Bioorg Med Chem 2006;14:1653-9.
- Majid T, Hasan A, Alireza K, Ahmad T. Rosmarinic acid ameliorates diabetic nephropathy in uninephrectomized diabetic rats. Iran J Basic MedSci 2011;14:275-83.
- Saber AS, Hawazen AL. Protective effect of Rosemary (RosmarinusOfficinalis) leaves extract on carbon tetrachloride-inducednephrotoxicity in Albino rats. Life Sci J 2012;9:779-85.
- Haraguchi H, Ohmi I, Sakai S, Fukuda A, Toihara Y, Fujimoto T, et al. Effect of Polygonumhydropiper sulfated flavonoids on lens aldose reductase and related enzymes. J Nat Prod 1996;59:443-5.
- Li R, Yuan C, Dong C, Shuang S, Choi MM. In vivoantioxidative effect of isoquercitrin on cadmium-induced oxidative damage to mouse liver and kidney. NaunynSchmiedebergs Arch Pharmacol 2011;383:437-45.
- Haraguchi H, Ohmi I, Fukuda A, Tamura Y, Mizutani K, Tanaka O, et al. Inhibition of aldose reductase and sorbitol accumulation byastilbin and taxifolindihydroflavonols in Engelhardtiachrysolepis. BiosciBiotechnolBiochem 1997;61:651-4.
- Chen L, Lan Z, Zhou Y, Li F, Zhang X, Zhang C, et al. Astilbin attenuates hyperuricemia and ameliorates nephropathy in fructose-induced hyperuricemic rats. Planta Med 2011;77:1769-73.
- Yoshikawa M, Morikawa T, Murakami T, Toguchida I, Harima S, Matsuda H. Medicinal flowers. I. Aldose reductase inhibitors and three new eudesmane-type sesquiterpenes, kikkanols A, B, and C, from the flowers of Chrysanthemum indicumL. Chem Pharm Bull 1999;47:340-5.
- Wang GG, Lu XH, Li W, Zhao X, Zhang C. Protective Effects of Luteolin on Diabetic Nephropathy in STZ-Induced Diabetic Rats. EvidBased Complement Alternat Med 2011;2011:3231-71.
- Murata M, Iries J, Homma S. Aldose reductase inhibitors from green tea. J Food SciTechnol 1994;27:401-5.
- Hase M, Babazono T, Karibe S, Kinae N, Iwamoto Y. Renoprotectiveeffects of tea catechin in streptozotocin- induced diabetic rats. IntUrolNephrol 2006;38:693-9.
- Yamabe N, Yokozawa T, Oya T, Kim M. Therapeutic potential of (-)-epigallocatechin 3-O-gallate on renal damage in diabetic nephropathy model rats. J PharmacolExpTher 2006;319:228-36.
- Morikawa T, Kishi A, Pongpiriyadacha Y, Matsuda H, Yoshikawa M. Structures of new friedelane-type triterpenes and eudesmane-type sesquiterpene and aldose reductase inhibitors from Salaciachinensis. J Nat Prod 2003;66:1191-6.
- Li X, Cui X, Sun X, Li X, Zhu Q, Li W. Mangiferin prevents diabetic nephropathy progression in streptozotocin-induced diabetic rats. Phytother Res 2010;24:893-9.
- Goodarzi MT, Zal F, Malakooti M, Safari MR, Sadeghian S. Inhibitory activity of flavonoids on the lens aldose reductase of healthy and diabetic rats. Acta Med Iran 2006;44:41-5.
- Sharma AK, Bharti S, Ojha S, Bhatia J, Kumar N, Ray R, et al.Up-regulation of PPARγ, heat shock protein-27 and -72 by naringin attenuates insulin resistance, β-cell dysfunction, hepatic steatosisand kidney damage in a rat model of type 2 diabetes. Br J Nutr2011;106:1713-23.
- Du ZY, Bao YD, Liu Z, Qiao W, Ma L, Huang ZS, et al. Curcuminanalogs as potent aldose reductase inhibitors. Arch Pharm (Weinheim) 2006;339:123-8.
- Suresh Babu P, Srinivasan K. Amelioration of renal lesions associated with diabetes by dietary curcumin in streptozotocin diabetic rats. MolCell Biochem 1998;181:87-96.
- Chiu J, Khan ZA, Farhangkhoee H, Chakrabarti S. Curcumin prevents diabetes-associated abnormalities in the kidneys by inhibiting p300 and nuclear factor-kappaB. Nutrition 2009;25:964-72.
- Jung SH, Lee YS, Lee S, Lim SS, Kim YS, Shin KH. Isoflavonoidsfrom the rhizomes of Belamcandachinensis and their effects on aldose reductase and sorbitol accumulation in streptozotocin induced diabetic rat tissues. Arch Pharm Res 2002;25:306-12.
- Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T, et al. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int 2005;67:1890-8.
- Ueda H, Kuroiwa E, Tachibana Y, Kawanishi K, Ayala F, Moriyasu M. Aldose reductase inhibitors from the leaves of Myrciariadubia(H. B. and K.) McVaugh. Phytomedicine 2004;11:652-6.
- Chao CY, Mong MC, Chan KC, Yin MC. Antiglycative and antiinflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. MolNutr Food Res 2010;54:388-95.
- Kato A, Higuchi Y, Gato H, Kizu H, Okamoto T, Asano N, et al. Inhibitory effects of Zingiberofficinale Roscoe derived components on aldose reductase activity in vitro and in vivo. J Agric Food Chem 2006;54:6640-4.
- Qattan KA, Thomson M, Muslim A. Garlic (Allium sativum) and ginger (Zingiberofficinale) attenuate structural nephropathy progression in streptozotocin-induced diabetic rats. e-SPEN, Eur e-J ClinNutrMetabol 2008;3:e62-e71.
- Chethan S, Dharmesh SM, Malleshi NG. Inhibition of aldose reductase from cataracted eye lenses by finger millet (Eleusinecoracana) polyphenols. Bioorg Med Chem 2008;16:10085-90.
- Choi R, Kim BH, Naowaboot J, Lee MY, Hyun MR, Cho EJ, et al.Effects of ferulic acid on diabetic nephropathy in a rat model of type 2 diabetes. ExpMol Med 2011;43:676-83.
- Lee EH, Song DG, Lee JY, Pan CH, Um BH, Jung SH. Inhibitory effect of the compounds isolated from Rhusverniciflua on aldose reductaseand advanced glycationendproducts. Biol Pharm Bull 2008;31:1626-30.
- Kang DG, Lee AS, Mun YJ, Woo WH, Kim YC, Sohn EJ, et al. Butein ameliorates renal concentrating ability in cisplatin-induced acute renal failure in rats. Biol Pharm Bull 2004;27:366-70.
- Lee HS. Rat lens aldose reductase inhibitory activities of Coptisjaponica root- derived isoquiniline alkaloids. J Agric Food Chem2002;50:7013-6.
- Wu D, Wen W, Qi CL, Zhao RX, Lü JH, Zhong CY, et al.Ameliorative effect of berberine on renal damage in rats with diabetes induced by high-fat diet and streptozotocin. Phytomedicine 2012;19:712-8.
- Boel E, Selmer J, Flodgaard HJ, Jensen T. Diabetic late complications: Will aldose reductase inhibitors or inhibitors of advanced glycosylation endproduct formation hold promise J Diabetes Complications 1995;9:104-29.
- Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: mechanisms to management. PharmacolTher 2008;120:1-34.
- Oka M, Kato N. Aldose reductase inhibitors. J Enzyme Inhib 2001;16:465-73.
- Anjaneyulu M, Chopra K. Quercetin, a bioflavonoid, attenuates thermal hyperalgesia in a mouse model of diabetic neuropathic pain. ProgNeuropsychopharmacolBiol Psychiatry 2003;27:1001-5.
- Je HD, Shin CY, Park SY, Yim SH, Kum C, Huh IH, et al. Combination of vitamin C and rutin on neuropathy and lung damage of diabetes mellitus rats. Arch Pharm Res 2002;25:184-90.
- Stavniichuk R, Drel VR, Shevalye H, Maksimchyk Y, Kuchmerovska TM, Nadler JL, et al. Baicalein alleviates diabetic peripheral neuropathy through inhibition of oxidative-nitrosative stress and p38 MAPK activation. ExpNeurol 2011;230:106-13.
- Bagdas D, Cinkilic N, Ozboluk HY, Ozyigit MO, Gurun MS. Antihyperalgesic activity of chlorogenic acid in experimental neuropathic pain. J Nat Med 2013;67:698-704.
- Tourandokht B, Mehrdad R. Chronic Oral Epigallocatechin-gallatealleviates streptozotocin-induced diabetic neuropathic hyperalgesia in rat: Involvement of oxidative stress. Iran J Pharm Res 2012;11:1243-53.
- Uzar E, Alp H, Cevik MU, F?rat U, Evliyaoglu O, Tufek A, et al.Ellagic acid attenuates oxidative stress on brain and sciatic nerve and improves histopathology of brain in streptozotocin-induced diabetic rats. NeurolSci 2012;33:567-74.
- Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia 2012;83:650-9.
- Xie Y, Jingdan S, Wenpu C. Effects of Puerarin injection on diabetic peripheral neuropathy: Analysis of 31 cases. J Guangdong Med Coll 1998-Z1. Available at http://en.cnki.com.cn/Article_en/CJFDTOTAL-GDYY8Z1.017.htm [Last accessed on 2014 Jan 17].
- Sharma S, Kulkarni SK, Agrewala JN, Chopra K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol 2006;536:256-61.
- Yanhu D, Linan P, Xiujun W. Primary observation of therapeutic effect of baicalin on diabetic peripheral neuropathy. Chin J Diabetes 1999:06. Available at http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZGTL199906011.htm [Last accessed on 2014 Jan 17].
- Nangle MR, Gibson TM, Cotter MA, Cameron NE. Effects of eugenol on nerve and vascular dysfunction in streptozotocin-diabetic rats. Planta Med 2006;72:494-500.
- Logendra S, Ribnicky DM, Yang H, Poulev A, Ma J, Kennelly EJ, et al. Bioassay-guided isolation of aldose reductase inhibitors fromArtemisia dracunculus. Phytochemistry 2006;67:1539-46.
- Watcho P, Stavniichuk R, Tane P, Shevalye H, Maksimchyk Y, Pacher P, et al. IG. Evaluation of PMI-5011, an ethanolic extract of ArtemisiadracunculusL., on peripheral neuropathy in streptozotocin-diabeticmice. Int J Mol Med 2011;27:299-307.
- Nanjundan PK, Arunachalam A, Thakur RS. Antinociceptive property of Trigonellafoenumgraecum (Fenugreek seeds) in high fat diet-fed/low dose streptozotocin-induced diabetic neuropathy in rats. Pharmacologyonline 2009;2:24-36.
- Saraswat M, Muthenna P, Suryanarayana P, Petrash JM, Reddy GB. Dietary sources of aldose reductase inhibitors: Prospects for alleviating diabetic complications. Asia Pac J ClinNutr 2008;17:558-65.
- Celia G, Cummings E, David AP, Jaipaul S. Beneficial effect and mechanism of action of Momordicacharantia in the treatment of diabetes mellitus: A mini review. Int J Diabetes Metab2003;11:46-55.
- Kaeidi A, Esmaeili-Mahani S, Sheibani V, Abbasnejad M, Rasoulian B, Hajializadeh Z, et al. Olive (Oleaeuropaea L.) leaf extract attenuates early diabetic neuropathic pain through prevention of high glucose-induced apoptosis: In vitro and in vivo studies. J Ethnopharmacol 2011;136:188-96.
- Ederer F, Hiller R, Taylor HR. Senile lens changes and diabetes in two population studies. Am J Ophthalmol 1981;91:381-95.
- Ahmed N. Advanced Glycation end products-role in pathology of diabetic complications. Diabetes Res ClinPract 2005;67:3-21.
- Vinson JA, Zhang J. Black and green teas equally inhibit diabetic cataracts in a streptozotocin-induced rat model of diabetes. J Agric Food Chem 2005;53:3710-3.
- Gacche RN, Dhole NA. Aldose reductase inhibitory, anticataract and antioxidant potential of selected medicinal plants from the Marathwada region, India. Nat Prod Res 2011;25:760-3.
- Halder N, Joshi S, Gupta SK. Lens aldose reductase inhibiting potential of some indigenous plants. J Ethnopharmacol 2003;86:113-6.
- Huseini HF, Zaree AB, Zarch AB, Heshmat R. The effect of herbal medicine Silybummarianum (L.) Gaertn. seed extract on galactose-induced cataract formation in rat. J Med Plants 2004;3:58-62.
- Ajani EO, Salako AA, Sharlie PD, Akinleye WA, Adeoye AO, SalauBA, et al. Chemopreventive and remediation effect ofHydrocotylbonariensisComm. Ex Lam (Apiaceae) leave extract in galactose-induced cataract. J Ethnopharmacol 2009;123:134-42.
- Suryanarayana P, Saraswat M, Mrudula T, Krishna TP, KrishnaswamyK, Reddy GB. Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Invest Ophthalmol Vis Sci 2005;46:2092-9.
- Suryanarayana P, Saraswat M, Petrash JM, Reddy GB. Emblicaofficinalisand its enriched tannoids delay streptozotocin-induceddiabetic cataract in rats. Mol Vis 2007;13:1291-7.
- Rosler KH, Goodwin RS, Mabry TJ, Varma SD, Norris J. Flavonoids with anticataract activity from Brickelliaarguta. J Nat Prod 1984;47:316-9.
- Chung YS, Choi YH, Lee SJ, Choi SA, Lee JH, Kim H, et al. Water extract of Aralia elata prevents cataractogenesisin vitro and in vivo. J Ethnopharmacol 2005;101:49-54.
- Lija Y, Biju PG, Reeni A, Cibin TR, Sahasranamam V, AbrahamA. Modulation of selenite cataract by the flavonoid fraction of Emilia sonchifoliain experimental animal models. Phytother Res2006;20:1091-5.
- Isai M, Sakthivel M, Ramesh E, Thomas PA, Geraldine P. Prevention of selenite-induced cataractogenesis by rutin in Wistar rats. Mol Vis 2009;15:2570-7.
- Yao K, Zhang L, Zhang Y, Ye P, Zhu N. The flavonoid fisetin inhibits UV radiation-induced oxidative stress and the activation of NF-kappaB and MAPK signaling in human lens epithelial cells. Mol Vis 2008;14:1865-71.
- Sakthivel M, Elanchezhian R, Ramesh E, Isai M, Jesudasan CN, Thomas PA, et al. Prevention of selenite-induced cataractogenesis in Wistar rats by the polyphenol, ellagic acid. Exp Eye Res 2008;86:251-9.
- Huang R, Shi F, Lei T, Song Y, Hughes CL, Liu G. Effect of the isoflavonegenistein against galactose-induced cataracts in rats. ExpBiol
- Med 2007;232:118-25.Cornish KM, Williamson G, Sanderson J. Quercetin metabolism in the lens: Role in inhibition of hydrogen peroxide induced cataract. FreeRadicBiol Med 2002;33:63-70.
- Mohan M, Gupta SK, Agnihotri S, Joshi S, Uppal RK. Anticataract action of topical quercetin and myricetin in galactosemic rats. Med Sci Res 1988;6:685-6.
- Varma SD, Mizuno A, Kinoshita JH. Diabetic Cataracts and Flavonoids. Science 1977;195:205-6.
- Kim NH, Kim YS, Lee YM, Jang DS, Kim JS. Inhibition of aldose reductase and xylose-induced lens opacity by puerariafuran from the roots of Puerarialobata. Biol Pharm Bull 2010;33:1605-9.
- Hao LN, He SZ, Shen YH, Zhang YQ, Wang ZY, Wang YH. Protective effects of puerarin on lens epithelial cells in rat diabetic cataract. Zhonghua Yan KeZaZhi 2011;47:320-6.
- Frank RN. Etiologic mechanisms in diabetic retinopathy. In: Ryan SJ, editor. Retina. 2nd ed. St. Louis: CV Mosby; 1994. p. 1243-76.
- ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Eng J Med 2010;363:233-44.
- Funada M, Okamoto I, Fujinaga Y, Yamana T. Effects of aldose reductase inhibitor (M79175) on ERG oscillatory potential abnormalities in streptozotocin fructose- induced diabetes in rats. Jpn J Ophthalmol 1987;31:305-14.
- Engerman RL, Kern TS. Aldose reductase inhibition fails to prevent retinopathy in diabetic and galactosemic dogs. Diabetes 1993;42:820-5.
- Halim EM, Mukhopadhyay AK. Effect of Ocimum sanctum (Tulsi) and Vitamin E on biochemical parameters and retinopathy in streptozotocininduced diabetic rats. Indian J ClinBiochem 2006;21:181-8.
- Agrawal SS, Naqvi S, Gupta SK, Srivastava S. Prevention and management of diabetic retinopathy in STZ diabetic rats by Tinosporacordifoliaand its molecular mechanisms. Food ChemToxicol2012;50:3126-32.
- Yuan YX, Wang SQ. Effects of Ganodermalucidum Spores on Antioxidatizing Reaction in the Retinal Tissue of Diabetic Rats. Chin Arch Tradit Chin Med 2008;26:637-8.
- Yang LP, Sun HL, Wu LM, Guo XJ, Dou HL, Tso MO, et al. Baicaleinreduces inflammatory process in a rodent model of diabetic retinopathy. Invest Ophthalmol Vis Sci 2009;50:2319-27.
- Kowluru RA, Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. NutrMetab 2007;4:8.
- Kumar B, Gupta SK, Srinivasan BP, Nag TC, Srivastava S, Saxena R. Hesperetin ameliorates hyperglycemia induced retinal vasculopathy via antiangiogenic effects in experimental diabetic rats. VasculPharmacol 2012;57:201-7.
- Chen Y, Li XX, Xing NZ, Cao XG. Quercetin inhibits choroidal and retinal angiogenesis in vitro. Graefes Arch ClinExpOphthalmol 2008;246:373-8.
- Kim JH, Lee BJ, Kim JH, Yu YS, Kim MY, Kim KW. Rosmarinic acid suppresses retinal neovascularization via cell cycle arrest with increase of p21 (WAF1) expression. Eur J Pharmacol 2009;615:150-4.
- Kern TS, Engerman RL. Aldose reductase and the development of renal disease in diabetic dogs. J Diabetes Complications 1999;13:10-6.