- *Corresponding Author:
- S. Lankalapalli
Raghu college of pharmacy, Dakamarri, Bheemunipatnam, Visakhapatnam 531 162, India
E-mail: lsrinivas2001@yahoo.com
| Date of Submission | 09 March 2009 |
| Date of Revision | 11 August 2009 |
| Date of Acceptance | 20 August 2009 |
| Indian J Pharm Sci, 2009, 71 (5): 481-487 |
Abstract
Over the past several years, great advances have been made towards novel drug delivery systems. The phenomena of interpolymer interactions and formation of polyelectrolyte complexes have been the focus of intensive fundamental and applied research. Interpolyelectrolyte complexes combine unique physicochemical properties with high biocompatibility. Studies have been carried out on many different polymer blends and types. Such combinations may possess unique properties that are different from those of individual component. The present review emphasizes on the applicability of polyelectrolyte complexes in drug delivery technology.
Keywords
Polyelectrolyte complex, Polymer, Drug delivery, Polyion, Electrostatic interaction
Current state of art is witnessing a revolution in new techniques for drug delivery. These techniques are capable of controlling the rate of drug delivery, sustaining the duration of therapeutic activity and/ or targeting the drug to specific tissues. These advancements led to the development of several novel drug delivery systems, revolutionizing the medication with several advantages.
Recent decades witnessed the appearance of polymers that respond in some desired way to changes in temperature, pH, electric or magnetic field. The driving force behind these transitions include stimuli like neutralization of charged groups by either a pH shift or the addition of an oppositely charged polymer, changes in the eff ciency of hydrogen bonding with an increase in temperature or ionic strength and collapse of hydrogels and interpenetration of polymer network. These types of polymers not only convert the active substances into a non-deleterious form which can be administered, but also have specif c effect on the biodistribution, bioavailability or absorption of the active substances and hence increasingly gaining importance in modern pharmaceutical technology. The interaction between two oppositely charged polymers results in the formation of a complex, termed as polyelectrolyte complex [1]. These polyelectrolyte complexes meet the profile of requirements of biocompatible polymer systems and can be adapted to meet the various requirements like carrier substances and components for active substances.
Polyelectrolyte complexes (PECs) are the association complexes formed between oppositely charged particles (e.g. polymer-polymer, polymer-drug and polymer-drug-polymer). These are formed due to electrostatic interaction between oppositely charged polyions. This avoids the use of chemical cross linking agents, thereby reducing the possible toxicity and other undesirable effects of the reagents. The polyelectrolyte complexes formed between a poly acid and poly base are little affected by the pH variation of the dissolution medium. This concept of complexation, between DNA and chitosan [2], has extensively been studied in the development of delivery vehicle for gene therapy and oral vaccination.
The occurrences of charge-charge interactions between ionic polymers and drugs were considered to be a negative event when the ionic polymers are used as excipients in pharmaceutical formulations. In these systems release of drugs may be strongly affected by the occurrence of charge-charge interactions. However, in recent years these negative events of polymer-drug and polymer-polymer interactions have been exploited positively for controlled drug release [3,4].
Polyelectrolytes
The polymers that contain a net negative or positive charge at near neutral pH are called polyelectrolytes [5]. They are generally soluble in water. Their solubility is driven by the electrostatic interactions between water and the charged monomer. Examples of such polymers include DNA, protein, certain derivatives of cellulose polymers and carragenan.
Classif cation of polyelectrolytes [6]
The polyelectrolytes are classified into various types. Based on origin they are classified as natural polyelectrolytes, synthetic polyelectrolytes and chemically modif ed biopolymers. Based on composition they are homopolymers and copolymers. Based on molecular architecture linear, branched and cross linked. Based on electrochemistry they are classified as polyacids/polyanions, polybases/polycations and polyampholytes. Some of the important polyelectrolytes are exemplif ed in Table 1.
| Name | Category(based on thecharge type) |
|---|---|
| Natural Polyelectrolytes | |
| Nucleic acids | Polyanion |
| Poly (L-lysine) | Polycation |
| Poly (L-glutamic acid) | Polyanion |
| Carrageenan | Polyanion |
| Alginates | Polyanion |
| Hyaluronic acid | Polyanion |
| Chemically modifed biopolymers | |
| Pectin | Polyanion |
| Chitosan (deacetylation of chitin) | Polycation |
| Cellulose-based | Polyanion or polycation |
| Starch-based | Polyanion or polycation |
| Dextran-based | Polyanion or polycation |
| Synthetic polyelectrolytes | |
| Poly (vinylbenzyltrialkyl ammonium) | Polycation |
| Poly (4-vinyl-N-alkyl-pyridimiun) | Polycation |
| Poly (acryloyl-oxyalkyl-trialkyl ammonium) | Polycation |
| Poly (acryamidoalkyl-trialkyl ammonium) | Polycation |
| Poly (diallydimethyl-ammonium) | Polycation |
| Poly (styrenesulfonic acid) | Polyanion |
| Poly (vinylsulfonic acid0 | Polyanion |
| Poly (acrylic or methacrylic acid) | Polyanion |
| Poly (itaconic acid) | Polyanion |
| Maleic acid/ diallyamine copolymer | Polyampholytic |
Table 1: Some of the important polyelectrolytes
Theoretical aspects of PECs
Many researchers extensively investigated the properties of the polyelectrolytes [6,7] and the formation of PECs [8-10]. There have been theories proposed based on the electrostatic forces and Flory-Huggins mixing free energies of the polyelectrolytes to explain the mechanism of formation of PECs [11-13]. In general the backbones of the two polymers are not compatible and repel to each other, however, the charge fraction of the polymers determines the type of interaction going to occur between the polymers. When the charge fraction is low, the polymer backbone repulsion (Flory interaction parameter) is dominant and the solution separates in to two phases each containing mostly one of the polymers. At high charge fraction, the attractive electrostatic interactions between the polymers dominate and they precipitate to form a complex. In an intermediate range of charge fraction, the equilibrium state can be a meso phase where the two polymers only separate microscopically. Depending on the stoichiometry of the mixture (the relative concentrations, the relative chain lengths and charge densities), one observes mainly two types of complex formations, a macroscopic phase separation between the solvent and the polymers or a partial aggregation of the polymer chains [14].
Formation of PECs
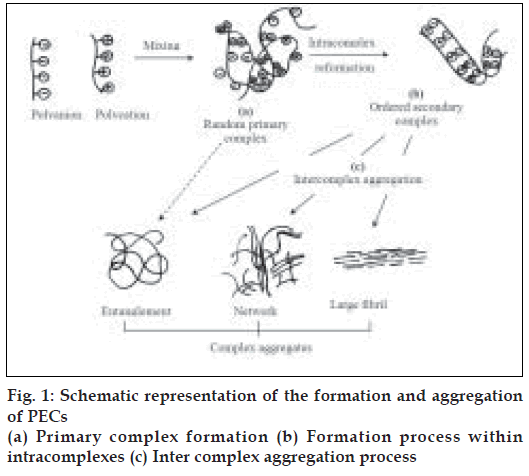
This process involves mainly 3 steps as shown in fig. 1 [15]. First step is primary complex formation and Coulomb forces are responsible for this step. Second step is formation process within intracomplexes. It involves formation of new bonds and/or the correction of the distortions of the polymer chains. Third is intercomplex aggregation process, which involves the aggregation of secondary complexes mainly through hydrophobic interactions.
Factors affecting the formation of PECs
A number of parameters are known to inß uence the formation of PECs [16]. These are ion site, charge density, polyelectrolyte concentration, pH, ionic strength, solvents and temperature. Several workers evaluated the factors effecting the formation of polyelectrolyte complexes with different polymeric blends [17,18]. Precipitation is caused by effective attractions due to charge fluctuations and by short range attractions between monomers. Interesting in the context of the polyion stoichiometry are studies of layer formation from strongly asymmetric pairs of polyions. Employing polyions with a reduced charge density along the chain, i.e. consisting of charged and uncharged co-monomers, it was observed that a minimum charge density [19] is required for polyelectrolyte adsorption. Changing the ionic strength by addition of salt [20] can modulate the electrostatic interactions in a polyelectrolyte solution. The electrostatic interactions can be weakened by addition of inorganic salts into the solutions. Thus, an increase of the ionic strength of the solution depresses the complexation between polyions, because of the screening of opposite charges of the macromolecules by low molecular weight ions. By varying the pH environment during PEC formation, the degree of ionization of weak polyelectrolytes can be controlled [21,22]. This was found to affect multilayer properties such as layer thickness, the degree of interpenetration between layers, surface wettability and number of unbound functional groups. Therefore, by choosing the right pH conditions, a platform may be found with properties that are advantageous for loading charged small molecules into the film via electrostatic interactions.
Characterization of PECs
Various methods have been used to investigate interactions between polymers [23]. Measurements of turbidity, pH and ionic strength [24,25] as a function of weight ratio of polymer in the media [26], viscosity [27], light scattering [28,29], infrared spectroscopy, NMR, thermal analysis, pKa and powder X-ray diffraction [30] were employed to evaluate interpolymer complexation.
Applications of PECs
PECs have gained much attention in the past few years because of their potential applications. These can be used as membranes [31-33], for coating on f lms and f bers [34], for isolation and fractionation of proteins [35,36], for isolation of nucleic acid [37-39], for binding pharmaceutical products [40], as supports for catalyst [41] and for preparation of microcapsules for drug delivery [42,43]. Many of the applications are based on the functional properties of the polyelectrolyte. The functional applications of PECs are summarized in Table 2.
| Functional property | Application | |
|---|---|---|
| Interaction with counter ions | Support of Þltration process- removal of counter ions [61,62] | |
| Gelation process-bridging with multivalent counter ions [63] | ||
| Analytical methods-counter ion exchange [64] | ||
| Interaction with surfactants | Insoluble polyion surfactant complex for low energy surface modification [65] | |
| Highly ordered structures (micelle) formation [66,67] | ||
| Interaction with charged low molar mass molecules | Polyion drug complexes-soluble or insoluble [68] | |
| Interaction with charged particles | Floculation-waste water treatment [69] | |
| Dewatering-sludge, pulp and paper production [70] | ||
| Flotation-mining | ||
| Retention-paper production | ||
| Interaction with charged surfaces | Displacement chromatography-separation and concentration of biomolecules [38] | |
| ModiÞcation of surfaces and interfaces-coating (antistatic, sensors, | ||
| multi-layer) [71,72] | ||
| Additive (cosmetics, detergents) |
Table 2: Practical application of polyelectrolyte complexes
The concept of PECs in the design of drug delivery systems may be useful due to the advancements made during the last two decades [44-47]. The active components will be encapsulated in the polymer matrix at molecular level. They offer greater advantages for the drug substances through improving/ and altering physicochemical characters like stability and dissolution. The active substance can be incorporated in to PECs by four ways [48]. In the f rst case the active substance will be entrapped from the solution during precipitation of the complex. The active substance will be absorbed from the solution and gets incorporated in to the already formed complex on contact in the second case. In the third case the active substance may be chemically bound to at least one complex partner and precipitates during complexation. In the last case the active compound itself may act as poly ion and form PEC. The active substance from these PECs will be released either by solution equilibration or by ion exchange mechanism or by charge interaction and slow decomplexation as well as breakdown and dissolution of the complex.
There are several reports on different methods of preparation and applications of PECs in pharmacy. Kawashima et al. [49] developed a novel method for the preparation of theophylline granules coated with a PEC of sodium tripolyphosphate and chitosan. The theophylline granules containing sodium tripolyphosphate were stirred in an HCl solution of chitosan. During the mixing, the dissolved sodium tripolyphosphate in the granules moved to the surface and reacted with the chitosan, resulting in the formation of a PEC f lm. The drug-release pattern of the coated granules followed zero-order kinetics and the release rates were signif cantly reduced compared with that of the original granules.
Shiraishi et al. [50] studied the controlled drug release behaviour of indomethacin by chitosan-PEC. They also optimized the formulation conditions and reported its in-vivo/in-vitro evaluation studies. They prepared the PEC of indomethacin by using complexation of sodium tripolyphosphate and chitosan. Here the effects of the molecular weights of chitosan hydrolysates on the release and absorption rates of indomethacin from gel beads were examined. The release rates of indomethacin decreased with increasing of molecular weight and indomethacin content. A negative correlation was observed between the molecular weight and release rate constant (r=-0.983).
Jimenez-Kairuz et al. [51] developed and characterized swellable drug-polyelectrolyte matrices (SDPM) using carbomer and different basic drugs like atenelol, lidocaine and metoclopramide. The authors concluded that drugs can be loaded in a high proportion on to the polymer and therefore the resulting SDPM material could be diluted with other polymers to modulate delivery properties of SDPM. Matrices of atenolol and lidocaine exhibited robust delivery properties with regard to change in proportion of loading drug. Liao et al. [52] prepared drug-loaded chitosan-alginate f bers by interfacial polyelectrolyte complexation technique. Depending on the component properties, the release time of encapsulated components from these fi bers could range from hours to weeks. Dexamethasone was completely released within 2 h, whereas charged compounds such as bovine serum albumin, PDGF-bb, and avidin showed sustained release for 3 w. In this study, interfacial polyelectrolyte complexation demonstrated to be a promising technique for producing drug-loaded fibers with high encapsulation efficiency, sustained release kinetics, and capacity to retain the bioactivity of the encapsulants.
Tapia et al. [53] evaluated the possibility of using mixtures of PECs from both chitosan (CS)-alginate and CS-carrageenan as prolonged release systems. Different dissolution prof les for diltiazem clorhydrate were obtained by changing the polymer matrix system (CS-alginate or CS-carrageenan) and the method used to include these polymers into the formulation (physical mixture or PEC). Drug dissolution prof les from the matrices have been discussed by considering the swelling behavior of the polymers used. They reported that CS-alginate systems were considered to be better in prolonging the release when compared to CS-carrageenan systems.
Paloma et al. [54] prepared polyionic complexes of CS and poly(acrylic acid) (PAA) in a wide range of copolymer composition and with two kinds of drugs. Release of amoxicillin trihydrate and amoxicillin sodium from these different complexes was studied. The swelling behavior of and solute transport in swellable hydrogels were investigated to check the effect of polymer/polymer and polymer/drugs interactions. The electrostatic polymer/polymer interactions took place between the cationic groups from CS and the anionic ones from PAA. The diffusion of amoxicillin trihydrate was controlled only by the swelling/eroding ratio of the polyionic complexes. The swelling degree of amoxicillin sodium hydrogels was more extensive when compared to the swelling degree of amoxicillin trihydrate formulations. It was concluded that the water uptake was mainly governed by the degree of ionization. Restriction of amoxicillin sodium diffusion could be achieved by polymer/ionized-drug interaction that retards the drug release. Win et al. [55] developed PEC gel beads based on phosphorylated chitosan (PCS) for controlled release of ibuprofen in oral administration. The PCS gel beads were prepared from soluble phosphorylated chitosan by using an ionotropic gelation with counter polyanion, tripolyphosphate (TPP) at pH 4. Surface morphology studies for the prepared beads were done by using SEM. The percentage release of ibuprofen from PCS gel beads was found to be increased as the pH of the dissolution medium increased. The release rate of ibuprofen at pH 7.4 was higher than the release rate at pH 1.4 due to the ionization of phosphate group and higher solubility of ibuprofen at pH 7.4 medium. The ability of the prepared copolymer to be used as drug carrier for colonspecific drug delivery system was estimated using ketoprofen as model drug.
Albeno et al. [56] obtained a patent for preparation of stable water insoluble complexes of poorly soluble compounds molecularly dispersed in water insoluble ionic polymers. The compounds were micro precipitated in the ionic polymers in amorphous form. The complexes according to the present invention significantly increased the bioavailability of poorly soluble therapeutically active compounds.
Rolfes et al. [57] reported a method of making a solid interpolymer complex for use as a controlled release matrix for oral administration. The process involved mixing of two oppositely charged polymers and spray-dried to evaporate the solvent and to prepare solid particles of interpolymer complex. An active agent such as drug can be preferably embedded or encapsulated in the interpolymer complex before spray drying or may be incorporated by suitable means at a later stage. Mi et al. [58] employed enzyme hydrolyzed CS to prepare CS tripolyphosphate and CS polyphosphoric acid gel beads using a polyelectrolyte complexation method for the sustained release of anticancer agent, 6-mercaptopurine. Nandini and Cherng-Ju [59] developed drug PECs with poly(acrylamido-2-methyl-1-propansulfonate sodiumco- methyl-methacrylate. They studied and reported that the release kinetics were strongly dependent on the drug solubility rather than on the type of amine in the drug. The release of drugs from the tablets of drug-poly(acrylamide-2-methyl-1-propane sulfonate sodium-co-methyl methacrylate complex were well described by the dissociation/erosion mechanism. Petzold et al. [60] prepared different PECs from poly(diallyl-dimethyl-ammoniumchloride) and two different polyanions and characterized their application as flocculants. The results showed that the most important advantages of PEC were the high velocity of sedimentation and a very broad range of the optimum flocculation concentration.
The review summarized emerging popularity and valuable potential offered by the polyelectrolyte complexes in the field of drug delivery. They represent an attractive class of polymer-based materials finding an irreplaceable role in many areas of the everyday life used for the preparation of biodegradable and biocompatible three-dimensional membranes, microcapsules, nano-sized formulations and various types of controlled release drug delivery systems.
Conclusion
An extensive research is going on in the area of polyelectrolytes and polyelectrolyte complexes. There is a great potential in utilizing these PECs in ecology, biotechnology, medicine and pharmaceutical technology. However these techniques are not effectively applied for the development of drug delivery systems. They may efficiently modify the release; improve the stability and character of the drug substances due to their capacity to entrap the drug at molecular level. Hence the polyelectrolyte complexes have great potential in the design of novel drug delivery systems.
Acknowledgements
One of the authors Mr. S. Lankalapalli is thankful to the Council of Scientif c and Industrial Research (CSIR), India for the award of Senior Research Fellowship to carryout the research work.
References
- Philipp B, Dautzenberg H, Linow K, Kotz J, Dawydoff W. Polyelectrolyte complexes-recent developments and open problems. Prog Polym Sci 1989;14:91-172.
- Krishnendu R, Hai-Quan M, Shau-Ku H, Kam WL. Oral gene delivery with chitosan-DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nature Med 1999;5:387-91.
- Genta I, Perugini P, Modena T, Pavanetto F, Castelli F, Muzzarelli RA, et al. Miconazole-loaded 6-oxychitin-chitosan microcapsules. CarbohydrPolym 2003;52:11-8.
- Macleod GS, Collett JH, Fell JT. The potential use of mixed flms of pectin, chitosan and HPMC for bimodal drug release. J Control Release 1999;58:303-10.
- Kubisa P. Terminology of Polymers Containing Ionizable or Ionic Groups and of Polymers Containing Ions, IUPAC Recommendations 2004, (DRAFT 23 December 2004).
- Available from: http://scgc.epß.ch/load/courschim/chwanreypart.5.pdf. [cited in 2004].
- Joanny JF, Castelnovo M. Polyelectrolyte adsorption and multiplayer formation. In: Decher G. Schlenoff JB, editors. Multilayer Thin Films. Weinheim: Wiley-VCH; 2002. p. 87-97.
- Webster L, Huglin MB, Robb ID. Complex formation between polyelectrolytes in dilute aqueous solution. Polymer 1997;38:1373-80.
- Webster L, Huglin MB. Observations on complex formation between polyelectrolytes in dilute aqueous solution. Eur Polym J 1997;339:1173-7.
- Burgess DJ. Practical analysis of complex coacervate systems. J ColloidInterfacial Sci 1990;140:227-38.
- Overbeek JT, Voorn MJ. Phase separation in polyelectrolyte solutions:Theory of complex coacervation. J Cell Comp Physiol 1957;49:7-26.
- Voorn MJ. Phase separation in polymer solutions. Fortschr Hochpolym-Forsc 1959;1:192-233.
- Xavier C, Jean-Francois J. Adsorption of polyelectrolyte solutions onsurface: A Debye-Huckel theory. J Phys II France 1996;6:1669-86.
- Tsuchida E. Formation of polyelectrolyte complexes and their structures.J Macromol Sci Pure Appl Chem 1994;A31:1-15.
- Kokufuta E. Colloid titration behavior of poly (ethyleneimine).Macromolecules 1979;12:350-3.
- Monika S, Layered polyelectrolyte complexes: Physics of formation andmolecular properties. J Phys Condens Matter 2003;15:R1781-808.
- Maarten P, Biesheuvel, Martien A, Cohen S. Cylindrical cell model forthe electrostatic free energy of polyelectrolyte complexes. Langmuir2004;20:4764-70.
- Fredheim GE, Christensen BE. Polyelectrolyte complexes: Interactionsbetween lignosulfonate and chitosan. Biomacromol 2003;4:232-9.
- Weinbreck F, de Vries R, Schrooyen P, de Kruif CG. Complexcoacervation of whey proteins and gum arabic. Biomacromol2003;4:293-303.
- Alexander K, Monica OD. Precipitation of oppositely chargedpolyelectrolytes in salt solutions. J Chem Phys 2004;20:404-12.
- Gamzazade AI, Nasibov SM. Formation and properties ofpolyelectrolyte complexes of chitosan hydrochloride and sodiumdextransulfate. Carbohydr Polym 2002;50:339-43.
- Shiratori SS, Rubner MF. pH-Dependent thickness behavior ofsequentially adsorbed layers of weak polyelectrolytes. Macromol2000;33:4213-9.
- Kumar V, Yang T, Yang Y. Interpolymer complexation. I.Preparation and characterization of a polyvinyl acetate phthalate-polyvinylpyrrolidone (PVAP-PVP) complex. Int J Pharm 1999;188:221- 32.
- Arguelles-Monal W, Cabrera G, Peniche C, Rinaudo M. Conductimetricstudy of the interpolyelectrolyte reaction between chitosan andpolygalacturonic acid. Polymer 2000;41:2373-8.
- Herman P, Van L, Rob FM, Cleven, Pavel V. Conductometric analysisof polyelectrolytes in solution. Pure App Chem 1991;63:1251-68.
- Takayama K, Nagai T. Application of interpolymer complexation ofpolyvinylpyrrolidone/carboxyvinyl polymer to control of drug release Chem Pharm Bull 1987;35:4921-7.
- Zhang LM. Synergistic blends from aqueous solutions of two cellulosederivatives. Colloid Polym Sci 1999;277:886-90.
- Herbert D, Light scattering studies on polyelectrolyte complexes.Macromolecular Symposia 2001;162:1-22.
- Natalia VP, Nickolay VT. Structure and dynamics of the polyelectrolytecomplex formation. Macromol 1997;30:4897-904.
- Anlar S, Çapan Y, Güven O, Gögüs A, Dalkara T, Hincal A.Formulation and in-vitro-in-vivo evaluation of buccoadhesive morphinesulfate tablets. Pharm Res 1994;11:231-6.
- Senuma M, Kuwabara S, Kaeriyama K, Hase F, Shimura Y. Polymercomplex from copolymers of acrylonitrile and ionic vinyl benzylcompounds. J Appl Polym Sci 1986;31:1687-97.
- Sato H, Maeda M, Nakajima A. Mechanochemistry and permeability ofpolyelectrolyte complex membranes composed of poly (vinyl alcohol)derivatives. J Appl Polym Sci 1979;23:1759-67.
- Harris EL, Angal S. Protein purifcation methods: A practical approach.New York: Oxford University Press; 1993.
- Yamamoto H, Horita C, Senoo Y, Nishida A, Ohkawa K. Polyioncomplex fber and capsule formed by self-assembly of chitosan andgellan at solution interfaces. Macromol Chem Phys 2000;201:84-92.
- Hirouki Y, Takeshi K. Adsorption of BSA on cross-linked chitosan: Theequilibrium isotherm. Chem Eng Japan 1989;41:B11-5.
- Dubin PL, Gao J, Mattison K. Protein purifcation by selective phaseseparation with polyelectrolytes. Sep Purif Methods 1994;23:1-16.
- Cordes RM, Sima WB, Glatz CE. Precipitation of nucleic acids withpoly (ethyleneimine). Biotechnol Prog 1990;6:283-5.
- Atkinson JG. Precipitation of nucleic acids with polyethyleneimine andthe chromatography of nucleic acids on immobilized polyethyleneimine.Biochim Biophys Acta 1973;308:41-52.
- Jendrisak J. In: Burgerss R, editors Protein purification: Micro tomacro. New York: Alan R Liss Inc; 1987. p. 75-97.
- Chen J, Jo S, Park K. Polysaccharide hydrogels for protein drugdelivery. Carbohydr Polym 1995;28:69-76.
- Dautzenberg H, Kotz J, Linow KJ, Philipp B, Rother G. Static lightscattering of polyelectrolyte complex solutions. In: Dubin P, Bock J,Davis R, Schulz DN, Thies C, editors. Macromolecular complexes inchemistry and biology. Berlin: Springer Verlag; 1994. p. 119-33.
- Artur B, David H. Carrageenan-oligochitosan microcapsules:Optimization of the formation process. Colloids Surf B: Biointerfaces2001;21:285-98.
- Murakami R, Takashima R. Mechanical properties of the capsules ofchitosan-soy globulin polyelectrolyte complex. Food Hydrocolloids2003;17:885-8.
- Kabanov VA. In: Dubin P, Bock J, Davis R, Schulz DN, Thies C,editors. Macromolecular Complexes in Chemistry and Biology. NewYork: Springer; 1994.
- Leclercq L, Boustta M, Vert M. A physico-chemical approach ofpolyanion-polycation interactions aimed at better understanding thein vivo behaviour of polyelectrolyte-based drug delivery and genetransfection. J Drug Targeting 2003;11:129-38.
- Kekkonen J, Lattu H, Stenius P. Adsorption kinetics of complexesformed by oppositely charged polyelectrolytes. J Colloid Interface Sci2001;234:384-92.
- Shojaei AH. Buccal mucosa as route for systemic drug delivery: Areview. J Pharm Pharmaceut Sci 1998;1:15-30.
- Krone V, Magerstadt M, Walch A, Groner A, Hoffmann D.Pharmacological composition containing polyelectrolyte complexes inmicroparticulate form and at least on active agent. United States patent5,700,459, Dec 23, 1997.
- Kawashima Y, Handa T, Kasai A, Takenaka H, Lin SY, Ando Y. Novelmethod for the preparation of controlled-release theophylline granulescoated with a polyelectrolyte complex of sodium polyphosphate-chitosan. J Pharm Sci 1985;74:264-8.
- Shiraishi S, Imai T, Otagiri M. Controlled release of Indomethacin bychitosan polyelectrolyte complex: Optimization and in vivo/in vitroevaluation. J Control Release 1993;25:217-25.
- Jimenez-Kairuz AF, Llabot JM, Allemandi DA, Manzo RH. Swellabledrug-polyelectrolyte matrices (SDPM): Characterization and deliveryproperties. Int J Pharm 2005;288:87-99.
- Liao I-C, Wan ACA, Yim EK, Leong KW. Controlled release fromfbers of polyelectrolyte complexes. J Control Release 2005;104:347-58.
- Tapia C, Escobar Z, Costa E, Sapag-Hagar J, Valenzuela F, Basualto C,et al. Comparative studies on polyelectrolyte complexes and mixturesof chitosan-alginate and chitosan-carrageenan as prolonged diltiazemclorhydrate release systems. Eur J Pharm Biopharm 2004;57:65-75.
- Paloma M, de la T, Yewande E, Guillermo T, Susana T. Release ofamoxicillin from polyionic complexes of chitosan and poly(acrylicacid): Study of polymer/polymer and polymer/drug interactions withinthe network structure. Biomaterials 2003;24:1499-506.
- Win PP, Shin-ya Y, Hong KJ, Kajiuchi T. Formulation andcharacterization of pH sensitive drug carrier based on phosphorylatedchitosan (PCS). Carbohydr Polym 2003;53:305-10.
- Albano AA, Phuapradit W, Sandhu HK, Shah NH. Stable complexesof poorly soluble compounds in ionic polymers. United States Patent6,350,786, 2002.
- Rolfes H, Van Der Merve TL, Truter PA. Method of making controlledrelease particles of complexed polymers. United States Patent6,221,399, 2001.
- Mi FL, Shyu SS, Kuan CY, Lee ST, Lu KT, Jang SF. ChitosanPolyelectrolyte complexation for the preparation of gel beads andcontrolled release of anticancer drug: I: Effect of phosphorouspolyelectrolyte complex and enzymatic hydrolysis of polymer. J ApplPolym Sci 1999;74:1868-79.
- Nandini K, Cherng-ju K. Drug release from drug-polyanion complextablets: Poly(acrylamido-2-methyl-1-propanesulfonate sodium-co-methylmethacrylate). J Control Release 1999;57:141-50.
- Petzold G, Nebel A, Buchhammer H-M, Lunkwitz K. Preparationand characterization of different polyelectrolyte complexes and theirapplication as ßocculants. Colloid Polym Sci 1998;276:125-30.
- Dainiak MB, Izumrudov VA, Muronetz VI, Galaev IY, Mattiasson B.Affnity precipitation of monoclonal antibodies by nonstoichiometricpolyelectrolyte complexes. Bioseparation 1998;7:231-40.
- Izumrudov VA, Galaev IY, Mattiasson B. Polycomplexes--potential forbioseparation. Bioseparation 1998;7:207-20.
- Kim B, Peppas NA. Analysis of molecular interactions in poly(methacrylic acid-g-ethylene glycol) hydrogels. Polym 2003;44:3701-7.
- Pirogov AV, Shpak AV, Shpigun OA. Application of polyelectrolytecomplexes as novel pseudo-stationary phases in MEKC. Anal BioanalChem 2003;375:1199-203.
- Barreiro-Iglesias R, Alvarez-Lorenzo C, Concheiro A. Controlled releaseof estradiol solubilized in carbopol/surfactant aggregates. J ControlRelease 2003;93:319-30.
- Lee J, Cho EC, Cho K. Incorporation and release behavior ofhydrophobic drug in functionalized poly (lactide)-block-poly (ethyleneoxide) micelles. J Control Release 2004;94:323-35.
- Swanson VM, Dubin PL, Almgren M, Li Y. Cryo-TEM ofpolyelectrolyte-micelle complexes. J Colloid Interface Sci1997;186:414-9.
- Cherng-Ju K, Yvonne NN. Drug release from an erodible drug-polyelectrolyte complex. Eur Polym J 1995;31:937-40.
- Anders L, Charlotte W, Staffan W. Flocculation of cationic polymersand nanosized particles. Colloids Surfaces A Physicochem Eng Aspects1999;159:65-76.
- Janne L, Lindström T. Topo chemical modifcation of cellulosic fberswith bipolar activators: An overview of some technical applications. SciTechnol 2001;1:40-5.
- Mendelsohn JD, Barrett CJ, Chan VV, Pal AJ, Mayes AM, Rubner MF.Fabrication of microporous thin flms from polyelectrolyte multilayers.Langmuir 2000;16:5017-23.
- Yan X, Khor E, Lim LY. PEC flms prepared from Chitosan-Alginate coacervates. Chem Pharm Bull 2000;48:941-6.